Abstract
Inhibition of uncontrolled epidermal growth factor receptor (EGFR) is one of the approaches for the treatment of breast and lung cancers. We designed oligopeptides consisting of amino-acid sequences of the major (Y1068, Y1148, and Y1173) and minor (Y992) autophosphorylation sites of EGFR. These peptides may be exogenous substrates or pseudosubstrates that interfere with the autophosphorylation of EGFR. The effects of the peptides on autophosphorylation of EGFR were studied.
Purified EGFR was phosphorylated in vitro with EGF in the presence of various synthetic peptides. The phosphorylation level of EGFR was then evaluated after SDS–PAGE separation, followed by Western blot analysis with antiphosphotyrosine antibody.
Ac-VPEYINQ-NH2 (Y1068) and Ac-DYQQD-NH2 (Y1148) showed the most potent inhibitory effects, followed by Ac-ENAEYLR-NH2 (Y1173). These peptides at 4 mM suppressed phosphorylation to 30–50%.
Combination of the three kinds of peptides much more strongly inhibited autophosphorylation. The 50% inhibitory concentration (IC50) value was 0.5 mM as a mixture and was comparable to that of AG1478 (IC50, 0.3 mM) at 0.2 mM ATP.
Neither Ac-DIYET-NH2 or Ac-KIYEK-NH2, designed previously based on the amino-acid sequence of an autophosphorylation site of insulin receptor, nor their related (Ac-KIFMK-NH2) or unrelated (Ac-LPFFD-NH2) peptides showed an inhibitory effect. These results suggest that the small peptides that originated from the autophosphorylation sites of EGFR interact solely with EGFR.
The peptides containing the sequences surrounding Y1068, Y1148, and Y1173 may be a promising seed for the development of therapeutic agents for breast and lung cancers.
Keywords: AG1478, DYQQD, epidermal growth factor, EGF, EGFR, ENAEYLR, lignocaine, phosphorylation, tyrosine kinase, VPEYINQ
Introduction
Receptor tyrosine kinases (RTKs) play a crucial role in the regulation of cell proliferation, cell differentiation, and intracellular signaling processes (Hubbard et al., 1998; Hunter, 1998). Uncontrolled and elevated RTK activity resulting from overexpression is associated with a range of human malignant tumors (Levitzki & Gazit, 1995). Therefore, inhibition of these receptors represents a potent approach for the treatment of various cancers.
Epidermal growth factor receptor (EGFR) is one of the RTKs that regulate cell proliferation (Jorissen et al., 2003). EGFR has extracellular ligand-binding domains to which epidermal growth factor (EGF) binds. Binding ligands lead to the formation of homo- and heterodimers of EGFR and other EGFR family members (ErbB2 (Yamamoto et al., 1986), ErbB3 (Kraus et al., 1989), and ErbB4 (Plowman et al., 1993)). Subsequently, their intracellular autophosphorylation domains in the C-terminal region are trans-autophosphorylated and the catalytic domains of EGFR are activated. Active EGFR recruits and phosphorylates signaling proteins, such as Grb2, which links cell surface receptors to the Ras/MAPK signaling cascade. Ultimately, intranuclear mitogenesis, followed by cell proliferation, is caused via the activation of the Ras/MAPK signaling pathway (Schlessinger, 2000).
The pentapeptide KIFMK (Ac-KIFMK-NH2), a synthetic peptide containing the IFM motif in the sodium channel inactivation gate on the cytoplasmic linker between domains III and IV (III–IV linker), is known to restore fast inactivation to mutant sodium channels having a defective inactivation gate (Eaholtz et al., 1994). Both the N- and C-terminal Lys residues are important factors to allow fast inactivation (Eaholtz et al., 1999). As there are acidic amino acids only near the IFM motif in the amino acid sequence of the III–IV linker (QDIFMTEEQ1486–1494, rat brain type-IIA sodium channel), it may be considered that KIFMK binds to the DIFMTEE moiety of the III–IV linker of the sodium channel (Kuroda et al., 1999).
We found substantial homology between the amino-acid sequence of the III–IV linker of the sodium channel and that of the activation loop (A loop) of the insulin receptor (IR) (Hirose et al., 2002). Based on this finding, we investigated the effects of KIFMK and its related peptides on tyrosine autophosphorylation of IR (Hirose et al., 2004). We found that not only KIFMK, but also its related peptides, DIYET and KIYEK, suppress tyrosine autophosphorylation. The amino-acid sequence of DIYET, including the IYE motif, was taken from that of the autophosphorylation site surrounding Y1158 in the A loop of IR (RDIYETDYY1155–1163). The crystal structure of the unphosphorylated state of IR reveals that Y1162, which is located near the Y1158, in the A loop acts as a pseudosubstrate inhibitor (Hubbard et al., 1994). Accordingly, we considered that DIYET, KIYEK, and KIFMK were recognized as pseudosubstrate inhibitor peptides (Kemp & Pearson, 1991). Presently, we investigated whether small peptides derived from the sequence of the autophosphorylation sites of EGFR may be pseudosubstrate-based peptide inhibitors.
EGFR has six autophosphorylation sites (Y992, Y1045, Y1068, Y1086, Y1148, and Y1173) (Downward et al., 1984; Hsuan et al., 1989; Margolis et al., 1989; Walton et al., 1990; Levkowitz et al., 1999; Marmor & Yarden, 2003), which regulate the activity of EGFR. It has been reported that Y1068, Y1148, and Y1173 are major (Downward et al., 1984) and Y992 (Walton et al., 1990), Y1045 (Levkowitz et al., 1999), and Y1086 (Margolis et al., 1989) are minor autophosphorylation sites. In this study, we focused on the three major and one minor (Y992) phosphorylation sites. We designed 5- to 7-mer synthetic peptides originating from the amino-acid sequences of those sites (Table 1). When the sequence included an acidic (Asp, Glu) or a basic (Lys, Arg) amino acid at the peptide N- or C-terminal end or both, a peptide containing the opposite charge was also prepared. We investigated the effects of the synthetic peptides on the phosphorylation of EGFR. We also investigated the peptides that could (KIFMK, DIYET, and KIYEK) or could not (LPFFD) suppress the autophosphorylation of IR, and tyrphostin AG1478, which is known to be a potent EGFR inhibitor (Levitzki & Gazit, 1995), for comparison purposes.
Table 1.
Sequences of autophosphorylation sites of EGFR and the synthetic peptides derived from them
| Y992 | D | A | D | E | Y | L | I | P | Q |
| D | E | Y | L | I | |||||
| K | E | Y | L | I | |||||
| Y1068 | P | V | P | E | Y | I | N | Q | S |
| V | P | E | Y | I | N | Q | |||
| Y1148 | D | N | P | D | Y | Q | Q | D | F |
| D | Y | Q | Q | D | |||||
| K | Y | Q | Q | K | |||||
| Y1173 | E | N | A | E | Y | L | R | V | A |
| E | N | A | E | Y | L | R | |||
| K | N | A | E | Y | L | E |
Methods
Materials
Antiphosphotyrosine antibody 4G10 was obtained from Upstate Biotechnology Inc. (Lake Placid, NY, U.S.A.). Anti-phospho-EGFR (pY1068) antibody and anti-phospho-EGFR (pY1148) antibody were obtained from Abcam Ltd. (Cambridge, U.K.) and anti-phospho-EGFR (pY1173) antibody was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, U.S.A.). Purified EGFR derived from human carcinoma cells A431 and EGF were obtained from Sigma Chemical Co. (St Louise, MO, U.S.A.). An EGFR inhibitor, AG1478, was obtained from Calbiochem (Cambridge, MA, U.S.A.).
