Abstract
Based on pharmacological criteria, we previously suggested that in the mouse aorta, endothelium-dependent relaxation by nucleotides is mediated by P2Y1 (adenosine diphosphate (ADP)), P2Y2 (adenosine triphosphate (ATP)) and P2Y6 (uridine diphosphate (UDP)) receptors. For UTP, it was unclear whether P2Y2, P2Y6 or yet another subtype was involved. Therefore, in view of the lack of selective purinergic agonists and antagonists, we used P2Y2-deficient mice to clarify the action of UTP.
Thoracic aorta segments (width 2 mm) of P2Y2-deficient and wild-type (WT) mice were mounted in organ baths to measure isometric force development and intracellular calcium signalling.
Relaxations evoked by ADP, UDP and acetylcholine were identical in knockout and WT mice, indicating that the receptors for these agonists function normally.
P2Y2-deficient mice showed impaired ATP- and adenosine 5′[γ-thio] triphosphate (ATPγS)-evoked relaxation, suggesting that in WT mice, ATP and ATPγS activate predominantly the P2Y2 subtype.
The ATP/ATPγS-evoked relaxation and calcium signals in the knockout mice were partially rescued by P2Y1, as they were sensitive to 2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate (MRS2179), a P2Y1-selective antagonist.
In contrast to ATP, the UTP-evoked relaxation was not different between knockout and WT mice. Moreover, the action of UTP was not sensitive to MRS2179. Therefore, the action of UTP is probably mediated mainly by a P2Y6(like) receptor subtype.
In conclusion, we demonstrated that ATP-evoked relaxation of the murine aorta is mainly mediated by P2Y2. But this P2Y2 receptor has apparently no major role in UTP-evoked relaxation. The vasodilator effect of UTP is probably mediated mainly by a P2Y6(like) receptor.
Keywords: P2Y2 receptor, knockout mouse, ATP, UTP, P2Y6 receptor, aorta, endothelium
Introduction
Nucleotides are important regulators of various biological functions. They are released in physiological and patho-physiological circumstances. Hypoxia, damage of the endothelial lining and platelet degranulation give rise to massive release of adenosine triphosphate (ATP), adenosine diphosphate (ADP) and uridine triphosphate (UTP) into the circulation (Lazarowski et al., 2000; Lazarowski & Boucher, 2001; Di Virgilio & Solini, 2002). These nucleotides act through cell-surface receptors, which can be divided into the P2Y and the P2X receptor families (Ralevic & Burnstock, 1998). P2X receptors are membrane ion channels made by the assembly of subunits of the same (homo-oligomers) or different (hetero-oligomers) subtypes. The P2X receptor family has seven subtypes (P2X1–7) that are all mainly activated by ATP (North, 2002). P2Y receptors consist of seven membrane spanning domains, and eight subtypes have been identified: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14 (Van Der Giet et al., 2002; Abbracchio et al., 2003). P2Y receptors are coupled via G-proteins to phospholipase C, resulting in inositol 1,4,5-trisphosphate generation and Ca2+ release from intracellular stores, or in stimulation/inhibition of adenylate cyclase (Boarder & Hourani, 1998).
In humans, endothelial P2Y receptors mediate vasodilatation by releasing nitric oxide (NO), prostanoids or endothelium-derived hyperpolarizing factor (Wihlborg et al., 2003), whereas in rat and mouse, P2Y receptors mediate endothelium-dependent relaxation mainly via the NO pathway (Buvinic et al., 2002; Guns et al., 2005). In contrast, vascular smooth muscle cells express multiple P2Y and P2X receptors (Kunapuli & Daniel, 1998) that cause contraction. As a consequence, nucleotides are important regulators of the arterial tone.
