Abstract
The mechanism of flecainide-induced unexpected death remains uncertain. Phase-2 ventricular arrhythmias occur during infarct evolution. We examined whether flecainide (0.74 and 1.48 μM, representing the peak unbound plasma and total blood concentrations, respectively, at ‘therapeutic' dosage) has proarrhythmic activity on phase-2 arrhythmia susceptibility during infarct evolution.
To achieve this, we used the Langendorff-perfused rat heart preparation (n=8 per group) in which baseline phase-2 arrhythmia susceptibility is low. Left main coronary occlusion evoked phase-1 (acute ischaemia-induced) ventricular arrhythmias including fibrillation (VF) in all hearts. By 90 min, hearts were relatively arrhythmia-free.
Randomized and blinded switch of perfusion to flecainide at 90 min caused no increase over baseline in the incidence of VF, tachycardia (VT) or premature beats (VPB) during the following 150 min of ischaemia, or during reperfusion (begun 240 min after the onset of ischaemia).
In separate hearts, catecholamines (313 nM norepinephrine and 75 nM epinephrine) were co-perfused with flecainide from 90 min of ischaemia. Catecholamine perfusion increased heart rate, coronary flow and QT interval, and shortened PR interval (all P<0.05), actions that were not altered by flecainide. Catecholamine perfusion caused a weak nonsignificant increase in phase-2 VPB, VT and VF incidence, but there was no proarrhythmic interaction with flecainide.
In conclusion, the present findings suggest that the increased risk of death associated with clinical use of flecainide is not due to facilitation of phase-2 ventricular arrhythmias.
Keywords: Class I antiarrhythmics, Langendorff preparation, myocardial infarction, phase-2 arrhythmias, proarrhythmia, reperfusion, safety-pharmacology, ventricular fibrillation
Introduction
Flecainide increased mortality following myocardial infarction (MI) in the Cardiac Arrhythmias Suppression (CAST) trial (Echt et al., 1991) in which patients surviving acute MI (and who are characteristically at high risk of further acute MI) were randomized to receive maintenance antiarrhythmic therapy or placebo. The mechanism responsible was not determined, although it has been speculated that flecainide exacerbated the risk of ventricular fibrillation (VF) either during the early phase of ischaemia or later, during the infarct evolution phase (Greenberg et al., 1995; Hallstrom et al., 1995). It would be of value to explore this in more detail. It is no longer possible to establish the mechanism by further clinical investigation.
Animal models of one-stage maintained coronary obstruction (to mimic severe acute MI) allow investigation of the arrhythmias, including VF, that occur in discrete bursts during the early phase of ischaemia and, later, during the infarct evolution phase. A first peak occurs during the first 15 min of ischaemia (Phase-1a), a second between 15 and 45 min (phase-1b) and a third (phase-2) following a hiatus and coinciding with infarct evolution beginning approximately 90 min after the start of ischaemia (Clark et al., 1980; Meesmann, 1982; Curtis et al., 1987; Pugsley et al., 1995). With temporary ischaemia, reperfusion can evoke arrhythmias too, with susceptibility dependent on ischaemia duration (Manning & Hearse, 1984). It is inherent (and supported by experimental evidence) that these different manifestations of arrhythmias, occurring at different stages of MI pathology, have different underlying mechanisms, and are likely to respond differently to antiarrhythmic and proarrhythmic drugs (Curtis & Hearse, 1989a, 1989b; Clements-Jewery et al., 2005).
Animal studies conducted to evaluate flecainide's possible proarrhythmic effects have focused largely on phase-1 arrhythmias, and the outcomes have been inconsistent. In an early study in dogs, a strong proarrhythmic effect of flecainide on phase-1a VF was identified, yet reperfusion-induced VF was suppressed (Wenger et al., 1985). More recently, using a perfused rat heart preparation to allow controlled concentration–response analysis, we observed a suppression of phase-1b VF with flecainide, yet saw a possible proarrhythmic hastening of phase-1b VF onset, and no suppression of reperfusion-induced VF (Farkas & Curtis, 2002). During stable MI (72 h after the onset of ischaemia) in dogs, flecainide was reported to cause marked proarrhythmia, but substantial proarrhythmia was also reported in healthy control dogs (Ranger & Nattel, 1995).
The notion that a drug may suppress one phase of VF yet fail to protect against, or even potentiate, another phase of VF has been of limited clinical interest (Campbell, 1987; Greenberg et al., 1995), but has recently received renewed attention since it has been argued that for a drug to be of real value in suppressing VF it would need to be effective and safe in all clinical circumstances post-MI, circumstances that encompass acute ischaemia, evolving and stable infarction and reperfusion (Clements-Jewery et al., 2005). This also has potential relevance to the emerging field of safety pharmacology (Clements-Jewery et al., 2005).
In view of this, we have adapted the model used in our previous studies on flecainide's actions during acute ischaemia (Farkas & Curtis, 2002) to examine whether flecainide has proarrhythmic activity during phase-2 arrhythmia susceptibility, and during susceptibility to ‘late' reperfusion-induced arrhythmias after sustained (240 min) ischaemia. The isolated perfused rat heart is suitable for this because the baseline susceptibilities to phase-2 arrhythmias, and late reperfusion-induced arrhythmias, are low, allowing scope for detection of proarrhythmic activity (Ravingerova et al., 1995; Clements-Jewery et al., 2002). The main focus of the present study was phase-2 arrhythmias, but we were able to additionally examine susceptibility to immediate reperfusion-induced arrhythmias, which may have relevance to reperfusion arrhythmia susceptibility following in-hospital thrombolysis, as the timing of reperfusion (at 4 h) fell within the entry criteria timeframe typically used in thrombolysis trials (e.g., within 6 h after the onset of chest pain; Brener et al., 2005).