Synthesis and purification of peptides
Peptides were synthesized automatically by the solid-phase method using Fmoc chemistry on an ABI 433A Peptide Synthesizer. Their N-termini were acetylated (Ac-) and C-termini were amidated (-NH2). After cleavage with trifluoroacetic acid (TFA), the peptides were purified on a reverse-phase C18 HPLC column using a gradient 95% A, 5% B–60% A, and 40% B in 35 min, where A is 0.1% TFA in water and B is 0.1% TFA in acetonitrile. The peptides were characterized by mass spectrometry on a Perkin-Elmer SCIEX API III mass spectrometer. Ac-ENAEYLR-NH2: m/z calculated 934.45 (monoisotope), 935.00 (average), found 936.5 (MH+); Ac-ENAEALR-NH2: m/z calculated 842.42 (monoisotope), 842.91 (average), found 844.0 (MH+); Ac-KNAEYLE-NH2: m/z calculated 906.44 (monoisotope), 907.00 (average), found 908.0 (MH+); Ac-DEYLI-NH2: m/z calculated 692.34 (monoisotope), 692.77 (average), found 694.0 (MH+); Ac-KEYLI-NH2: m/z calculated 705.41 (monoisotope), 705.85 (average), found 707.5 (MH+); Ac-DYQQD-NH2: m/z calculated 708.27 (monoisotope), 708.68 (average), found 709.5 (MH+); Ac-DpYQQD-NH2 (pY, phosphorylated tyrosine): m/z calculated 788.24 (monoisotope), 788.66 (average), found 790.0 (MH+); Ac-DAQQD-NH2: m/z calculated 616.25 (monoisotope), 616.58 (average), found 617.5 (MH+); Ac-KYQQK-NH2: m/z calculated 734.41 (monoisotope), 734.85 (average), found 736.0 (MH+); Ac-VPEYINQ-NH2: m/z calculated 902.45 (monoisotope), 903.00 (average), found 904.0 (MH+); Ac-VPEAINQ-NH2: m/z calculated 810.42 (monoisotope), 810.90 (average), found 812.0 (MH+); Ac-KIFMK-NH2: m/z calculated 706.42 (monoisotope), 706.94 (average), found 707.0 (MH+); Ac-DIYET-NH2: m/z calculated 680.30 (monoisotope), 680.71 (average), found 680.5 (MH+); Ac-KIYEK-NH2: m/z calculated 720.42 (monoisotope), 720.87 (average), found 721.0 (MH+); Ac-LPFFD-NH2: m/z calculated 678.34 (monoisotope), 678.79 (average), found 680.0 (MH+).
In vitro phosphorylation of EGFR in the presence of synthetic peptides or AG1478
Purified EGFR (20 μg ml−1) was phosphorylated with EGF (100 ng ml−1) and with the synthetic peptides (0.004, 0.04, 0.4, or 4 mM) or AG1478 (0.0004, 0.004, 0.04, 0.4, or 4 mM) for 5 min at 37°C in 50 μl of incubation buffer, including 50 mM HEPES, pH 7.4, 125 mM NaCl, 1 mM EDTA, 10 mM MgCl2, 5 mM MnCl2, 5 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and ATP, respectively. The concentration of ATP was 0.2 mM, unless otherwise noted. After the reactions were stopped by the addition of Laemmli sample buffer and boiling for 5 min, EGFRs were separated by SDS–PAGE in 7.5% (v v−1) acrylamide gels and transferred to a PVDF membrane (Amersham Biosciences Ltd, Piscataway, NJ, U.S.A.). The membranes were blocked in 5% (w v−1) dried milk in PBS-Tween 20 at room temperature overnight, and were then immunoblotted with antiphosphotyrosine antibody 4G10, anti-phospho-EGFR (pY1068) antibody, anti-phospho-EGFR (pY1148) antibody, or anti-phospho-EGFR (pY1173) antibody. The antigen antibody complexes were visualized by ECL Western blotting detection reagents (Amersham Biosciences Ltd, Piscataway, NJ, U.S.A.). The bands were exposed to X-ray films and images obtained were analyzed by the use of Scion Image Software (Scion Co., Frederick, MD, U.S.A.).
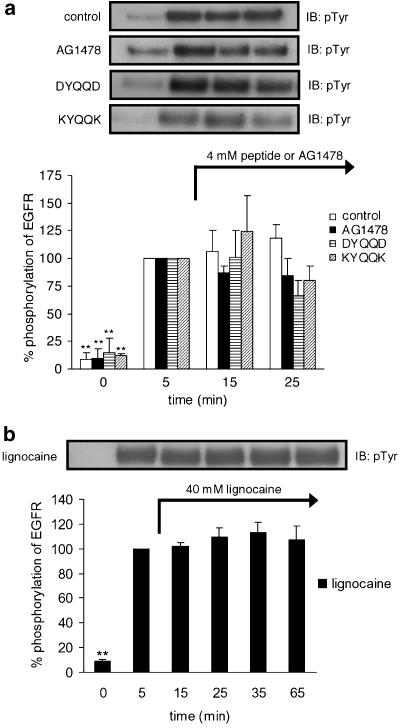
Effects of synthetic peptides (DYQQD and KYQQK), lignocaine, or AG1478 on pre-phosphorylated tyrosine residues of EGFR in vitro
Purified EGFR (20 μg ml−1) was incubated in 50 μl of incubation buffer for 0, 5, 15, or 25 min at 37°C with EGF (100 ng ml−1). The reactions were stopped by the addition of Laemmli sample buffer at 0 or 5 min after EGF stimulation in the samples that had been scheduled for 0- or 5-min incubation. In the samples scheduled for 15- or 25-min incubation, 4 mM of DYQQD or KYQQK, 40 mM of lignocaine, or 4 mM of AG1478 was added at 5 min after EGF stimulation, and Laemmli sample buffer was added at 15 or 25 min after EGF stimulation to stop the reactions. All samples were boiled for 5 min, followed by Western blot analysis with antiphosphotyrosine antibody. EGF-stimulated responses of EGFR at 5 min were considered to be 100%.
Statistical analysis
Data were analyzed by one-way analysis of variance with Dunnett post hoc analysis, using Kaleida Graph (Synergy Software Technologies Inc., Reading, PA, U.S.A.). The statistical significance was established at the P<0.05 level. All values are reported as means±s.d.