In the past, we demonstrated that nucleotides evoked complete (ATP, UTP, uridine diphosphate (UDP); >90%) or partial (ADP) relaxation of phenylephrine precontracted thoracic aortic rings of wild-type (WT; C57Bl6) mice. These results pointed to the presence of functional P2Y1 (ADP>ATP), P2Y2 (ATP and UTP) and P2Y6 (UDP) receptors. However, apparent pKb values of purinergic antagonists such as suramin and pyridoxal-phosphate-6-azophenyl-2′-4′-disulphonic acid suggested that the action of UTP was not exclusively due to the P2Y2 receptor subtype (Guns et al., 2005). Therefore, we used P2Y2-deficient mice to investigate further the action of UTP. In addition, the vasodilator effects of the nucleotides ATP, adenosine 5′[γ-thio] triphosphate (ATPγS), ADP, UDP and of acetylcholine (ACh) were tested on the thoracic aorta segments. Further, we determined the effect of the dinucleotides P1,P4-di(uridine 5′-) triphosphate (Up3U) and P1,P4-di(uridine 5′-) tetraphosphate (Up4U (INS365)), which are less rapidly degraded by ectonucleotidases than UTP and UDP, and exhibit relative P2Y6 and P2Y2 selectivity respectively (Pendergast et al., 2001; Yerxa et al., 2002). Finally, we evaluated the effect of the P2Y1-selective antagonist 2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate (MRS2179) on the relaxation responses as well as on the intracellular calcium signalling in intact aortic rings upon stimulation with ATP, ATPγS, ADP and UTP.
Methods
Mice
The studies were approved by the Ethical Committee of the University of Antwerp, and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. P2Y2−/− and the corresponding WT mice (both B6D2/SV129 strain, age 12–18 weeks and body weight 21.9±2.8 and 22.0±1.7 g respectively) were used. Genotyping was carried out using two primer sets as previously described (Homolya et al., 1999). P2Y2−/− mice, on a B6D2 genetic background (Homolya et al., 1999), were provided as breeder pairs by B.H. Koller. They were crossed with the SV129 strain by J. Leipziger (Matos et al., 2005).
Isolating and mounting of blood vessels
After anaesthesia (sodium pentobarbital, 75 mg kg−1, i.p.), the aorta was carefully removed, stripped of adherent tissue and dissected systematically. The thoracic aorta was divided into five sequential 2 mm wide segments (TA1 → TA5) starting 3 mm from the origin of the left subclavian artery (where the azygos vein crosses the aorta) down to the diaphragm (Crauwels et al., 2003). Segments were mounted between two parallel tungsten wire hooks in 10 ml organ baths and tension was measured isometrically with a Statham UC2 force transducer (Gould) connected to a data acquisition system (Moise 3, EMKA Technologies). Vessels were immersed in Krebs–Ringer solution (37°C, and continuously aerated with 95% O2/5% CO2, pH 7.4) with (in mM) NaCl 118, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, CaEDTA 0.025 and glucose 11.1. Segments of the thoracic aorta were gradually stretched until a stable loading tension of 20 mN was attained (Crauwels et al., 2003; Guns et al., 2005).
Vasomotor studies
Indomethacin (10 μM) was present in all studies to avoid any vasomotor interference due to contractile prostanoids (Vanhoutte et al., 2005). Between each concentration–response curve, the Krebs–Ringer solution was replaced three times to wash out the agents. Rings were first contracted with a depolarizing potassium solution (50 mM). After three washing steps, a cumulative concentration–response curve was drawn for phenylephrine (3 nM–30 μM) and the concentration (EC50) resulting in 50% of the maximal contraction was assessed for each vessel segment. At the highest phenylephrine concentration, segments were exposed for 4 min to ATP (7 × 10−5 M) followed by three washing steps. This ATP pretreatment was used to minimize the shift between the first and second curve seen with all purinergic agonists (Guns et al., 2005). In relaxation studies, vessels were precontracted with their individual EC50 of phenylephrine, followed by cumulative concentration–response curves.