Phase-2 arrhythmias in the perfused rat heart preparation are weakly (nonsignificantly) facilitated by catecholamine challenge (Clements-Jewery et al., 2002). Furthermore, it has been speculated that an interaction between catecholamines and flecainide contributes to flecainide's proarrhythmic propensity (Packer et al., 1997). Therefore, the actions of flecainide were additionally examined during catecholamine perfusion.
Methods
Animals and general experimental methods
All methods have been described previously (Clements-Jewery et al., 2002; Farkas & Curtis, 2002). Male Wistar rats (180–250 g; Bantin and Kingman, U.K.) were anaesthetized with pentobarbitone (60 mg kg−1 i.p.) mixed with 250 I.U. sodium heparin to prevent blood clot formation in the coronary vasculature. Once surgical-depth anaesthesia had been reached (normally 5–10 min after administration of pentobarbitone and heparin), hearts were excised and placed into ice-cold control solution containing (in mM), NaCl 118.5, NaHCO3 25.0, MgSO4 1.2, NaH2PO4 1.2, CaCl2 1.4, KCl 3 and glucose 11.1, then perfused according to Langendorff, with warmed and gassed control solution (37°C and pH 7.4). All control and test solutions were filtered (5 μm pore size) before use. Perfusion pressure was maintained constant at 70 mmHg. A unipolar electrogram (ECG) was recorded from the centre of the region to become ischaemic (against an electrode attached to the metal aortic cannula). A traction-type coronary occluder consisting of a silk suture (Mersilk, 4/0) threaded through a polythene guide was positioned loosely around the left main coronary artery beneath the left atrial appendage, and tightened and loosened, respectively, to achieve regional ischaemia and reperfusion.
Experimental protocol
Six groups of hearts were used (n=8 per group). All hearts were perfused for an initial 10 min with control solution, and then regional ischaemia was induced by tightening the coronary ligature. At 90 min of ischaemia, after the appearance and subsequent disappearance of phase-1 arrhythmias (Clements-Jewery et al., 2002), the perfusion solution was switched in a randomized and blinded fashion from control Krebs' to one of six modified Krebs' solutions: control (vehicle), flecainide 0.74 or 1.48 μM, catecholamine solution (313 nM norepinephrine plus 75 nM epinephrine) or catecholamines plus 0.74 or 1.48 μM flecainide. The recipes for these modifications and the rationale for their inclusion are given below. The choice of solution was made by reference to a randomisation table. After a further 150 min of ischaemia (making a total of 240 min continuous ischaemia), the occluder was released to achieve reperfusion.
Measurement of involved zone size and coronary flow
At the end of 5 min of reperfusion, the size of the involved zone (the region subjected to ischaemia and reperfusion) was quantified using the disulphine blue dye exclusion method (Curtis & Hearse, 1989a, 1989b) and expressed as per cent of total ventricular weight. Coronary flow was measured by timed collection of coronary effluent. Values of coronary flow in the uninvolved and the reperfused tissues were calculated from the total coronary flow and the weights of the involved zone and the uninvolved zone, as described previously (Curtis & Hearse, 1989a, 1989b).
Arrhythmia diagnosis and ECG analysis
The ECG was recorded using a PowerLab™ system. Arrhythmias were defined according to the Lambeth Conventions (Walker et al., 1988). Ventricular premature beats (VPBs) were defined as discrete and identifiable premature QRS complexes and a run of four or more VPBs was defined as ventricular tachycardia (VT). VF was defined as a signal from which individual QRS deflections varied in amplitude and coupling interval on a cycle to cycle basis. From the ECG, the incidence and the time to onset of ventricular arrhythmias, the PR interval, RR interval and the QT interval (measured at the point of 90% repolarization with on-screen cursors) were obtained. QT interval was not corrected for heart rate as it is not rate-dependent in perfused rat hearts (Rees & Curtis, 1993) (nor is it in rats in vivo; Hayes et al., 1994). Measurement of all variables was performed in a blinded manner.
Rationale for choice of drug concentrations and recipes for preparation of solutions
The choice of flecainide concentration was made as before (Farkas & Curtis, 2002) and was based on clinical data on the mean peak unbound and mean peak total plasma concentrations, taking into account plasma protein binding (Woosley et al., 1984; Benet et al., 1996). The concentrations of norepinephrine and epinephrine were chosen on the basis of their ability to restore heart rate (values of which are always lower in perfused hearts compared with values in vivo; Curtis, 1998) to values similar to those typical for conscious rats (Clements-Jewery et al., 2002) and provide a catecholamine concentration ratio that is within the range encountered in man under conditions of stress (Baumgartner et al., 1985; Ratge et al., 1986; Coplan et al., 1989).
Drug stocks were prepared fresh each week and perfusion solutions were prepared fresh each day. ‘Vehicle stock' was an 8 ml solution containing 6 ml ethanol and 2 ml water. The control solution contained 0.24 ml of ‘vehicle stock' in 1 l of modified Krebs (i.e., 0.018% ethanol). The drug solutions were prepared from 8 ml of a ‘drug stock'. Drug stock for the low concentration consisted of 11.7 mg flecainide dissolved in 6 ml ethanol plus 2 ml water, while drug stock for the high concentration consisted of 23.4 mg flecainide dissolved in 6 ml ethanol plus 2 ml water, such that 0.24 ml of these stocks dissolved in 1 l of modified Krebs obtained the drug solutions (flecainide 0.74 or 1.48 μM), while, all solutions contained the same amount of ethanol (0.018%).
All salts were reagent grade chemicals from Sigma Chemical Co. Water for preparing perfusion solution was supplied using a reverse osmosis system (Maxima Ultrapure™, Elga) and had a specific resistivity of more than 18 M Ohm.
Exclusion criteria
Any heart with a sinus rate of less than 250 beats min−1, or a coronary flow more than 23 ml min−1 g−1or less than 7.5 ml min−1 g−1 5 min before the onset of ischaemia, or an involved zone of less than 30% or more than 50% of total ventricular weight was excluded. All excluded hearts were replaced to maintain equal group sizes. Any heart not in sinus rhythm during the 2 s before the start of reperfusion is normally excluded from the reperfusion sample and replaced, but in the present study this was not necessary.
