Abstract
Bisphosphonates are currently the most important class of antiresorptive drugs used for the treatment of diseases with excess bone resorption. On the basis of their molecular mechanism of action, bisphosphonates can be divided into two pharmacological classes; nitrogen-containing (N-BPs) and non-nitrogen-containing bisphosphonates (non-N-BP). Both classes induce apoptosis but they evoke it differently; N-BPs by inhibiting the intracellular mevalonate pathway and protein isoprenylation, and non-N-BPs via cytotoxic ATP analog-type metabolites. N-BPs are not metabolized to ATP analogs, but we report here that these bisphosphonates can induce formation of a novel ATP analog (ApppI) as a consequence of the inhibition of the mevalonate pathway in cells. We also investigated whether ApppI is involved in the apoptosis induced by N-BPs.
Mass spectrometry and NMR were used to identify ApppI in N-BP treated osteoclasts, macrophages and glioma cells. The potency of different bisphosphonates to promote ApppI production was tested in J774 macrophages. The effects of ApppI on ADP/ATP translocase in isolated mitochondria and its capability to induce apoptosis in osteoclasts were also studied.
ApppI production correlated well with the capacity of N-BPs to inhibit mevalonate pathway. ApppI inhibited the mitochondrial ADP/ATP translocase and caused apoptosis in osteoclasts.
In conclusion, these findings provide the basis for a new mechanism of action for N-BPs. Some of these very potent bisphosphonates, such as zoledronic acid, represent a third class of bisphosphonates that can act both via the inhibition of the mevalonate pathway and by the blockade of mitochondrial ADP/ATP translocase, which is known to be involved in the induction of apoptosis.
Keywords: Bisphosphonate, mevalonate pathway, FPP synthase, ATP analog, ANT, apoptosis
Introduction
Bisphosphonates (BPs) are a class of drugs developed for the treatment of bone diseases with a high bone turnover, such as Paget's disease (Roux & Dougados, 1999), tumor-associated osteolysis (Coleman, 2001) and postmenopausal osteoporosis (Delmas, 2002). Despite their widespread clinical use, the exact biochemical mechanism of action of bisphosphonates was poorly understood until recently. According to current knowledge, bisphosphonates can be divided into two pharmacological classes with distinct molecular mechanisms of action.
Bisphosphonates that contain a nitrogen function in their structure (nitrogen-containing bisphosphonates, N-BPs), such as zoledronic acid and risedronate, appear to inhibit at least one enzyme of the intracellular mevalonate pathway. The main target enzyme in the mevalonate pathway is currently considered to be farnesyl pyrophosphate synthase (FPP synthase). Inhibition of this enzyme prevents the modification of important signaling proteins with isoprenoid lipids, and the subsequent lack of prenylated proteins leads to a loss of osteoclast function, and consequently, indirect apoptotic cell death (Rogers, 2003).
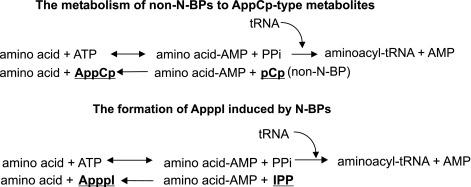
The less potent bisphosphonates that lack a nitrogen function (non-N-BPs), such as clodronate, do not inhibit the mevalonate pathway and protein isoprenylation, but are metabolized intracellularly to AppCp-type metabolites, which are cytotoxic analogs of ATP. These metabolites accumulate in the cell cytoplasm and directly evoke apoptosis by inhibiting the mitochondrial adenine nucleotide translocase (ANT) (Lehenkari et al., 2002). N-BPs are not metabolized to ATP analogs (Auriola et al., 1997; Benford et al., 1999).