Studies on phosphorylation of DYQQD, ENAEYLR, and VPEYINQ by mass spectrometry
Purified EGFR (20 μg ml−1) was incubated for 30 min at 37°C with EGF (100 ng ml−1) and ATP (0.2 mM) and with DYQQD, ENAEYLR, and VPEYINQ (1 μM, each). The reaction mixture was desalted with the solid-phase extraction method using Discovery DSC-18 (Supelco, Bellefonte, PA, U.S.A.). The eluent was 80% A, 20% B for the mixture of DYQQD, ENAEYLR, and VPEYINQ, and 70% A, 30% B for the mixture of ENAEYLR and VPEYINQ, where A is 0.1% TFA in water and B is 0.1% TFA in acetonitrile. The molecular masses of peptides in the sample solution were analyzed with a Perkin-Elmer SCIEX API III mass spectrometer.
Results
In vitro autophosphorylation of EGFR in the presence of synthetic peptides or AG1478
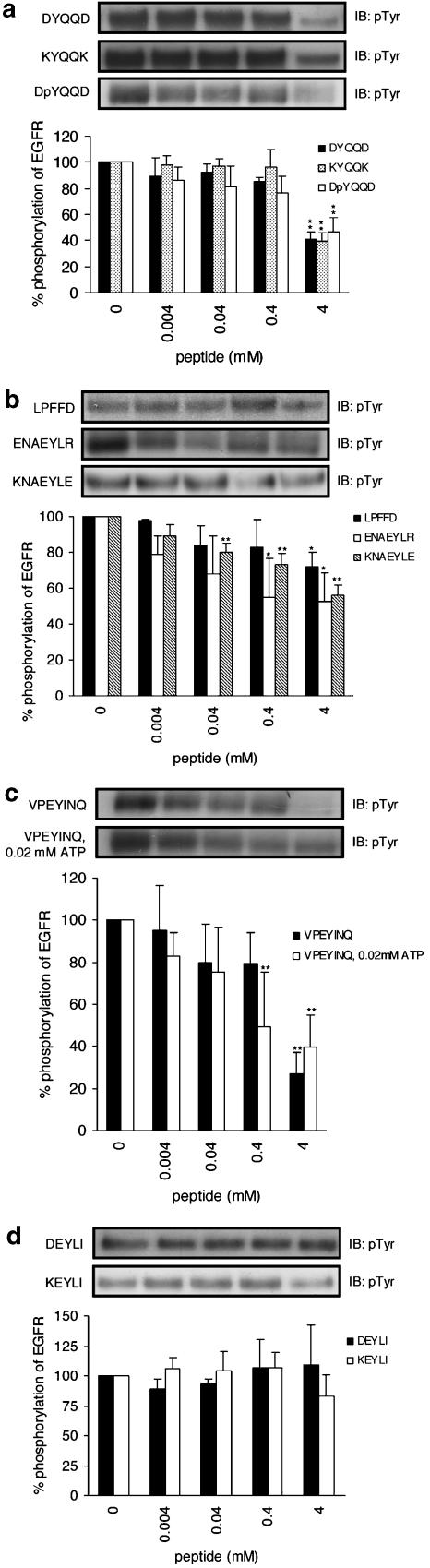
We investigated the effects of synthetic peptides on the phosphorylation of EGFR. Results are shown in Figure 1, where the EGF-stimulated responses of EGFR without peptides at 5 min were taken as the control and considered to be 100%. In all, 4 mM each of DYQQD and KYQQK, which contain Y1148, suppressed the autophosphorylation of EGFR to 41.2±5.5 and 39.3±6.6%, respectively (Figure 1a). In order to study the effect of an oligopeptide containing a phosphorylated tyrosine (pY) on the autophosphorylation of EGFR, we also investigated DpYQQD (Figure 1a). Also, 4 mM of DpYQQD suppressed autophosphorylation to 46.4±10.9%. The inhibitory effects of ENAEYLR and KNAEYLE, which contain Y1173, were less potent than those derived from Y1148; 4 mM of ENAEYLR suppressed autophosphorylation to 52.5±16.1% and 4 mM of KNAEYLE to 55.8±5.7% (Figure 1b). The inhibitory effects of VPEYINQ, which contain Y1068, appear to be the most potent; VPEYINQ suppressed autophosphorylation to 27.2±9.7% (Figure 1c). Conversely, no inhibitory effects were seen in the peptides containing Y992 (DEYLI and KEYLI) (Figure 1d). LPFFD, which is an unrelated control peptide and was anticipated to show no inhibitory effect, slightly suppressed autophosphorylation (Figure 1b). These observations show that peptides derived from the major phosphorylation site (Y1148, Y1173, or Y1068) inhibit autophosphorylation much more effectively than that from the minor site (Y992) or unrelated peptides with regard to the amino-acid sequences of the autophosphorylation sites of EGFR.
Figure 1.
Phosphorylation of purified EGFR in the presence or absence of peptides. (a) Peptides including Y1148 (pY, phosphorylated tyrosine), (b) peptides including Y1173 and the control peptide, Ac-LPFFD-NH2, (c) a peptide including Y1068, and (d) peptides including Y992. EGFR was incubated with or without peptides for 5 min at 37°C in the buffer containing 0.2 mM of ATP, except open bars shown in (c), where 0.02 mM of ATP was used. Results displayed on the top panels represent typical immunoblots (IB). *P<0.05, **P<0.01 versus EGF-stimulated tyrosine phosphorylation at 5-min incubation without peptides; n=4 for each lane.
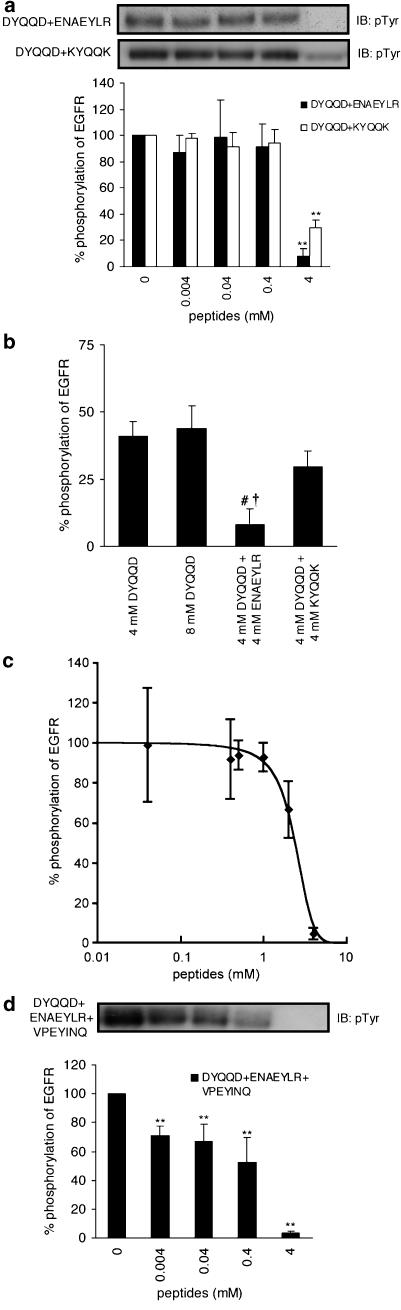
As the oligopeptides in Table 1 were designed by taking into account the amino-acid sequences at the autophosphorylation sites of EGFR, it might be anticipated that the peptides originating from the different autophosphorylation sites would interact at different sites of EGFR. If this was indeed true, it might be expected that a mixture of, for example, two kinds of peptides, different in origin, would inhibit autophosphorylation more effectively than would twice the concentration of each kind of peptide. Interestingly, this was in fact observed. A mixture of DYQQD and ENAEYLR, 4 mM each, inhibited the autophosphorylation of EGFR to 8.1±5.9% (Figure 2a). In contrast, 8 mM DYQQD or a mixture of 4 mM DYQQD and 4 mM KYQQK, each of which originates from the same site at Y1148, showed no significant differences in the extent of phosphorylation as compared with that of 4 mM DYQQD (Figure 2b). These results unambiguously show that DYQQD and ENAEYLR are interacting with EGFR at different sites. Since the inhibitory effects of the mixed peptides are not dose-dependent, but effective abruptly at 4 mM (Figure 2a), we have added three data points between 0.4 and 4 mM (0.5, 1, and 2 mM for each component of the peptides) and determined the 50% inhibitory concentration (IC50) for the mixed peptides (Figure 2c). The IC50 value of the mixed peptides thus determined was 2.4 mM. Furthermore, we also studied three different kinds of mixed peptides, VPEYINQ, DYQQD, and ENAEYLR, expecting much more potent inhibitory effects on phosphorylation than were achieved by the two kinds of peptides (Figure 2d). This was, in fact, observed. Moreover, the combined use of the three kinds of peptides suppressed autophosphorylation in a dose-dependent manner. A volume of 4 mM each of the mixed peptides suppressed phosphorylation to 3.5±1.4%; the IC50 value as mixed peptides was 0.5 mM.