Statistics
Gaussian distributed variables, expressed as mean±s.e.m., were subjected to analysis of variance. If treatment constituted a significant source of variance, flecainide-perfused hearts were compared with flecainide-free controls, and catecholamine-perfused hearts were compared with catecholamine-free hearts, using Tukey's test. The incidences of arrhythmias were compared using Fisher's exact probability test with Bonferoni correction, that is, the P-values of Fisher's exact probability test were multiplied by 6 (the number of groups in each set of experiments) to allow for multiple comparisons (Altman, 1991). P<0.05 was taken as indicative of a statistically significant difference between values.
Results
Ischaemia and reperfusion arrhythmia incidences
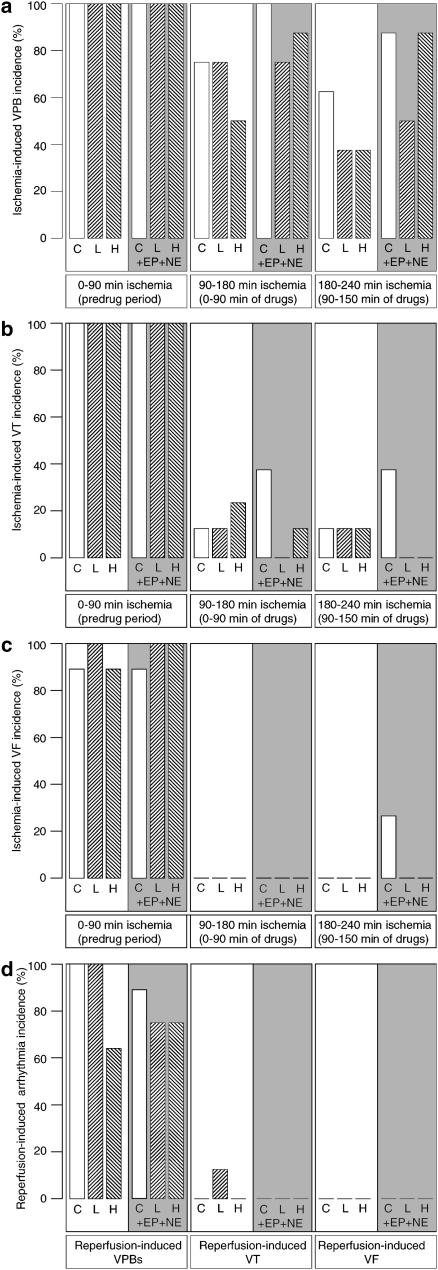
Phase-1 arrhythmias occurred during the first 90 min of ischaemia in all hearts in all groups, as expected (all hearts were drug-free during this period). This is shown in Figure 1a–c. Phase-2 arrhythmias were initially recorded in consecutive 15 min time blocks, but arrhythmia occurrence was too low within most blocks to make analysis worthwhile. Therefore, phase-2 arrhythmias were analysed in two time blocks: the first 90 min of drug perfusion (during 90–180 min after the start of ischaemia) and during the final 60 min (during 180–240 min after the start of ischaemia).
Figure 1.
Arrhythmias (expressed as percentage of each group of hearts expressing each type of arrhythmia) during myocardial ischaemia (0–90 min), infarct evolution (90–180 and 180–240 min) and reperfusion. Parts (a–c) show VPBs (a), VT (b) and VF (c). Part (d) shows reperfusion-induced arrhythmias following 240 min ischaemia. In each graph, perfusion was changed to the test solution at 90 min. Test solutions were vehicle control (C), low (L) and high (H) concentrations of flecainide or these same solutions co-perfused with epinephrine and norepinephrine (EP+NE). There were no significant differences between groups within any of the three time bins.
In comparison with susceptibility to phase-1 equivalents, control group susceptibility to phase-2 VPBs was slightly lower (Figure 1a), susceptibility to phase-2 VT was considerably lower (Figure 1b) and susceptibility to phase-2 VF was negligible (Figure 1c), as expected on the basis of previous findings (Clements-Jewery et al., 2002).
Flecainide had no significant proarrhythmic activity on phase-2 arrhythmias, and did not show even any trends toward proarrhythmic activity (Figure 1a–c).
Catecholamine perfusion caused a modest but statistically nonsignificant increase in susceptibility to ischaemia-induced VPBs and VT during the first and second blocks of the period of observation (Figure 1a and b), and facilitated the appearance of phase-2 VF (absent in controls) in several hearts during the final 60 min of the observation period (Figure 1c).
Flecainide's lack of proarrhythmic activity on phase-2 arrhythmias was not altered by co-perfusion with catecholamines, and no tendency for flecainide to increase arrhythmia susceptibility was unmasked (Figure 1a–c). The pattern during reperfusion was similar, with no evidence of proarrhythmia with flecainide with or without catecholamine perfusion (Figure 1d).
Other variables
Mean involved zone size was not affected by flecainide or catecholamine perfusion, and individual values ranged from 37 to 44% of the total ventricular weight (data not shown).