In the present studies, however, we found, that although N-BPs themselves are not metabolized into AppCp-type metabolites, cells treated with N-BPs do synthetize a new type of ATP analog. The IUPAC name for this molecule is triphosphoric acid 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester, abbreviated to ‘ApppI'. The inhibition of FPP synthase leads to the accumulation of isopentenyl pyrophosphate (IPP), which then seems to be converted to ApppI via aminoacyl-tRNA-synthetases (Figure 1), the same enzyme that catalyzes the formation of AppCp-type metabolites from non-N-BPs (Rogers et al., 1996). However, ApppI does not contain BP in its structure as is the case for the AppCp-type metabolites of non-N-BPs. After identification of ApppI induced by N-BPs, we investigated whether ApppI inhibits the mitochondrial ANT and evokes apoptosis as the ATP analogs of non-N-BPs. Our results indicate that ApppI induced apoptosis by the same mechanism as the ATP analogs of non-N-BPs, that is, via inhibition of ANT. The data suggest that very potent N-BPs, such as zoledronic acid, represent a new class of bisphosphonates that evoke apoptosis both indirectly via the inhibition of protein isoprenylation and directly via inhibition of ANT by ApppI.
Figure 1.
Mevalonate pathway. The compounds (italicized) used in this study and the affected enzymes are indicated in the boxes. Biosynthesis of ApppI results from inhibition of FPP synthase by N-BPs and consequent accumulation of IPP.
Methods
Synthesis of ApppI
Triphosphoric acid 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester (ApppI): phosphoric acid mono-(3-methyl-but-3-enyl) ester disodium salt (500 mg, 2.4 mmol) was synthesized from 3-methyl-bute-3-en-1-ol and OPCl3 following a published procedure (Eggerer & Lynen, 1960), treated with 1 M HCl (5.5 ml) for 5 min, and this mixture was evaporated to dryness under high vacuum. The residue was dissolved in dry dimethylformamide (20 ml) and dry ADP (200 mg, 0.5 mmol, Sigma A-2754), tributylamine (220 mg, 1.1 mmol) and dicyclohexylcarbodiimide (500 mg, 2.4 mmol) were added under an argon atmosphere. After 48 h, the mixture was evaporated to dryness and the product was purified by anion exchanging chromatography in a SAX column using ammonium acetate as an eluent. 1H NMR (D2O): 8.53 (1H, s), 8.27 (1H, s), 6.14 (1H, d, J=5.8 Hz), 4.55 (1H, m), 4.39 (1H, m), 4.27–4.22 (3H, m), 4.00 (2H, m), 2.24 (2H, t, J=6.5 Hz), 1.64 (3H, s), C=CH2 signals under HDO-line at 4.8.
Liquid chromatography conditions for IPP, ApppI and AppCCl2p
On-line HPLC-ESI-MS measurements were carried out with a Rheos 4000 (Flux Instruments, Danderyd, Sweden), Ultimate (LC Packings, Netherlands) or Surveyor (Thermo Electron Corporation, San Jose, CA, U.S.A.) pump and a Rheodyne 7725 (Cotati, CA, U.S.A.), Famos (LC Packings) or Surveyor (Thermo Electron Corporation) autosampler with a 20–50 μl loop. The reversed phase column was a Genesis C18 (50 × 2 mm) (Jones Chromatography, Lakewood, CO, U.S.A.), which was eluted with a mobile phase at a flow-rate of 100–200 μl min−1. IPP, ApppI and AppCCl2p were separated by using an ion-pairing HPLC method as described by Mönkkönen et al. (2000). Briefly, dimethylhexylamine (DMHA) was used as the ion-pairing agent and methyleneadenosine 5′-triphosphate (AppCp) as internal standard. We obtained a good retention time and high signal intensity by using DMHA. The compounds were eluted from the column in 50–80% methanol and 2 mM DMHA, which is a favorable composition for the ionization process (Mönkkönen et al., 2000).
Tandem mass spectrometry
Negative ion mass spectra were acquired using an LCQ or LTQ quadrupole ion trap mass spectrometer equipped with an electrospray ionization (ESI) source (Thermo Electron Corporation, San Jose, CA, U.S.A.). The total eluent flow of 100–200 μl min−1 was directed to the ESI source. Other conditions have been described previously by Mönkkönen et al. (2000).