To assess the function of the P2Y2 subtype, two different protocols were used. In the first protocol, consecutive concentration–response curves were performed for ATP, UTP, ADP/ACh, UDP/ATPγS and ATP/ADP in the presence of MRS2179. In these experiments, we observed a decrease of maximal relaxation capacity during the experiment; therefore, in a second experiment, ATPγS, UTP, UDP, Up3U and Up4U were tested simultaneously on a particular thoracic aorta segment followed by a second cumulative concentration–response curve in the presence of MRS2179 (10 μM). MRS2179 (10 μM) was added to the organ bath 20 min before the nucleotide cumulative concentration–response curve. Interference of regional differences among the different segments was excluded by using a rotation system. In this manner, each agonist was tested once on each of the five thoracic aorta segments.
Calcium measurements
After mounting in a wire myograph (Danish Myo Technology A/S, Denmark), the relaxed aorta segments (1 mm) were incubated for 2.5 h in a Krebs–Ringer solution with 0.02% pluronic F-127, 0.01% bovine serum albumin and 10 μM Fura2-acetoxymethyl ester (Fura2-AM). After incubation, the tissue was rinsed with regular Krebs–Ringer solution for 0.5 h (5 ml min−1). Excitation wavelengths (340 and 380 nm) were delivered at a frequency of 1 Hz with a DeltaRam Multiwavelengths Illuminator (Photon Technology International, PTI, U.S.A.). Emission (dichroic mirror, emission filter XF2031, XF3007, Omega optical Inc., Germany) was measured with a microscope photometer (D-104, PTI, U.S.A.). The emission ratio, measured at dual excitation wavelengths (340/380 ratio), was calculated with Felix software (PTI, U.S.A.) and used as a relative measure of free [Ca2+]i. Before starting an experiment, the mean background emission values for excitation at 340 and 380 nm were determined before loading with Fura2-AM over a period of 60 s. These background values were real-time subtracted from the emission values during the experiment. Intracellular calcium signals were measured upon stimulation of aorta segments from WT or P2Y2-deficient mice with ATP and UTP (both 10 μM) for 5 min in the absence or presence of MRS2179 (10 μM). After each stimulation, the agonist was washed out by perfusion with Krebs–Ringer solution (5 ml min−1) for 10 min.
Statistical analysis
All results are expressed as mean±s.e.m.; n represents the number of mice. Statistical analysis was performed by SPSS package for windows (version 11.5). The knockout and WT mice were compared by using a Student's t-test.
Materials
Sodium pentobarbital (Nembutal®) was purchased from Sanofi (Brussels, Belgium) and indomethacin was from Federa (Brussels, Belgium). Further, phenylephrine hydrochloride, all nucleotides and MRS2179 were obtained from Sigma (Bornem, Belgium). Up3U and Up4U were provided by Inspire Pharmaceuticals (Durham, NC, U.S.A.).
Results
Agonists
The relaxation capacity of the different nucleotides was tested in the phenylephrine precontracted thoracic aorta of P2Y2-knockout mice and the corresponding WT mice. ADP evoked in both strains transient relaxations. At higher ADP concentrations, ADP became less active and contractile effects were sometimes observed. ATP and ATPγS, on the other hand, evoked sustained relaxation in WT mice, but transient responses in P2Y2-deficient mice (Figure 1).
Figure 1.
Representative tracings of cumulative concentration–response curves (3 × 10−8–1 × 10−4 M) of (a) ADP, (b) ATP and (c) ATPγS of phenylephrine-precontracted aortic rings from P2Y2-deficient or WT mice.
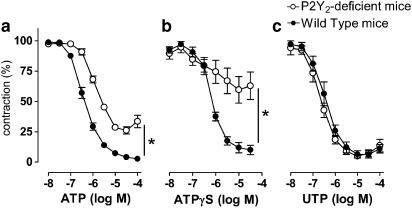
Furthermore, in cumulative concentration–response curves, ATP displayed a greater sensitivity and effectiveness in WT mice in comparison to the P2Y2-knockout mice (Figure 2a; Table 1). The relaxation evoked by ATPγS was severely impaired in the P2Y2-knockout mice, whereas the WT mice showed complete relaxation.