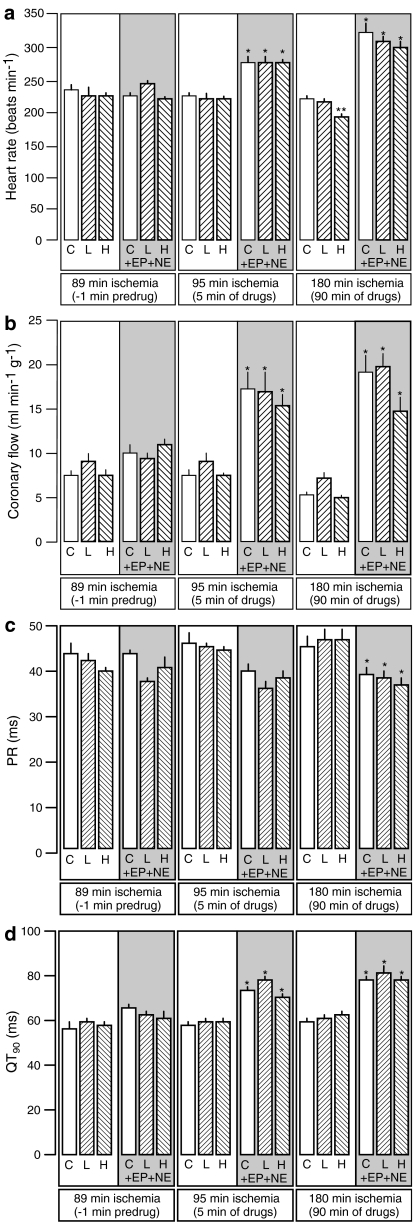
Values of heart rate, coronary flow, PR and QT interval are presented in Figure 2 for the critical time points: 1 min before introduction of drug perfusion (after 89 min of ischaemia), after 5 min of drug perfusion (95 min of ischaemia) and at the middle of the phase-2 observation period after 90 min of drug perfusion (180 min after the start of ischaemia). Flecainide caused a concentration-dependent, slowly developing and insubstantial (but statistically significant) bradycardic effect (Figure 2a) similar to that observed previously in rat heart perfused with flecainide during brief (30 min) ischaemia (Farkas & Curtis, 2002). Flecainide had no other effects. Catecholamine perfusion caused an immediate increase in heart rate (Figure 2a) and coronary flow (Figure 2b) together with a less marked shortening of PR interval (Figure 2c) and QT prolongation (Figure 2d), effects all similar to those seen previously (Clements-Jewery et al., 2002). Catecholamine effects were similar in flecainide-perfused hearts, meaning that flecainide's only significant effect (bradycardia) was surmounted by catecholamine perfusion.
Figure 2.
Heart rate (beats min−1; a), coronary flow (ml min−1 g−1; b), PR interval (ms; c) and QT at 90% repolarization (QT90; ms; d) 89 min after the onset of ischaemia (1 min before introduction of test solution), 95 min after the onset of ischaemia (5 min after the start of perfusion with test solution) and 180 min after the onset of ischaemia (90 min after the start of perfusion with test solution). Test solutions were vehicle control (C), low (L) and high (H) concentrations of flecainide or these same solutions co-perfused with epinephrine and norepinephrine (EP+NE). *P<0.05 versus test-matched solution not containing epinephrine and norepinephrine (i.e., C versus C, L versus L or H versus H). **P<0.05 versus control (C) within the catecholamine-free or catcholamine-containing blocks of groups.
Discussion
Reasons for examining flecainide's phase-2 proarrhythmic liability
Proarrhythmic liability detected during safety pharmacology assessment is now a major reason for the failure of new cardiac and noncardiac drugs to gain approval for clinical use. However, the study of drug-induced proarrhythmia is a notoriously difficult research area, since there is no consensus on the most appropriate model choice for preclinical detection, even for the most intensively researched form of proarrhythmia, drug-induced torsades de pointes (Bass et al., 2005; FDA, 2005; Friedrichs et al., 2005).
Flecainide's proarrhythmic liability has been explored from a cellular electrophysiological standpoint, and a proarrhythmic action was predicted on this basis by Hondeghem (1987) prior to the publication of the CAST findings. However, at the level of the whole organ (especially the diseased organ), the mechanism of the proarrhythmia caused by flecainide in the CAST trial remains ill-defined. This is particularly important since an as-yet undefined interaction between some aspect of the tissue milieu during an as-yet uncertain moment following coronary obstruction has been suggested to be a critical determinant of flecainide's proarrhythmic liability (Greenberg et al., 1995). In view of this, we examined whether the proarrhythmic effects of flecainide depend upon the identity of the distinct phases of postischaemic myocardial pathology that change over time (from reversible ischaemia through infarct evolution to irreversible injury) and that appear to have different arrhythmia mechanisms and different profiles of susceptibility to modification by drugs (Clements-Jewery et al., 2005). With studies on phase-1 arrhythmias now complete (Farkas & Curtis, 2002), we turned our attention to phase-2 arrhythmias.
Main findings
In the present study, flecainide, at clinically relevant concentrations (Farkas & Curtis, 2002), possessed no proarrhythmic activity during the phase-2 period of arrhythmogenesis following coronary ligation, or during the initial stages of reperfusion following sustained (240 min) ischaemia. It has been suggested previously that proarrhythmic effects of Class I drugs during ischaemia and reperfusion are easier to evoke in in vivo models compared with isolated perfused hearts, and that the presence of an autonomic nervous system may account for this (Farkas & Curtis, 2002). In in vivo studies (autonomic nervous system intact), flecainide increased ischaemia-induced (phase-1) VF incidence in anesthetized dogs (Wenger et al., 1985) and reduced the mean time to onset of phase-1 ischaemic VF in anesthetized pigs (Bergey et al., 1982; Gout et al., 1995). However, the characteristic lack of sympathetic drive was not sufficient to account for the lack of apparent proarrhythmic activity of flecainide during the phase-2 period in the present study, since proarrhythmic activity was not facilitated by catecholamine perfusion sufficient to cause rapid and significant restoration of sinus rate and other variable to levels equivalent to those in vivo by mechanisms shown previously to involve activation of cardiac β1 and α1 adrenoceptors (Clements-Jewery et al., 2002). Together, these findings suggest that facilitation of phase 2 arrhythmias is unlikely to be the reason for the findings of the CAST study.