NMR spectroscopy
1H and 31P NMR spectra were recorded on a Bruker Avance DRX500 NMR spectrometer (Bruker, Rheinstetter, Germany) operating at 500.13 and 202.45 MHz, respectively. Samples were prepared in D2O using 21.5 mM TSP (Me3SiCD2CD2CO2Na) as an internal reference standard for 1H, and 85% H3PO4 as an external standard for 31P NMR chemical shifts.
Animals
Sprague–Dawley rats from the stock of the laboratory animal center of the University of Oulu were used and treated according to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
ApppI production in primary osteoclasts cultured on coated bone slices
Bone slices used in this study were prepared from frozen bovine cortical bone shafts. Bone was cut with a diamond saw into 200 μm thick slices, sterilized with sonication and given a brief rinse in 70% ethanol. Before culture, bovine bone slices were coated with N-BP by rinsing with 2 mM zoledronic acid for 1 min. Control bone slices were rinsed with 0.9% saline. Hereafter, the bovine slices were transferred into 24-well plates containing fresh 37°C medium. Osteoclasts from the long bones (femur, tibia and humerus) of 1- to 2-day old newborn rats were isolated after decapitation. Procedures for osteoclast culture have been described previously in detail by Boyde et al. (1984) and Chambers et al. (1984). Briefly, osteoclasts were scraped with a scalpel blade from the endosteal surface of the split long bones into α-MEM, centrifuged at 1000 r.p.m. for 10 min and final medium added at 50 μl per bone slice. Cells were allowed to attach at 37°C in 5% CO2 and 95% air for 30 min and non-attached cells were washed with PBS. Attached cells were cultured in 24-well plates in α-MEM buffered with 20 mM HEPES, 2 mM L-glutamine, penicillin (100 IU ml−1), streptomycin (100 μg ml−1) and 10% heat-inactivated fetal calf serum in the culture atmosphere mentioned above.
After 24 h, the cultured cells were scraped off the bone slices and washed in ice-cold PBS. Extracts from cells were prepared by using ice-cold acetonitrile as previously described (Mönkkönen et al., 2000). The molar amount of ApppI was determined in cell extracts by HPLC negative ion electrospray ionization mass spectrometry (HPLC-ESI-MS) (Mönkkönen et al., 2000).
ApppI production in J774 macrophages and C6 glioma cells
J774 macrophages were obtained from the European Collection of Cell Cultures (Salisbury, U.K.) and C6 glioma cells were from the American Type Culture Collection (Manassas, VA). J774 cells were cultured at 37°C in Dulbecco's modified Eagle's medium (Sigma, St Louis, MO, U.S.A.), supplemented with 10% of fetal bovine serum (Gibco, Grand Island, NY, U.S.A.), 100 IU ml−1 of penicillin and streptomycin (Gibco) and 1 mM L-glutamine (Gibco) in a 7% CO2 atmosphere. C6 cells were cultured at 37°C in Ham's F12K medium (Gibco), supplemented with 2.5% of fetal bovine serum (Gibco), 15% of horse serum (Gibco), 100 IU ml−1 of penicillin and streptomycin (Gibco) in a 7% CO2 atmosphere. J774 cells were harvested by scraping, and C6 cells by using trypsin (Gibco) and EDTA. Plastics were supplied by Nunc (Roskilde, Denmark).
For studies on ApppI production in J774 macrophages, the cells were seeded into six-well plates (Nunc, Roskilde, Denmark) at a density of 3 × 106 cells per well and left to adhere for 4 h, then treated with different free BPs for 24 h. After treatment, the cultured cells were scraped off the wells and washed in ice-cold PBS. Extracts from cells were prepared and the molar amount of ApppI was determined in cell extracts as described above.