Figure 2.
Cumulative concentration–response curves of P2Y2-deficient versus WT mice for ATP (a), ATPγS (b) and UTP (c). Results are expressed as mean±s.e.m.; n=5. *P2Y2-deficient mice different from WT mice (P<0.005).
Table 1.
The pD2 values (−log M) of nucleotide agonists inducing relaxation of the thoracic aorta of WT and P2Y2-deficient mice
| WT | P2Y2-deficient mice | P-valuea | |
|---|---|---|---|
| ATP | 6.41±0.04 | 5.90±0.05 | <0.0001 |
| ATPγS | 6.17±0.05 | —b | |
| ADP | 6.44±0.13 | 6.32±0.17 | 0.573 |
| UTP | 6.46±0.07 | 6.65±0.07 | 0.109 |
| UDP | 6.78±0.14 | 6.80±0.13 | 0.914 |
| Up3U | 5.88±0.14 | 6.12±0.11 | 0.227 |
| Up4U | 5.88±0.17 | 5.79±0.12 | 0.692 |
Values represent mean±s.e.m. of five mice.
WT versus P2Y2-deficient mice (Student's t-test).
Curve fitting was not appropriate.
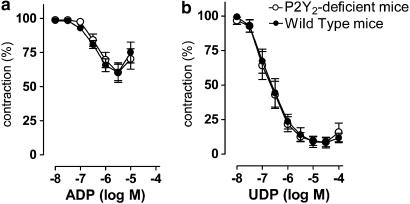
The cumulative concentration–response curves of P2Y2-knockout mice for UTP, ADP, UDP and ACh (not shown) were not different from those of WT mice with respect to potency and maximal vasodilator capacity (Figures 2 and 3; Table 1). Finally, the vasodilator potency of the dinucleotides Up3U and Up4U was evaluated. Both compounds were less potent and less effective than UTP and UDP, and their action did not differ between the P2Y2-knockout mice and WT mice (Figure 4).
Figure 3.
Cumulative concentration–response curves of P2Y2-deficient and WT mice for ADP (a) and UDP (b). Results are expressed as mean±s.e.m.; n=5.
Figure 4.
Cumulative concentration–response curves of P2Y2-deficient and WT mice for Up3U (a) and Up4U (b). Results are expressed as mean±s.e.m.; n=5.
Antagonist
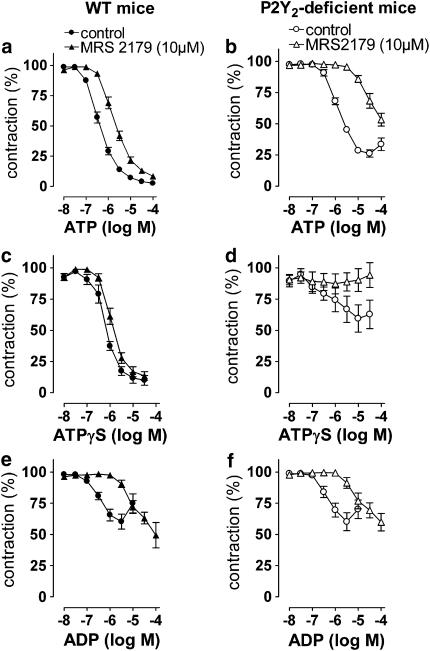
MRS2179 was used to determine the contribution of the P2Y1 receptor. In the presence of MRS2179, the concentration–response curve of ATP showed a larger rightward shift in the P2Y2-knockout mice than in the WT mice (Figure 5). The responses of ATPγS were even completely inhibited in the knockout mice, whereas MRS2179 hardly influenced the relaxation in the WT mice (Figure 5). The rightward shift of the concentration–response curve for ADP was of the same magnitude in the knockout and WT mice (Figure 5). The responses of UTP were not influenced by MRS2179 in either the knockout mice or in the WT mice (not shown).