Advantages and disadvantages of the experimental model
The present study is the first to use the perfused rat heart for the chosen purpose. Perfused hearts have obvious advantages in that drug concentrations can be controlled exactly for precise determination of concentration–response relationships. In addition, rat hearts are ideal for examining the rhythm consequences of coronary obstruction because they have reproducible coronary anatomy and negligible collateral flow resulting in uniform involved zone sizes and a reproducible susceptibility to arrhythmias following coronary occlusion (Curtis, 1998). Although conscious rats are ideal for the study of antiarrhythmic drug actions because they exhibit a very high susceptibility to phase-2 arrhythmias (Curtis et al., 1987), this makes them unsuitable for examination of possible proarrhythmic drug activity in the phase-2 setting. In contrast, isolated rat hearts have an advantage owing to t heir low baseline susceptibility to phase-2 arrhythmias, which is convenient for unmasking proarrhythmic drug activity, were a drug to possess such activity.
The reason for the low susceptibility to phase-2 arrhythmias in control isolated rat hearts is a subject of interest that remains to be resolved (Clements-Jewery et al., 2005) and is beyond the scope of the present investigation. However, it is not a reflection of insufficient severity of cardiac injury since most control perfused rat hearts develop phase-1 VF (Curtis, 1998), and all develop regional necrosis and electron microscopic evidence of typical infarction during 240 min coronary ligation in our hands (Ravingerova et al., 1995) nor does it appear to be due to the lack of catecholamine drive in the preparation (Clements-Jewery et al., 2002).
The main issue of potential concern with the use of the perfused rat heart as a model relates to the heart rate, which is five times the resting human heart rate (Clements-Jewery et al., 2005). The high heart rate means that residual drug binding to Na+ channels during diastole, cumulative drug binding and steady state INa block are all likely to be higher in rat ventricle than in man, in accordance with the modulated receptor hypothesis (Hondeghem, 1987). This means that if INa block is responsible for class I (especially IC) proarrhythmia, then this is likely to be exaggerated in the rat heart. Whether this is a disadvantage of the model is a moot point. Since, despite its notoriety, Class IC-induced serious proarrhythmia is uncommon (if it were common then class 1C use would have long since been ended), then an exaggerated susceptibility to class IC proarrhythmia in a preclinical model is perhaps an advantage in terms of permitting a qualitative detection of a potential liability. Nevertheless, the point is academic, since flecainide did not elicit arrhythmias in the present study.
A further possible limitation of the present model is the absence of functional IKr in the rat heart (Rees & Curtis, 1993). This means that if IKr block contributed to flecainide's proarrhythmic action in the CAST study, then it would not be possible to model this in the present or any other type of rat preparation. Flecainide does block IKr, but this was reported in a study that tested only a very high flecainide concentration of 10 μM (Follmer & Colatsky, 1990). In isolated perfused rabbit hearts, 2 μM flecainide had no significant effects on refractoriness or monophasic action potential duration (Eckardt et al., 1998). Collectively, these data suggest that the lack of IKr in rat hearts does not constitute a limitation to the present findings since IKr block by flecainide requires concentrations beyond the clinically relevant range examined in the present study (see justification for choice of drug concentrations in Methods).
The lack of apparent proarrhythmic interaction between catecholamines and flecainide in the present study might appear surprising given the conclusions of Cragun et al. (1997) who suggested that a synergy between catecholamines and flecainide to reduce INa may facilitate arrhythmogenesis in ischaemia. The evidence for this proposition came from superfusion studies with Purkinje fibres in which isoproterenol exacerbated the depolarization caused by a clinically relevant concentration (1 μM) of flecainide, provided the fibres were bathed in a high K+ solution (which mimics the early ischaemic milieu and the milieu of the border zone of the evolving infarct; Hill & Gettes, 1980; Hirche et al., 1980). However, an enhanced degree of Na+ channel block is a potentially double-edged sword; the Purkinje fibre data imply that catecholamines may facilitate the ability of flecainide either to block conduction or to merely slow conduction velocity further, in the border zone of an evolving infarct – the first action being potentially antiarrhythmic and the other being potentially proarrhythmic (Hondeghem, 1987; Starmer et al., 1991). Therefore, the present findings contradict only the conclusion of Cragun et al. (1997) and are not at odds with the data that informed their conclusion. Certainly, depending on concentration and circumstances, flecainide can suppress arrhythmias following coronary ligation, as well as exacerbate them (Bergey et al., 1982; Gout et al., 1995; Farkas & Curtis, 2002).
The present data appear to be at odds with those of Ranger & Nattel (1995) who identified proarrhythmia with flecainide in an in vivo dog infarct model. However, direct comparison between this and the present study is unwise because we focused on the first 4 h of sustained ischaemia, whereas Ranger & Nattel used dogs with established fully evolved infarcts, 72 h after coronary ligation – which is many hours after susceptibility to spontaneous phase-2 arrhythmias has occurred (Clements-Jewery et al., 2005). Additionally, there is a strong possibility that this model, in contrast to the present, exaggerates the susceptibility to flecainide-induced proarrhythmia. This is because not only did 15 of 19 dogs with an infarct experience ventricular proarrhythmia, but so did four of 13 (31% of) normal healthy dogs. Flecainide is certainly proarrhythmic in man, but the incidence of ventricular tachyarrhythmias caused by flecainide in patients with normal healthy ventricles is less than a trivial fraction of 31%, which explains why the drug remains in use for treatment of supraventricular arrhythmias (Blomstrom-Lundqvist et al., 2003).
As a general point, it is probably too early to make meaningful value judgements about different models. Whether an exaggerated susceptibility to proarrhythmia is an advantage or disadvantage to a model is a vexing issue. On the one hand, an exaggerated susceptibility is necessary when the clinical susceptibility is low (in order to allow the model to function as a usable bioassay). On the other hand, if the susceptibility is exaggerated, the model could be regarded as being not clinically relevant. In the present case, only time and the generation of a comprehensive database with a range of models will clarify the value of each.