Figure 2.
Phosphorylation of purified EGFR in the presence or absence of mixtures of peptides. (a) A mixture of DYQQD and ENAEYLR and that of DYQQD and KYQQK (concentrations indicated are those of each component), (b) comparison of inhibitory effects by 8 mM DYQQD and two kinds of mixtures of peptides with that of 4 mM DYQQD, (c) determination of IC50 value for a mixture of DYQQD and ENAEYLR (concentrations indicated are those of each component), and (d) phosphorylation of purified EGFR in the presence or absence of the mixture of VPEYINQ, DYQQD, and ENAEYLR (concentrations indicated are that of each component). EGFR was incubated in the buffer with or without peptides for 5 min at 37°C. Results displayed on the top panels represent typical immunoblots (IB). **P<0.01 versus EGF-stimulated tyrosine phosphorylation at 5 min incubation without peptides; #P<0.01 versus EGF-stimulated tyrosine phosphorylation at 5 min incubation with 4 mM DYQQD; †P<0.01 versus EGF-stimulated tyrosine phosphorylation at 5 min incubation with 8 mM DYQQD; n=4 for each lane.
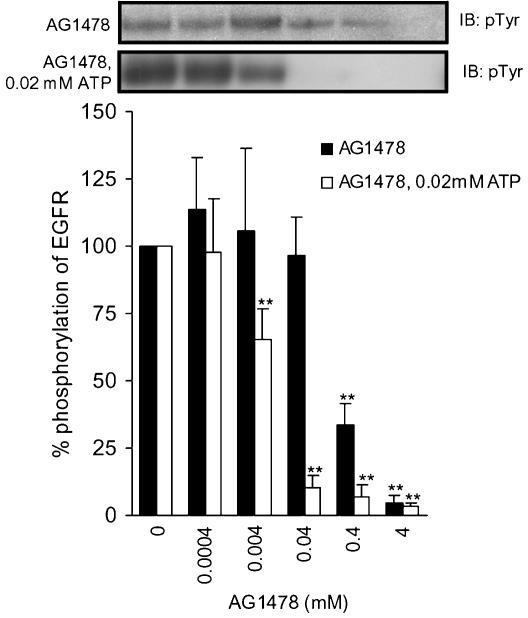
In order to compare the potency of the mixed peptides with that of the inhibitor reported so far, we investigated tyrphostin AG1478 (Levitzki & Gazit, 1995), since it is a well-known potent EGFR inhibitor (Figure 3). The AG1478 inhibited phosphorylation of EGFR in a dose-dependent manner; 4 mM of AG1478 suppressed the phosphorylation to 4.6±2.5%, with an IC50 of 0.3 mM. Interestingly, the inhibitory potency achieved by the combined use of VPEYINQ, DYQQD, and ENAEYLR was comparable to that attained by AG1478.
Figure 3.
Phosphorylation of purified EGFR in the presence or absence of AG1478. EGFR was incubated in the buffer with or without AG1478 for 5 min at 37°C with 0.2 mM of ATP (closed bars) or with 0.02 mM of ATP (open bars). Results displayed on the top panels represent typical immunoblots (IB). **P<0.01 versus EGF-stimulated Tyr phosphorylation at 5-min incubation without AG1478; n=4 for each lane.
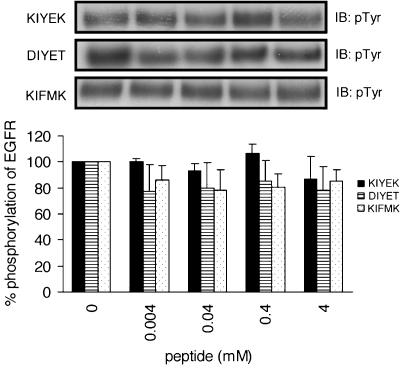
We also investigated the effects of KIFMK, DIYET, and KIYEK (Hirose et al., 2004) on the autophosphorylation of EGFR. The IFM motif in KIFMK was taken from the III–IV linker of the sodium channel and the amino-acid sequence of DIYET follows that of the activation loop of IR. These peptides, especially KIFMK and KIYEK, induced the complete suppression of insulin-stimulated autophosphorylation of IR at 4 mM (Hirose et al., 2004). If the peptides designed presently (Table 1) interact with the EGFR sequence specifically, it would be expected that KIFMK, DIYET, and KIYEK should have no effect on the autophosphorylation of EGFR. As shown in Figure 4, this was indeed true. No significant inhibitory effects were noted by these peptides on the autophosphorylation of EGFR.
Figure 4.
Phosphorylation of purified EGFR in the presence or absence of peptides, which can suppress phosphorylation of the IR. EGFR was incubated in the buffer with or without peptides for 5 min at 37°C. Results displayed on the top panels represent typical immunoblots (IB). n=4 for each lane.
Effects of the synthetic peptides (DYQQD and KYQQK), lignocaine or AG1478 on pre-phosphorylated tyrosine residues of EGFR in vitro
Previously, we found that KIYEK and DIYET, at 4 mM each, dephosphorylate pre-phosphorylated tyrosine residues of IR in vitro (Hirose et al., 2004). We also found that a local anesthetic, lignocaine, at 40 mM dephosphorylates phosphotyrosine residues (Hirose et al., 2004). In order to investigate whether the peptides DYQQD and KYQQK, lignocaine, and tyrphostin AG1478 dephosphorylate phosphotyrosine residues of EGFR, we incubated pre-phosphorylated EGFR together with these materials. As shown in Figure 5, all these materials did not dephosphorylate the pre-phosphorylated EGFR, although DYQQD at 25 min appears to have dephosphorylated EGFR slightly.