Figure 5.
Cumulative concentration–response curves of ATP (a and b), ATPγS (c and d) and ADP (e and f) in WT and P2Y2-deficient mice in the absence or presence of MRS2179 (10 μM).
Calcium measurements
ATP (10 μM) evoked a transient calcium peak in the knockout as well as in the WT mice. UTP, on the other hand, evoked a biphasic response (a transient spike followed by a sustained plateau) in WT mice, whereas the P2Y2-deficient mice showed only the sustained plateau, without the initial transient spike (Figure 6).
Figure 6.
Intracellular calcium signals (E 340/380 ratio) during stimulation (5 min) with ATP and UTP (both 10 μM) in P2Y2-deficient and WT mice. For each agonist, the calcium signal was measured twice in the absence of MRS2179 and once in the presence of MRS2179. Tracing is representative of three different experiments.
MRS2179 completely inhibited the ATP signal in P2Y2-knockout mice, whereas in WT mice it was only slightly inhibited. In contrast, the calcium peak evoked by UTP was not influenced by MRS2179 in either strain.
Discussion
Agonist
Previously, we documented the presence of P2Y1, P2Y2 and P2Y6 receptors in the murine aorta. The receptors involved in endothelium-dependent relaxation were tentatively identified based on pharmacological criteria: P2Y1 for ADP, P2Y2 for ATP and P2Y6 for UDP. For UTP it was unclear whether P2Y2, P2Y6 or yet another subtype was involved, but P2Y4 was excluded by experiments on P2Y4-null mice (Guns et al., 2005). In view of the lack of specific nucleotide agonists and antagonists, we used P2Y2 receptor-deficient mice to clarify the involvement of the P2Y2 receptor subtype in nucleotide-induced vasodilatation. The P2Y2-deficient mice model has been described and used previously (Homolya et al., 1999; Matos et al., 2005). First of all, it should be mentioned that relaxations evoked by non-P2Y2 agonists, such as ADP, UDP and ACh, were not different between P2Y2-deficient and WT mice. These findings indicate that the receptors for those agonists are functioning normally in the knockout mice. In fact, this makes the P2Y2-deficient mice an ideal and suitable model for investigating the role of P2Y2 receptors in nucleotide-induced vasodilatation. However, the pD2 values of ATPγS and particular ATP were lower in the present study (Table 1) in comparison with the previous one (Guns et al., 2005). This could be due to the different genetic background of the mice (B6D2/SV129 versus C57Bl6), which could lead to subtle differences in the activity of nucleotidases or other properties of the vessel wall. The cumulative concentration–response curves for ATP, ATPγS and UTP are most interesting, as they are all potential ligands for the P2Y2 subtype. In P2Y2-deficient mice, ATP was less potent and less efficient to evoke relaxation in comparison to the WT strain. These results clearly prove a disturbed ATP response in the absence of the P2Y2 receptor subtype. Moreover, the WT mice displayed a sustained vasodilator response to ATP and ATPγS, whereas in P2Y2-deficient mice these relaxations were only transient. As the P2Y1 agonist ADP evoked similar, transient relaxations in both strains, it seems likely that the ATP-evoked relaxation in the P2Y2-deficient mice is mediated directly or indirectly (upon conversion to ADP) by the P2Y1 receptor subtype. Furthermore, the concentration–response curve of ATPγS was severely impaired in P2Y2-deficient mice, much more than that of ATP. This can be explained by the fact that ATPγS is quite resistant to ATP-degrading enzymes. Therefore, we suppose that the (small) relaxing effect of ATPγS is due to (slow) conversion of ATPγS into ADP, whereas the relaxing effect of ATP is due to direct and/or indirect (upon conversion to ADP) interaction with the P2Y1 receptor. Indeed, the P2Y1-selective antagonist MRS2179 clearly antagonized the ATP- and ATPγS-evoked relaxation response in the P2Y2-deficient mice, whereas in the WT mice MRS2179 induced only a small rightward shift. This indicates that in P2Y2-deficient mice, the action of ATP and ATPγS is mediated by P2Y1 receptors. Furthermore, the involvement of the P2X4 receptor that is highly expressed in human endothelial cells (Yamamoto et al., 2000; Wang et al., 2002) seems unlikely, as MRS2179 clearly antagonized the ATP response in the knockout mice but is inactive at rat P2X4 (and P2X2) receptors (Brown et al., 2000). As expected, the effect of MRS2179 on the P2Y1-selective agonist ADP was similar for both strains.