Possible relevance to future drug development
Although flecainide and other IC agents have no future as ventricular antiarrhythmic agents, a better understanding of the proarrhythmic profile of flecainide may help to guide future drug development. This applies to avoidance of proarrhythmia with noncardiac drugs as well as to the search for new antiarrhythmics. In drug safety assessment, the appropriate preclinical programme necessary for ensuring a drug has no proarrhythmic liability remains a topic for debate (Bass et al., 2005; Friedrichs et al., 2005). It has been argued that evaluation of proarrhythmic risk in the phase-2 setting should be considered as part of the battery of tests for drug safety assessment (Clements-Jewery et al., 2005). At present, there is no accepted model for carrying out such work. In the present study, rats were chosen for the reasons given plus the fact that rats were used in our previous studies with flecainide (Farkas & Curtis, 2002). The guinea pig heart, so useful in preclinical torsades de pointes screening (Hamlin et al., 2004), would be unsuitable owing to its highly developed collateral vessel network that is sufficient to not only reduce ischaemia severity after coronary ligation but also to prevent entirely the appearance of infarction (Maxwell et al., 1987). Rabbit hearts may offer an alternative, being collateral-deficient like rat hearts (Rees & Curtis, 1993), although baseline phase-2 arrhythmia susceptibility has not been characterized in this model. If phase-2 proarrhythmia evaluation is to become part of standard safety pharmacology testing, then more work is required in order to identify the model of choice.
Conclusions
Potentiation of phase-2 ventricular arrhythmia susceptibility does not appear to be a likely mechanism to explain the proarrhythmia seen with flecainide in the CAST study. The perfused rat heart may serve as a useful model for evaluating drug-induced proarrhythmia in the phase-2 susceptible setting, but its value, like that of other models, will not become clear until there is a clinical template of drugs with known and quantified proarrhythmic liability in each pathological setting encountered post MI.
Acknowledgments
This work was funded, in part, by a BSc intercalated research project award from the British Pharmacological Society on behalf of G.S. Kanaganayagam, and in part by an A.J. Clark studentship awarded by the British Pharmacological Society on behalf of H. Clements-Jewery.
Abbreviations
- CAST
Cardiac Arrhythmia Suppression trial
- MI
myocardial infarction
- VF
ventricular fibrillation
- VPB
ventricular premature beat
- VT
ventricular tachycardia
References
- ALTMAN D.G. Practical Statistics for Medical Research. London: Chapman & Hall; 1991. [Google Scholar]
- BASS A.S., TOMASELLI G., BULLINGHAM R., III, KINTER L.B. Drugs effects on ventricular repolarization: a critical evaluation of the strengths and weaknesses of current methodologies and regulatory practices. J. Pharmacol. Toxicol. Methods. 2005;52:12–21. doi: 10.1016/j.vascn.2005.04.010. [DOI] [PubMed] [Google Scholar]
- BAUMGARTNER H., WIEDERMANN C.J., HORTNAGL H., MUHLBERGER V. Plasma catecholamines in arterial and capillary blood. Naunyn Schmiedebergs Arch. Pharmacol. 1985;328:461–463. doi: 10.1007/BF00692916. [DOI] [PubMed] [Google Scholar]
- BENET L.Z., OIE S., SCHWARTZ J.B.Design and optimization of dosage regimens; pharmacokinetic data Goodman & Gilman's the Pharmacologic Basis of Therapeutics 1996New York: McGraw-Hill; 1707–1792.ed. Hardman, J.G., Limbird, L.E., Molinoff, P.B., Ruddon, R.W. & Gilman, A.G. pp [Google Scholar]
- BERGEY J.L., NOCELLA K., MCCALLUM J.D. Acute coronary artery occlusion-reperfusion-induced arrhythmias in rats, dogs and pigs: antiarrhythmic evaluation of quinidine, procainamide and lidocaine. Eur. J. Pharmacol. 1982;81:205–216. doi: 10.1016/0014-2999(82)90438-1. [DOI] [PubMed] [Google Scholar]
- BLOMSTROM-LUNDQVIST C., SCHEINMAN M.M., ALIOT E.M., ALPERT J.S., CALKINS H., CAMM A.J., CAMPBELL W.B., HAINES D.E., KUCK K.H., LERMAN B.B., MILLER D.D., SHAEFFER C.W., JR., STEVENSON W.G., TOMASELLI G.F., ANTMAN E.M., SMITH S.C., JR., ALPERT J.S., FAXON D.P., FUSTER V., GIBBONS R.J., GREGORATOS G., HIRATZKA L.F., HUNT S.A., JACOBS A.K., RUSSELL R.O., JR., PRIORI S.G., BLANC J.J., BUDAJ A., BURGOS E.F., COWIE M., DECKERS J.W., GARCIA M.A., KLEIN W.W., LEKAKIS J., LINDAHL B., MAZZOTTA G., MORAIS J.C., OTO A., SMISETH O., TRAPPE H.J. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias – executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias) Circulation. 2003;108:1871–1909. doi: 10.1161/01.CIR.0000091380.04100.84. [DOI] [PubMed] [Google Scholar]
- BRENER S.J., LINCOFF A.M., BATES E.R., JIA G., ARMSTRONG P.W., GUETTA V., HOCHMAN J.S., SAVONITTO S., WILCOX R.G., WHITE H.D., TOPOL E.J. The relationship between baseline risk and mortality in ST-elevation acute myocardial infarction treated with pharmacological reperfusion: insights from the Global Utilization of Strategies To open Occluded arteries (GUSTO) V trial. Am. Heart J. 2005;150:89–93. doi: 10.1016/j.ahj.2005.01.030. [DOI] [PubMed] [Google Scholar]