Figure 5.
Effects of peptides, AG1478, or lignocaine on EGF-stimulated Tyr phosphorylation of EGFR at different time points. EGFR was incubated in the buffer for (a): 0, 5, 15, or 25 min at 37°C, respectively and for (b): 0, 5, 15, 25, 35, or 65 min at 37°C, respectively. Each peptide, AG1478, or lignocaine was added at 5 min after EGF stimulation, (a) in the samples of 15- or 25-min incubation and (b) in the samples of 15-, 25-, 35- or 65-min incubation. The ‘control' sample included no peptide, AG1478, or lignocaine. One point was made for each time, resulting in different incubations with or without peptides, AG1478, or lignocaine. Results displayed on the top panel represent typical immunoblots (IB). **P<0.01 versus EGF-stimulated Tyr phosphorylation at 5-min incubation without peptides, AG1478 or lignocaine; n=4 for each lane.
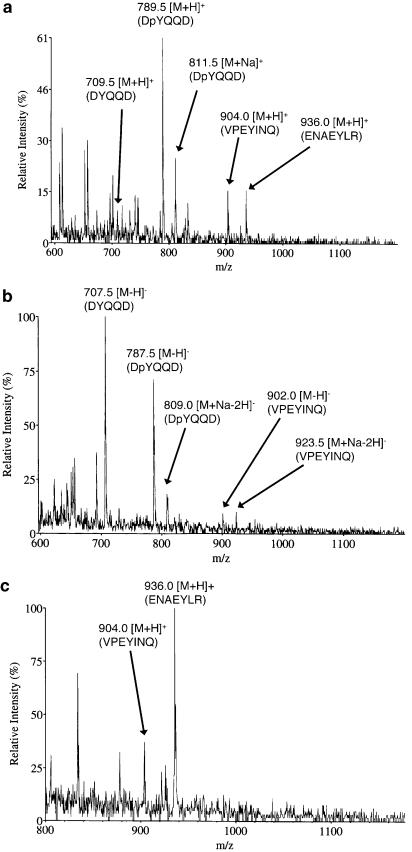
Detection of the phosphorylated peptide DpYQQD, ENAEpYLR, and VPEpYINQ by mass spectrometry
To investigate whether the oligopeptide designed in Table 1 is phosphorylated as an exogenous peptide substrate during the suppression of autophosphorylation of EGFR, we tried to measure the mass spectra for the mixture of DYQQD, ENAEYLR, and VPEYINQ after incubation with EGFR, EGF, and ATP. The results are summarized in Table 2, and typical mass spectra are shown in Figure 6. While all the ions due to nonphosphorylated DYQQD, ENAEYLR, and VPEYINQ were found in both positive- and negative-ion modes of operation, the corresponding ions due to phosphorylated peptides were detected only for DpYQQD (Figure 6a and b). This result means that among the three kinds of peptides, only DYQQD was recognized as an exogenous peptide substrate and suffered from phosphorylation, while the remaining peptides were recognized as pseudosubstrates and inhibited autophosphorylation. Since there remains the possibility that the preferential phosphorylation of DYQQD had suppressed the phosphorylation of ENAEYLR and VPEYINQ, we observed mass spectra for a sample solution incubated for the two kinds of peptides with EGFR, EGF, and ATP. As shown in Figure 6c, again, no ions due to phosphorylated peptides were observed. Thus, it appears that the amino-acid sequences of the oligopeptides, ENAEYLR and VPEYINQ, are not suitable for phosphorylation by the kinase domain of EGFR.
Table 2.
Theoretical and observed m/z values in positive- and negative-ion mass spectra
| Theoretical (average) | Observeda | |||
|---|---|---|---|---|
| [M+H]+ | [M−H]− | [M+H]+ | [M−H]− | |
| DYQQD | 709.69 | 707.67 | 709.5 | 707.5 |
| DpYQQD | 789.67 | 787.65 | 789.5 | 787.5 |
| ENAEYLR | 936.01 | 933.99 | 936.0 | ND |
| ENAEpYLR | 1015.99 | 1013.98 | ND | ND |
| VPEYINQ | 904.01 | 901.99 | 904.0 | 902.0 |
| VPEpYINQ | 983.99 | 981.97 | ND | ND |
ND, not detected.
Figure 6.
Mass spectra of the solutions for mixtures of DYQQD, ENAEYLR, and VPEYINQ incubated with EGFR, EGF, and ATP. (a) Positive-ion mode mass spectrum for a mixture of DYQQD, ENAEYLR, and VPEYINQ, (b) negative-ion mode mass spectrum for a mixture of DYQQD, ENAEYLR, and VPEYINQ, (c) positive-ion mode mass spectrum for a mixture of ENAEYLR and VPEYINQ.
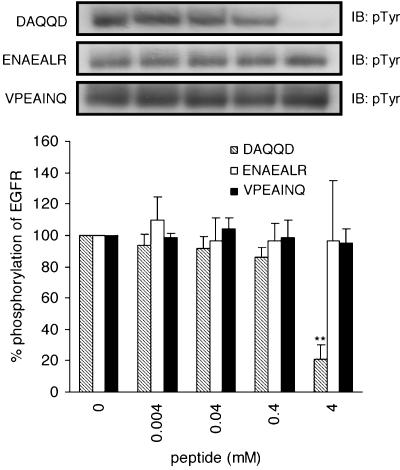
Effects of tyrosine to alanine mutation in DYQQD, ENAEYLR, and VPEYINQ on the inhibitory potencies for the autophosphorylation of EGFR
As revealed by the mass spectrometry, only DYQQD was phosphorylated during the suppression of autophosphorylation of EGFR. ENAEYLR and VPEYINQ were not phosphorylated, but they were able to suppress autophosphorylation, as described above. Moreover, Figure 1a showed that DpYQQD retained the inhibitory potency. These findings imply that phosphorylatable tyrosine residues are not necessary for the suppression of autophosphorylation. To make sure whether the presence of tyrosine residue is a primary requisite for the inhibition of autophosphorylation, we investigated tyrosine to alanine mutated peptides in DYQQD, ENAEYLR, and VPEYINQ (Figure 7). In contrast to our expectation, tyrosine to alanine mutation led to the complete elimination of inhibitory potencies for ENAEYLR and VPEYINQ, showing that tyrosine residues play a crucial role in inhibiting autophosphorylation. Conversely, substitution of the tyrosine of DYQQD with alanine led to an increased inhibitory potency, showing that the tyrosine residue is not very important in the inhibitory activity. DAQQD (4 mM) suppressed autophosphorylation to 20.1±8.7%. This value was markedly superior to that achieved by the parent DYQQD (41.2±5.5%).
Figure 7.
Effects of tyrosine to alanine mutation in DYQQD, ENAEYLR, and VPEYINQ on the inhibitory potencies for autophosphorylation of EGFR. EGFR was incubated in the buffer with or without peptides for 5 min at 37°C. Results displayed on the top panels represent typical immunoblots (IB). **P<0.01 versus EGF-stimulated tyrosine phosphorylation at 5-min incubation without peptides; n=4 for each lane.