In contrast to ATP, the relaxation evoked by UTP did not differ between P2Y2-deficient and WT mice, thereby proving that the UTP-evoked relaxation is not exclusively mediated by the P2Y2 subtype. Further, we evaluated the relaxation potency of the dinucleotides Up3U and Up4U. At recombinant human receptors, these compounds are P2Y2 (Up4U) and P2Y6 (Up3U) selective (Pendergast et al., 2001). Although they are less potent than UTP and UDP on those receptors, they have the advantage of being less rapidly degraded by ectonucleotidases than UTP or UDP. In accordance with the latter assumption, the Up3U action was not different in WT and P2Y2−/− mice. However, the current experiments also did not show differences between P2Y2-deficient mice and WT mice for Up4U. There are two possible explanations. Although Up4U is more resistant to ectonucleotidases than UTP, it could nevertheless be degraded into UDP to a significant extent (Yerxa et al., 2002). On the other hand, Up4U itself might be an agonist of the murine P2Y6 receptor.
Taken together, the action of UTP and Up4U, both described to be ligands of the P2Y2 receptor subtype, is not (exclusively) mediated by P2Y2 receptors. The involvement of P2Y4 receptors in the action of UTP has been excluded previously in experiments on P2Y4-null mice (Guns et al., 2005). Further, P2Y1 receptors can be excluded by the lack of effect of MRS2179 on UTP responses. Therefore, the action of UTP is possibly mediated by a P2Y6(like) receptor, as proposed previously (Vial & Evans, 2002; Malmsjo et al., 2003; Guns et al., 2005; Vonend et al., 2005). Fully compatible with this ‘P2Y6(like) receptor hypothesis' is the observation that the cumulative concentration–response curves of UTP and UDP are identical in P2Y2-deficient and WT mice.
In addition, intracellular calcium signalling was measured directly in endothelial cells in situ. In the WT mice, UTP evoked a biphasic response, a fast and transient initial spike, followed by a sustained plateau. In contrast, in the P2Y2-deficient strain, UTP evoked (only) a sustained plateau without the preceding fast initial peak. Hence, we suggest that the initial transient spike seen in the WT mice might be due to the P2Y2 receptor subtype, whereas the sustained plateau can probably be attributed to the P2Y6(like) receptor. This could be related to the particular time course of the P2Y6 receptor, characterized by a slow onset and little desensitization (Robaye et al., 1997). Moreover, these data suggest that although UTP might activate P2Y2 receptors and give rise to a calcium peak, this UTP–P2Y2 interaction does not seem to be related to the relaxation response, as the sustained plateau on its own (as demonstrated in the knockout mice) evoked complete relaxation.
Further, MRS2179 clearly inhibited the ATP-elicited calcium spike in P2Y2-deficient mice, confirming that ATP activates P2Y1 receptor subtypes in the knockout mice, whereas in WT mice, the ATP-evoked calcium spike was only slightly inhibited. In contrast, the UTP-evoked calcium signal was not influenced by MRS2179 in both strains. These results are fully compatible with the result of the vasomotor study.