- CAMPBELL R.W.F.Ventricular fibrillation: facts, fiction, and the future Life-Threatening Arrhythmias During Ischemia and Infarction 1987New York: Raven Press; 1–9.ed. Hearse, D.J., Manning, A.S. & Janse, M.J. pp [Google Scholar]
- CLARK C., FOREMAN M.I., KANE K.A., MCDONALD F.M., PARRATT J.R. Coronary artery ligation in anesthetized rats as a method for the production of experimental dysrhythmias and for the determination of infarct size. J. Pharmacol. Methods. 1980;3:357–368. doi: 10.1016/0160-5402(80)90077-7. [DOI] [PubMed] [Google Scholar]
- CLEMENTS-JEWERY H., HEARSE D.J., CURTIS M.J. Independent contribution of catecholamines to arrhythmogenesis during evolving infarction in the isolated rat heart. Br. J. Pharmacol. 2002;135:807–815. doi: 10.1038/sj.bjp.0704509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEMENTS-JEWERY H., HEARSE D.J., CURTIS M.J. Phase 2 ventricular arrhythmias in acute myocardial infarction: a neglected target for therapeutic antiarrhythmic drug development and for safety pharmacology evaluation. Br. J. Pharmacol. 2005;145:551–564. doi: 10.1038/sj.bjp.0706231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COPLAN N.L., GLEIM G.W., NICHOLAS J.A. Exercise-related changes in serum catecholamines and potassium: effect of sustained exercise above and below lactate threshold. Am. Heart J. 1989;117:1070–1075. doi: 10.1016/0002-8703(89)90864-8. [DOI] [PubMed] [Google Scholar]
- CRAGUN K.T., JOHNSON S.B., PACKER D.L. Beta-adrenergic augmentation of flecainide-induced conduction slowing in canine Purkinje fibers. Circulation. 1997;96:2701–2708. doi: 10.1161/01.cir.96.8.2701. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J. Characterisation, utilisation and clinical relevance of isolated perfused heart models of ischaemia-induced ventricular fibrillation. Cardiovasc. Res. 1998;39:194–215. doi: 10.1016/s0008-6363(98)00083-2. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., HEARSE D.J. Ischaemia-induced and reperfusion-induced arrhythmias differ in their sensitivity to potassium: implications for mechanisms of initiation and maintenance of ventricular fibrillation. J. Mol. Cell. Cardiol. 1989a;21:21–40. doi: 10.1016/0022-2828(89)91490-9. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., HEARSE D.J. Reperfusion-induced arrhythmias are critically dependent upon occluded zone size: relevance to the mechanism of arrhythmogenesis. J. Mol. Cell. Cardiol. 1989b;21:625–637. doi: 10.1016/0022-2828(89)90828-6. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., MACLEOD B.A., WALKER M.J. Models for the study of arrhythmias in myocardial ischaemia and infarction: the use of the rat. J. Mol. Cell. Cardiol. 1987;19:399–419. doi: 10.1016/s0022-2828(87)80585-0. [DOI] [PubMed] [Google Scholar]
- ECHT D.S., LIEBSON P.R., MITCHELL L.B., PETERS R.W., OBIAS-MANNO D., BARKER A.H., ARENSBERG D., BAKER A., FRIEDMAN L., GREENE H.L., HUNTER M.L., RICHARDSON D.W., THE CAST INVESTIGATORS. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- ECKARDT L., HAVERKAMP W., GOTTKER U., MADEJA M., JOHNA R., BORGGREFE M., BREITHARDT G. Divergent effect of acute ventricular dilatation on the electrophysiologic characteristics of d,l-sotalol and flecainide in the isolated rabbit heart. J. Cardiovasc. Electrophysiol. 1998;9:366–383. doi: 10.1111/j.1540-8167.1998.tb00925.x. [DOI] [PubMed] [Google Scholar]
- FARKAS A., CURTIS M.J. Limited antifibrillatory effectiveness of clinically relevant concentrations of class I antiarrhythmics in isolated perfused rat hearts. J. Cardiovasc. Pharmacol. 2002;39:412–424. doi: 10.1097/00005344-200203000-00013. [DOI] [PubMed] [Google Scholar]
- FDA ICH Harmonised Tripartite Guideline. The non-clinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals. S7B (draft) 2005.
- FOLLMER C.H., COLATSKY T.J. Block of delayed rectifier potassium current, IK, by flecainide and E-4031 in cat ventricular myocytes. Circulation. 1990;82:289–293. doi: 10.1161/01.cir.82.1.289. [DOI] [PubMed] [Google Scholar]
- FRIEDRICHS G.S., PATMORE L., BASS A. Non-clinical evaluation of ventricular repolarization (ICH S7B): results of an interim survey of international pharmaceutical companies. J. Pharmacol. Toxicol. Methods. 2005;52:6–11. doi: 10.1016/j.vascn.2005.05.001. [DOI] [PubMed] [Google Scholar]
- GOUT B., NICHOLS A.J., FEUERSTEIN G.Z., BRIL A. Antifibrillatory effects of BRL-32872 in anesthetized Yucatan minipigs with regional myocardial ischemia. J. Cardiovasc. Pharmacol. 1995;26:636–644. doi: 10.1097/00005344-199510000-00020. [DOI] [PubMed] [Google Scholar]