Effects of a low ATP concentration on inhibitory potencies of VPEYINQ and AG1478
The inhibition of autophosphorylation of EGFR by VPEYINQ and AG1478 at a low ATP concentration (0.02 mM) was investigated. The presently employed peptides appeared to have suppressed autophosphorylation of EGFR as substrate competitive inhibitors. Accordingly, the inhibitory potency of, for example, VPEYINQ is expected to not depend on ATP concentrations. On the contrary, the inhibitory potency of AG1478 is expected to depend on ATP concentrations, since it is a well-known ATP competitive inhibitor (Levitzki & Gazit, 1995). The results for VPEYINQ and AG1478 are shown in Figures 1c and 3, respectively. As expected, VPEYINQ showed no significant differences in inhibitory potencies between those at 0.2 and 0.02 mM of ATP. In contrast, the inhibitory potencies of AG1478 depended highly on the ATP concentrations. The IC50 value of AG1478 was drastically reduced from 0.3 mM to 4.7 μM on lowering the concentration of ATP. The value is comparable to those of tyrphostins determined at 2 μM of ATP (Osherov et al., 1993). Since the physiological concentrations of ATP in a cell are 1.5–5 mM (Traut, 1994), the IC50 value of AG1478 in a cell would far exceed 0.3 mM. Thus, it is conceivable that in a cell, the mixed peptides, which are composed of DYQQD, ENAEYLR, and VPEYINQ, may be more potent than AG1478.
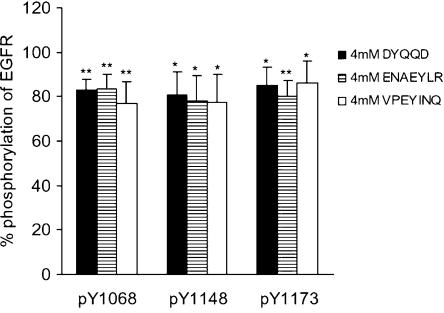
Detection of phosphorylation using site-specific antiphosphotyrosine antibodies against pY1068, pY1148, or pY1173
In order to investigate whether oligopeptides specifically inhibit autophosphorylation for the site from which they were derived, we observed phosphotyrosine residues using site-specific antiphosphotyrosine antibodies against pY1068, pY1148, or pY1173. The results for VPEYINQ, DYQQD, and ENAEYLR, 4 mM each, are summarized in Figure 8, where the EGF-stimulated responses of EGFR without peptides at 5 min were taken as the control and considered to be 100%. As shown in Figure 8, each of the peptides equally suppressed phosphorylation among the three autophosphorylation sites. These observations indicate that these oligopeptides are inhibiting a catalytic site of the kinase domain, irrespective of the origin of the oligopeptides.
Figure 8.
Detection of phosphorylation using site-specific antiphosphotyrosine antibodies against pY1068, pY1148, or pY1173. EGFR was incubated in the buffer with 4 mM of DYQQD, ENQEYLR, or VPEYINQ for 5 min at 37°C, followed by immunoblotting with three antibodies which recognize phosphorylated Y1068, Y1148, or Y1173 specifically. The EGF-stimulated responses of EGFR without peptides at 5 min were taken as the control and considered to be 100%. *P<0.05, **P<0.01 versus EGF-stimulated tyrosine phosphorylation at 5-min incubation without peptides; n=4 for each lane.
Discussion
The Ras/MAPK signaling pathway plays a role in the growth of cancer cells. Therefore, a blocker of Ras/MAPK signaling may be a promising candidate for an anti-cancer agent. The inhibition of tyrosine autophosphorylation of EGFR is one of a few crucial points to block Ras/MAPK signaling. Two major classes of EGFR inhibitors have been developed (Baselga, 2004): (1) monoclonal antibodies, that bind to the extracellular domain of EGFR and prevent the binding of ligands and (2) molecules that competitively inhibit the binding of ATP to the intracellular kinase domain, leading to the inhibition of the kinase activity of the EGFR and the prevention of downstream signaling. Tyrphostin AG1478, which was employed presently for comparative purposes, in potency belongs to category 2, and is an ATP competitive inhibitor (Levitzki & Gazit, 1995). In this study, we aimed to inhibit tyrosine autophosphorylation by the direct binding of peptides as a pseudosubstrate to a catalytic region of EGFR. The designing of the synthetic peptides in Table 1 was based on this strategy.
Some of the peptides studied, especially those that include the major phosphorylation sites, Y1068, Y1148, or Y1173, suppressed tyrosine phosphorylation of EGFR effectively (Figure 1). These findings indicate that C-terminal autophosphorylation sites of EGFR may be an autoinhibitory region that masks the catalytic activity (Kemp & Pearson, 1991). VPEYINQ, containing the sequence surrounding Y1068, and DYQQD and KYQQK, containing the sequence surrounding Y1148, showed the most potent inhibitory effects among the peptides. VPEYINQ (4 mM) and DYQQD (4 mM) or KYQQK suppressed the autophosphorylation of EGFR to approximately 30 and 40%, respectively (Figure 1a and c). The peptides containing the sequence surrounding Y1173 (ENAEYLR and KNAEYLE) were less potent than those peptides, but had an unambiguous potency to inhibit autophosphorylation (Figure 1b). All these peptides were derived from the major phosphorylation sites of EGFR. In contrast, the peptides derived from a minor phosphorylation site at Y992 (Figure 1d) or an unrelated peptide LPFFD (Figure 1b) essentially had no effect on the autophosphorylation of EGFR, indicating that the inhibitory effects of oligopeptides highly depend on their amino-acid sequences.
As higher concentrations of the peptides did not dissolve in water, we tried the combined use of two and three kinds of peptides originating from Y1148, Y1173, and Y1068 in order to achieve a more potent suppression of tyrosine phosphorylation. The mixture of DYQQD and ENAEYLR suppressed autophosphorylation of EGFR much more strongly than did each single kind of peptide (Figure 2a and b). In contrast, no significant difference in the inhibitory effect was seen for the mixture of DYQQD and KYQQK, as compared with 4 or 8 mM DYQQD (Figure 2b). The inhibitory effect of the mixture of 4 mM DYQQD and 4 mM ENAEYLR appeared to be comparable to that of the EGFR-specific inhibitor, AG1478 (Figure 3). However, the IC50 of the mixture was still an order of magnitude inferior to that of AG1478. Thus, we further tried mixed peptides consisting of three different kinds of peptides, in origin. The resulting IC50 value of the mixture was 0.5 mM, which is comparable to that of AG1478 (0.3 mM). These enhanced inhibitory effects by the use of two or three different kinds of peptides suggest that DYQQD, ENAEYLR, and VPEYINQ interact with EGFR at different sites. This finding is interesting, since if it can be proved that each oligopeptide is inhibiting the autophosphorylation site from which it is derived, it is expected that we can selectively inhibit a specific signaling pathway emitted from a specific autophosphorylation site. Towards this end, we have performed Western blot analysis using a site-specific antiphosphotyrosine antibody against pY1068, pY1148, or pY1173 (Figure 8). As shown in Figure 8, however, DYQQD, ENAEYLR, and VPEYINQ equally suppressed phosphorylation among Y1068, Y1148, and Y1173. This finding suggests that all these peptides are interacting with the catalytic site of the kinase domain. Since there are additive effects of different peptides when combined, the peptide substrate- or pseudosubstrate-binding pocket appears to be loosely constructed, and can accommodate peptides arising from different autophosphorylation sites in EGFR, while it is tightly constructed such that it can reject unrelated peptides (LPFFD) or peptides related to IR (KIFMK, DIYET, and KIYEK).