In conclusion, ATP- and ATPγS-evoked relaxation of the murine aorta is mediated by P2Y2 receptors, as the ATP and ATPγS responses were severely impaired in the knockout mice. Moreover, experiments with MRS2179 clearly showed that the residual ATP/ATPγS-induced relaxation in the P2Y2-deficient mice was mediated by the P2Y1 receptor subtype directly or indirectly, after conversion to ADP. In contrast, the UTP-evoked relaxation remained normal in the P2Y2-knockout mice and was not influenced by MRS2179. Therefore, it appears that the endothelium-dependent relaxation by UTP is mainly mediated by a receptor other than P2Y2, most probably the P2Y6 receptor.
Acknowledgments
Pieter-Jan Guns is a Research Assistant of the Fund for Scientific Research Flanders (Belgium – F.W.O.). We acknowledge the support by the Interuniversity Attraction Poles Programme – Belgian State – Federal Office for Scientific, Technical and Cultural Affairs, P5/02. We are grateful to B.H. Koller and J. Leipziger for the generous gift of P2Y2-knockout mice breeder pairs and we thank B. Yerxa (Inspire Pharmaceuticals) for the generous gift of Up3U and Up4U.
Abbreviations
- ACh
acetylcholine
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- ATPγS
adenosine 5′[γ-thio] triphosphate
- MRS2179
2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate
- NO
nitric oxide
- UDP
uridine diphosphate
- Up3U
P1,P4-di(uridine 5′-) triphosphate
- Up4U (INS365)
P1,P4-di(uridine 5′-) tetraphosphate
- UTP
uridine triphosphate
- WT
wild type
References
- ABBRACCHIO M.P., BOEYNAEMS J.M., BARNARD E.A., BOYER J.L., KENNEDY C., MIRAS-PORTUGAL M.T., KING B.F., GACHET C., JACOBSON K.A., WEISMAN G.A., BURNSTOCK G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol. Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOARDER M.R., HOURANI S.M. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol. Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BROWN S.G., KING B.F., KIM Y.-H., YANG S., BURNSTOCK G., JACOBSON K. Activity of novel adenine nucleotide derivatives as agonist and antagonists at recombinant rat P2X receptors. Drug Dev. Res. 2000;49:253–259. doi: 10.1002/1098-2299(200004)49:4<253::AID-DDR4>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUVINIC S., BRIONES R., HUIDOBRO-TORO J.P. P2Y1 and P2Y2 receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br. J. Pharmacol. 2002;136:847–856. doi: 10.1038/sj.bjp.0704789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAUWELS H.M., VAN HOVE C.E., HOLVOET P., HERMAN A.G., BULT H. Plaque-associated endothelial dysfunction in apolipoprotein E-deficient mice on a regular diet. Effect of human apolipoprotein AI. Cardiovasc. Res. 2003;59:189–199. doi: 10.1016/s0008-6363(03)00353-5. [DOI] [PubMed] [Google Scholar]
- DI VIRGILIO F., SOLINI A. P2 receptors: new potential players in atherosclerosis. Br. J. Pharmacol. 2002;135:831–842. doi: 10.1038/sj.bjp.0704524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNS P.J., KORDA A., CRAUWELS H.M., VAN ASSCHE T., ROBAYE B., BOEYNAEMS J.M., BULT H. Pharmacological characterization of nucleotide P2Y receptors on endothelial cells of the mouse aorta. Br. J. Pharmacol. 2005;146:288–295. doi: 10.1038/sj.bjp.0706326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOMOLYA L., WATT W.C., LAZAROWSKI E.R., KOLLER B.H., BOUCHER R.C. Nucleotide-regulated calcium signaling in lung fibroblasts and epithelial cells from normal and P2Y2 receptor (−/−) mice. J. Biol. Chem. 1999;274:26454–26460. doi: 10.1074/jbc.274.37.26454. [DOI] [PubMed] [Google Scholar]