- GREENBERG H.M., DWYER E.M.J., HOCHMAN J.S., STEINBERG J.S., ECHT D.S., PETERS R.W. Interaction of ischaemia and encainide/flecainide treatment: a proposed mechanism for the increased mortality in CAST I. Br. Heart J. 1995;74:631–635. doi: 10.1136/hrt.74.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLSTROM A.P., ANDERSON J.L., CARLSON M., DAVIES R., GREENE H.L., KAMMERLING J.M., ROMHILT D.W., DUFF H.J., HUTHER M. Time to arrhythmic, ischemic, and heart failure events: exploratory analyses to elucidate mechanisms of adverse drug effects in the Cardiac Arrhythmia Suppression Trial. Am. Heart J. 1995;130:71–79. doi: 10.1016/0002-8703(95)90238-4. [DOI] [PubMed] [Google Scholar]
- HAMLIN R.L., CRUZE C.A., MITTELSTADT S.W., KIJTAWORNRAT A., KEENE B.W., ROCHE B.M., NAKAYAMA T., NAKAYAMA H., HAMLIN D.M., ARNOLD T. Sensitivity and specificity of isolated perfused guinea pig heart to test for drug-induced lengthening of QTc. J. Pharmacol. Toxicol. Methods. 2004;49:15–23. doi: 10.1016/j.vascn.2003.08.003. [DOI] [PubMed] [Google Scholar]
- HAYES E., PUGSLEY M.K., PENZ W.P., ADAIKAN G., WALKER M.J. Relationship between QaT and RR intervals in rats, guinea pigs, rabbits, and primates. J. Pharmacol. Toxicol. Methods. 1994;32:201–207. doi: 10.1016/1056-8719(94)90088-4. [DOI] [PubMed] [Google Scholar]
- HILL J.L., GETTES L.S. Effect of acute coronary artery occlusion on local myocardial extracellular K+ activity in swine. Circulation. 1980;61:768–778. doi: 10.1161/01.cir.61.4.768. [DOI] [PubMed] [Google Scholar]
- HIRCHE H., FRANZ C., BOS L., BISSIG R., LANG R., SCHRAMM M. Myocardial extracellular K+ and H+ increase and noradrenaline release as possible cause of early arrhythmias following acute coronary artery occlusion in pigs. J. Mol. Cell. Cardiol. 1980;12:579–593. doi: 10.1016/0022-2828(80)90016-4. [DOI] [PubMed] [Google Scholar]
- HONDEGHEM L.M. Antiarrhythmic agents: modulated receptor applications. Circulation. 1987;75:514–520. doi: 10.1161/01.cir.75.3.514. [DOI] [PubMed] [Google Scholar]
- MANNING A.S., HEARSE D.J. Reperfusion-induced arrhythmias: mechanisms and prevention. J. Mol. Cell. Cardiol. 1984;16:497–518. doi: 10.1016/s0022-2828(84)80638-0. [DOI] [PubMed] [Google Scholar]
- MAXWELL M.P., HEARSE D.J., YELLON D.M. Species variation in the coronary collateral circulation during regional myocardial ischaemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc. Res. 1987;21:737–746. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]
- MEESMANN W.Early arrhythmias and primary ventricular fibrillation after acute myocardial ischaemia in relation to pre-existing coronary collaterals Early Arrhythmias Resulting from Myocardial Ischaemia 1982London, UK: McMillan; 93–112.ed. Parrat, J.R. pp [Google Scholar]
- PACKER D.L., MUNGER T.M., JOHNSON S.B., CRAGUN K.T. Mechanism of lethal proarrhythmia observed in the Cardiac Arrhythmia Suppression Trial: role of adrenergic modulation of drug binding. Pacing Clin. Electrophysiol. 1997;20:455–467. doi: 10.1111/j.1540-8159.1997.tb06204.x. [DOI] [PubMed] [Google Scholar]
- PUGSLEY M.K., RIES C.R., GUPPY L.J., HARVIE C.J., WALKER M.J. Effects of anipamil, a long acting analog of verapamil, in pigs subjected to myocardial ischemia. Life Sci. 1995;57:1219–1231. doi: 10.1016/0024-3205(95)02070-y. [DOI] [PubMed] [Google Scholar]
- RANGER S., NATTEL S. Determinants and mechanisms of flecainide-induced promotion of ventricular tachycardia in anesthetized dogs. Circulation. 1995;92:1300–1311. doi: 10.1161/01.cir.92.5.1300. [DOI] [PubMed] [Google Scholar]
- RATGE D., GEHRKE A., MELZNER I., WISSER H. Free and conjugated catecholamines in human plasma during physical exercise. Clin. Exp. Pharmacol. Physiol. 1986;13:543–553. doi: 10.1111/j.1440-1681.1986.tb00937.x. [DOI] [PubMed] [Google Scholar]
- RAVINGEROVA T., TRIBULOVA N., SLEZAK J., CURTIS M.J. Brief, intermediate and prolonged ischemia in the isolated crystalloid perfused rat heart: relationship between susceptibility to arrhythmias and degree of ultrastructural injury. J. Mol. Cell. Cardiol. 1995;27:1937–1951. doi: 10.1016/0022-2828(95)90016-0. [DOI] [PubMed] [Google Scholar]
- REES S.A., CURTIS M.J. Specific IK1 blockade: a new antiarrhythmic mechanism? Effect of RP58866 on ventricular arrhythmias in rat, rabbit, and primate. Circulation. 1993;87:1979–1989. doi: 10.1161/01.cir.87.6.1979. [DOI] [PubMed] [Google Scholar]
- STARMER C.F., LASTRA A.A., NESTERENKO V.V., GRANT A.O. Proarrhythmic response to sodium channel blockade. Theoretical model and numerical experiments. Circulation. 1991;84:1364–1377. doi: 10.1161/01.cir.84.3.1364. [DOI] [PubMed] [Google Scholar]
- WALKER M.J., CURTIS M.J., HEARSE D.J., CAMPBELL R.W., JANSE M.J., YELLON D.M., COBBE S.M., COKER S.J., HARNESS J.B., HARRON D.W., HIGGINS A.J., JULIAN D.G., LAB M.J., MANNING A.S., NORTHOVER B.J., PARRATT J.R., RIERMERSMA R.A., RIVA E., RUSSELL D.C., SHERIDAN D.J., WINSLOW E., WOODWARD B. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- WENGER T.L., LEDERMAN S.N., STRAUSS H.C. Effects of flecainide in dogs with coronary occlusion and reperfusion. Circulation. 1985;72 (Suppl.):III–225. [Google Scholar]
- WOOSLEY R.L., SIDDOWAY L.A., DUFF H.J., RODEN D.M. Flecainide dose–response relations in stable ventricular arrhythmias. Am. J. Cardiol. 1984;53:59B–65B. doi: 10.1016/0002-9149(84)90504-6. [DOI] [PubMed] [Google Scholar]