The pentapeptides KIFMK, DIYET, and KIYEK, which suppressed the autophosphorylation of IR (Hirose et al., 2004), showed no effect on the autophosphorylation of EGFR (Figure 4). In contrast to these results, Shoelson et al. have shown that an 11-mer peptide ([Tyr]peptide), which includes a DIYET motif and corresponds to the amino-acid sequence of the activation loop of IR (RDIYETDYYRK1155–1165) suppressed both IR and EGFR autophosphorylation with apparent IC50's of 1.0 and 3.0 mM, respectively (Shoelson et al., 1989). They also reported that the [Tyr]peptide is phosphorylated by both IR and EGFR kinases. The [Tyr]peptide served as an exogenous peptide substrate for both IR and EGFR. Their results suggest that in order for an exogenous peptide to become a substrate for another kind of tyrosine kinase, the peptide should be composed of more than 10 amino-acid residues. In other words, it appears that a short peptide could act as a specific exogenous peptide substrate for the tyrosine kinase, from which its amino-acid sequence was dissected.
DYQQD was found to be a substrate for EGFR by mass spectrometric analysis. This finding means that it approached the catalytic site of EGFR and inhibited the intrinsic autophosphorylation of EGFR. According to Hubbard, the A-loop of IR undergoes drastic conformational change upon autophosphorylation of the major phosphorylation sites at Y1158, Y1162, and Y1163 (Hubbard, 1997). This result implies that phosphorylated tyrosine residues are repelled from the catalytic site of the kinase domain of IR. It might, likewise, be expected that DpYQQD would be repelled from the catalytic site of EGFR and that it would not suppress autophosphorylation. However, this was not indeed the case. As shown in Figure 1a, DpYQQD showed nearly an identical extent of suppression of phosphorylation to that achieved by DYQQD. According to Tice et al. (1999), EGFR's kinase activity and its ability to activate an adapter protein are retained even after the mutation of Y845, the only tyrosine residue in the EGFR's activation loop, to phenylalanine. This finding means that, in contrast to IR, phosphorylation of the tyrosine residue on the activation loop of EGFR (Y845) does not alter the catalytic activity of EGFR. Thus, it appears that DpYQQD was not necessarily repelled from the catalytic site of EGFR and, consequently, it could inhibit autophosphorylation as was observed.
Previously, we reported that lignocaine and the pentapeptides DIYET and KIYEK, but not KIFMK, dephosphorylate phosphotyrosine residues of IR (Hirose et al., 2004). Since the IYE motif is taken from the A-loop of IR, we likewise examined the effects of DYQQD and KYQQK on the phosphorylation states of pre-phosphorylated EGFR. However, no dephosphorylation phenomena were observed with these peptides (Figure 5). Lignocaine and the peptides DIYET and KIYEK also had no dephosphorylation effects on the pre-phosphorylated EGFR. These results may be attributed to the structural differences between EGFR and IR: (1) inactive EGFR is a monomer and ligand binding to EGFR leads to formation of a homo- or heterodimer of EGFR (Lemmon et al., 1997), whereas IR has already formed disulfide-linked heterotetramers (α2β2) (Kasuga et al., 1982); and (2) the major autophosphorylation sites of the IR cluster in the A-loop, while those of EGFR disperse in the C-terminal tail. It will be interesting to investigate the effects of DIYET, KIYEK, and lignocaine on pre-phosphorylated insulin-like growth factor receptor-1 receptor (IGF-1R). This is because IGF-1R shares an amino-acid sequence and three-dimensional structure with IR (Blakesley et al., 1996). On the other hand, reconstitution of EGFR in a lipid phase would also be another attractive approach to study the dephosphorylation action of peptides and lignocaine. This is because the transmembrane domain of EGFR is reported to play an important role in dimerization and auto- or transphosphorylation (Chantry, 1995). The accelerated or stabilized dimerization of EGFR in the lipid phase may facilitate dephosphorylation action of peptides or lignocaine.
Besides the AG1478 employed presently, a number of drugs targeting the kinase activity of EGFR are being developed (Ranson, 2004). Among them, ZD1839 (Iressa) and OSI-774 (Tarceva) have already been approved (http://www.oncolink.com). AG1478, ZD1839, and OSI-774 belong to quinazolines and are ATP competitive inhibitors (Levitzki & Gazit, 1995). In contrast, the small peptides presently under investigation are considered to be substrate competitive inhibitors. As emphasized by Levitzki (2003), substrate competitive inhibitors may be superior to the ATP competitive inhibitors in that they bind to domains at the kinase site that are less conserved than the ATP-binding site and are therefore less likely to bind many other targets. Although peptides generally have poor cell membrane permeability, this drawback can be overcome by linking the peptides to the human immunodeficiency virus type 1 (HIV-1) Tat-(48–57) sequence (GRKKRRQRRR), which rapidly translocates inside cells (Vives et al., 1997). Conjugation of the peptide inhibitor to fatty acids (myristate or stearate) is also a useful modification to enable the permeation of peptides into cells (Kelemen et al., 2002). In conclusion, the mixed peptides consisting of three major phosphorylation sites of EGFR (Y1068, Y1148, and Y1173) may be a promising seed for the development of therapeutic agents for breast and lung cancers.