- KUNAPULI S.P., DANIEL J.L. P2 receptor subtypes in the cardiovascular system. Biochem. J. 1998;336:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., BOUCHER R.C. UTP as an extracellular signaling molecule. News Physiol. Sci. 2001;16:1–5. doi: 10.1152/physiologyonline.2001.16.1.1. [DOI] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., BOUCHER R.C., HARDEN T.K. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J. Biol. Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- MALMSJO M., HOU M., PENDERGAST W., ERLINGE D., EDVINSSON L. The stable pyrimidines UDPbetaS and UTPgammaS discriminate between contractile cerebrovascular P2 receptors. Eur. J. Pharmacol. 2003;458:305–311. doi: 10.1016/s0014-2999(02)02787-5. [DOI] [PubMed] [Google Scholar]
- MATOS J.E., ROBAYE B., BOEYNAEMS J.M., BEAUWENS R., LEIPZIGER J. K+ secretion activated by luminal P2Y2 and P2Y4 receptors in mouse colon. J. Physiol. 2005;564:269–279. doi: 10.1113/jphysiol.2004.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- PENDERGAST W., YERXA B.R., DOUGLASS J.G., III SHAVER S.R., DOUGHERTY R.W., REDICK C.C., SIMS I.F., RIDEOUT J.L. Synthesis and P2Y receptor activity of a series of uridine dinucleoside 5′-polyphosphates. Bioorg. Med. Chem. Lett. 2001;11:157–160. doi: 10.1016/s0960-894x(00)00612-0. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- ROBAYE B., BOEYNAEMS J.M., COMMUNI D. Slow desensitization of the human P2Y6 receptor. Eur. J. Pharmacol. 1997;329:231–236. [PubMed] [Google Scholar]
- VAN DER GIET M., GIEBING G., TOLLE M., SCHMIDT S. The role of P2Y receptors in the control of blood pressure. Drug News Perspect. 2002;15:640–646. doi: 10.1358/dnp.2002.15.10.740237. [DOI] [PubMed] [Google Scholar]
- VANHOUTTE P.M., FELETOU M., TADDEI S. Endothelium-dependent contractions in hypertension. Br. J. Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIAL C., EVANS R.J. P2X(1) receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol. Pharmacol. 2002;62:1438–1445. doi: 10.1124/mol.62.6.1438. [DOI] [PubMed] [Google Scholar]
- VONEND O., STEGBAUER J., SOJKA J., HABBEL S., QUACK I., ROBAYE B., BOEYNAEMS J.M., RUMP L.C. Noradrenaline and extracellular nucleotide cotransmission involves activation of vasoconstrictive P2X1,3- and P2Y6-like receptors in mouse perfused kidney. Br. J. Pharmacol. 2005;145:66–74. doi: 10.1038/sj.bjp.0706151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG L., KARLSSON L., MOSES S., HULTGARDH-NILSSON A., ANDERSSON M., BORNA C., GUDBJARTSSON T., JERN S., ERLINGE D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J. Cardiovasc. Pharmacol. 2002;40:841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- WIHLBORG A.K., MALMSJO M., EYJOLFSSON A., GUSTAFSSON R., JACOBSON K., ERLINGE D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br. J. Pharmacol. 2003;138:1451–1458. doi: 10.1038/sj.bjp.0705186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO K., KORENAGA R., KAMIYA A., ANDO J. Fluid shear stress activates Ca2+ influx into human endothelial cells via P2X4 purinoceptors. Circ. Res. 2000;87:385–391. doi: 10.1161/01.res.87.5.385. [DOI] [PubMed] [Google Scholar]
- YERXA B.R., SABATER J.R., DAVIS C.W., STUTTS M.J., LANG-FURR M., PICHER M., JONES A.C., COWLEN M., DOUGHERTY R., BOYER J., ABRAHAM W.M., BOUCHER R.C. Pharmacology of INS37217 [P1-(uridine 5′)-P4-(2′-deoxycytidine 5′)tetraphosphate, tetrasodium salt], a next-generation P2Y2 receptor agonist for the treatment of cystic fibrosis. J. Pharmacol. Exp. Ther. 2002;302:871–880. doi: 10.1124/jpet.102.035485. [DOI] [PubMed] [Google Scholar]