Abbreviations
- ATP
adenosine triphosphate
- EGFR
epidermal growth factor receptor
- IR
insulin receptor
- PAGE
polyacrylamide gel electrophoresis
- SDS
sodium dodecyl sulfate
References
- BASELGA J. The science of EGFR inhibition: A roadmap to improved outcomes. Signal. 2004;5:4–8. [Google Scholar]
- BLAKESLEY V.A., SCRIMGEOUR A., ESPOSITO D., ROITH D.L. Signaling via the insulin-like growth factor-I receptor: Does it differ from insulin receptor signaling. Cytokine Growth Factor Rev. 1996;7:153–159. doi: 10.1016/1359-6101(96)00015-9. [DOI] [PubMed] [Google Scholar]
- CHANTRY A. The kinase domain and membrane localization determine intracellular interactions between epidermal growth factor receptors. J. Biol. Chem. 1995;270:3068–3073. [PubMed] [Google Scholar]
- DOWNWARD J., PARKER P., WATERFIELD M.D. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984;311:483–485. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- EAHOLTZ G., COLVIN A., LEONARD D., TAYLOR C., CATTERALL W.A. Block of brain sodium channels by peptide mimetics of the isoleucine, phenylalanine, and methionine (IFM) motif from the inactivation gate. J. Gen. Physiol. 1999;113:279–293. doi: 10.1085/jgp.113.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAHOLTZ G., SCHEUER T., CATTERALL W.A. Restoration of inactivation and block of open sodium channels by an inactivation gate peptide. Neuron. 1994;12:1041–1048. doi: 10.1016/0896-6273(94)90312-3. [DOI] [PubMed] [Google Scholar]
- HIROSE M., KURODA Y., SAWA S., NAKAGAWA T., HIRATA M., SAKAGUCHI M., TANAKA Y. Suppression of insulin signaling by a synthetic peptide KIFMK suggests the cytoplasmic linker between DIII-S6 and DIV-S1 as a local anaesthetic binding site on the sodium channel. Br. J. Pharmacol. 2004;142:222–228. doi: 10.1038/sj.bjp.0705575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIROSE M., MARTYN J.A.J., KURODA Y., MARUNAKA Y., TANAKA Y. Mechanism of suppression of insulin signalling with lignocaine. Br. J. Pharmacol. 2002;136:76–80. doi: 10.1038/sj.bjp.0704691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSUAN J.J., TOTTY N., WATERFIELD M.D. Identification of a novel autophosphorylation site (P4) on the epidermal growth factor receptor. Biochem. J. 1989;262:659–663. doi: 10.1042/bj2620659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD S.R. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997;16:5572–5581. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD S.R., MOHAMMADI M., SCHLESSINGER J. Autoregulatory mechanisms in protein-tyrosine kinases. J. Biol. Chem. 1998;273:11987–11990. doi: 10.1074/jbc.273.20.11987. [DOI] [PubMed] [Google Scholar]
- HUBBARD S.R., WEI L., ELLIS L., HENDRICKSON W.A. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature. 1994;372:746–754. doi: 10.1038/372746a0. [DOI] [PubMed] [Google Scholar]
- HUNTER T. The phosphorylation of proteins on tyrosine: its role in cell growth and disease. Philos. Trans. R. Soc. London B Biol. Sci. 1998;353:583–605. doi: 10.1098/rstb.1998.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORISSEN R.N., WALKER F., POULIOT N., GARRETT T.P.J., WARD C.W., BURGESS A.W. Epidermal growth factor receptor: mechanisms of activation and signaling. Exp. Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- KASUGA M., ZICK Y., BLITH D.L., KARLSSON F.A., HARING H.U., KAHN C.R. Insulin stimulation of phosphorylation of the β subunit of the insulin receptor. J. Biol. Chem. 1982;257:9891–9894. [PubMed] [Google Scholar]
- KELEMEN B.R., HSIAO K., GOUELI S.A. Selective in vitro inhibition of mitogen-activated protein kinase activation using cell-permeable peptides. J. Biol. Chem. 2002;277:8741–8748. doi: 10.1074/jbc.M108459200. [DOI] [PubMed] [Google Scholar]
- KEMP B.E., PEARSON R.B. Intrasteric regulation of protein kinases and phosphatases. Biochim. Biophys. Acta. 1991;1094:67–76. doi: 10.1016/0167-4889(91)90027-u. [DOI] [PubMed] [Google Scholar]
- KRAUS M.H., ISSING W., MIKI T., POPESCU N.C., AARONSON S.A. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc. Natl. Acad. Sci. U.S.A. 1989;86:9193–9197. doi: 10.1073/pnas.86.23.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURODA Y., MAEDA Y., MIYAMOTO K., TANAKA K., KANAORI K., OTAKA A., FUJII N., NAKAGAWA T. 1H-NMR and circular dichroism spectroscopic studies on changes in secondary structures of the sodium channel inactivation gate peptides as caused by the pentapeptide KIFMK. Biophys. J. 1999;77:1363–1373. doi: 10.1016/S0006-3495(99)76985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMMON M.A., BU Z., LADBURY J.E., ZHOU M., PINCHASI D., LAX I., ENGELMAN D.M., SCHLESSINGER J. Two EGF molecules contribute additively to stabilization of the EGFR dimer. EMBO J. 1997;16:281–294. doi: 10.1093/emboj/16.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVITZKI A. Protein kinase inhibitors as a therapeutic modality. Acc. Chem. Res. 2003;36:462–469. doi: 10.1021/ar0201207. [DOI] [PubMed] [Google Scholar]
- LEVITZKI A., GAZIT A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1787. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- LEVKOWITZ G., WATERMAN H., ETTENBERG S.A., KATZ M., TSYGANKOV A.Y., ALROY I., LAVI S., IWAI K., REISS Y., CIECHANOVER A., LIPKOWITZ S., YARDEN Y. Uniquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- MARGOLIS B.L., LAX I., KRIS R., DOMBALAGIAN M., HONEGGER A.M., HOWK R., GIVOL D., ULLRICH A., SCHLESSINGER J. All autophosphorylation sites of epidermal growth factor (EGF) receptor and HER2/neu are located in their carboxyl-terminal tails. J. Biol. Chem. 1989;264:10667–10671. [PubMed] [Google Scholar]
- MARMOR M.D., YARDEN Y.EGF receptor family Handbook of Cell Signaling 2003New York: Academic Press; 405–408.eds. Bradshaw, R.A. & Dennis, E.A. Vol. 1, pp [Google Scholar]
- OSHEROV N., GAZIT A., GILON C., LEVITZKI A. Selective inhibition of the epidermal growth factor and HER2/Neu receptors by tyrphostins. J. Biol. Chem. 1993;268:11134–11142. [PubMed] [Google Scholar]
- PLOWMAN G.D., CULOUSCOU J.M., WHITNEY G.S., GREEN J.M., CARLTON G.W., FOY L., NEUBAUER M.G., SHOYAB M. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc. Natl. Acad. Sci. U.S.A. 1993;90:1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANSON M. Epidermal growth factor receptor tyrosine kinase inhibitors. Br. J. Cancer. 2004;90:2250–2255. doi: 10.1038/sj.bjc.6601873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLESSINGER J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- SHOELSON S.E., WHITE M.F., KAHN C.R. Nonphosphorylatable substrate analogs selectively block autophosphorylation and activation of the insulin receptor, epidermal growth factor receptor, and pp60v-src kinases. J. Biol. Chem. 1989;264:7831–7836. [PubMed] [Google Scholar]
- TICE D.A., BISCARDI J.S., NICKLES A.L., PARSONS S.J. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUT T.W. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- VIVES E., BRODIN P., LEBLEU B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- WALTON G.M., CHEN W.S., ROSENFELD M.G., GILL G.N. Analysis of deletions of the carboxyl terminus of the epidermal growth factor receptor reveals self-phosphorylation at tyrosine 992 and enhances in vivo tyrosine phosphorylation of cell substrates. J. Biol. Chem. 1990;265:1750–1754. [PubMed] [Google Scholar]
- YAMAMOTO T., IKAWA S., AKIYAMA T., SEMBA K., NOMURA N., MIYAJIMA N., SAITO T., TOYOSHIMA K. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature. 1986;319:230–234. doi: 10.1038/319230a0. [DOI] [PubMed] [Google Scholar]