For obtaining an NMR spectrum of biological ApppI, a large amount of C6 glioma cells was exposed to 100 μM risedronate for 24 h. The cells were seeded into three 75 cm2 flasks at a density of 1.5 × 107 cells per flask and incubated for 3 days (to confluence) before treatment. Extracts from cells were prepared as described above. Extracts from three separate flasks were combined to establish a single sample. The 1H NMR spectrum of ApppI was accumulated after purification of the sample by anion exchanging chromatography in a SAX column using ammonium acetate as an eluent.
ADP/ATP translocase
Rat liver mitochondria were isolated by standard differential centrifugation and suspended in 0.25 M sucrose, 0.5 mM EDTA and 5 mM Tris, pH 7.4. The ADP/ATP translocator activity was assayed in the ‘forward' direction as described by Paulson & Shug (1984). The reaction took place at 0°C in 100 mM KCl, 40 mM Tris and 1 mM EDTA, pH 7.4, was initiated by [8-14C]ATP and stopped after 10, 20, 30 or 60 s with 0–100 μM ApppI treatment by addition of 50 μM atractyloside. The mitochondria were then collected and washed in the same medium in the presence of 50 μM atractyloside, and solubilized in 0.2% sodium dodecylsulphate before radioactivity was measured by liquid scintillation counting.
Liposome encapsulation of compounds for osteoclast apoptosis assay
Stock solutions of 7 mM zoledronic acid, 64 mM clodronate, 6.2 mM ApppI and 7 mM AppCCl2p for liposome-encapsulation were prepared by dissolving the substances in MES-HEPES buffer (pH 7.2), with the osmotic pressure adjusted to 300 mOsm l−1 by NaCl (isotonic solution). We have found that negatively charged liposomes are more efficient for cellular drug delivery than neutral liposomes (Mönkkönen et al., 1994b), and thus, the negatively charged phospholipid DSPG was chosen for the liposome preparation. The molar ratio of phospholipid and cholesterol was 2 : 1. The compounds were encapsulated in liposomes by reverse-phase evaporation, as previously described (Mönkkönen et al., 1994a). The concentrations of zoledronic acid, clodronate, ApppI and AppCCl2p were measured spectrophotometrically, the lipid content of the liposomes was determined by phosphorus assay (Mönkkönen et al., 1994a), and the size distribution of liposomes was analyzed by the Nicomp Zeta Potential/Particle Sizer (model 380 XLS, NicompTM, Santa Barbara, CA, U.S.A.). The concentrations of liposome-encapsulated compounds were 0.56 mM (zoledronic acid), 2.79 mM (clodronate), 0.48 mM (ApppI) and 0.36 mM (AppCCl2p). The molar drug : phospholipid ratio was under 1.26 and a mean diameter of liposomes under 200 nm. Nonloaded liposomes were used as the control. Liposomes were diluted to MES-HEPES-NaCl buffer before treatment. The liposomes were used only for the osteoclasts apoptosis assay; all other experiments in this paper were carried out by using free compounds.
Osteoclast apoptosis assay
Osteoclasts were cultured similarly for apoptosis assay as in the studies of ApppI production described above, except that the attached osteoclasts were not treated with a bone-bound drug but with different concentrations (0.1, 1.0 μM) of liposome-encapsulated zoledronic acid, ApppI, AppCCl2p or (1.0, 10.0 μM) clodronate. After 24 h treatment, the cells were fixed with 3% paraformaldehyde and 2% sucrose for 5 min, and stained for tartrate resistant acid phosphatase (TRAP) using a histochemical kit (Sigma-Aldrich). TRAP positive giant cells with three or more nuclei were counted as osteoclasts. In order to define the number of apoptotic osteoclasts, the nuclei were stained with Hoechst 33258. Osteoclasts were marked as apoptotic if they showed strong staining for TRAP, a hallmark of cytoplasmic contraction, chromatin condensation and nuclear fragmentation, as previously described (Hughes et al., 1995). The amount of apoptotic cells was calculated as a percentage of all osteoclasts from six wells. This was an observer-blinded study.
Drugs and chemicals
Zoledronic acid (2-(imidazol-1-yl)-hydroxy-ethylidene-1,1-bisphosphonic acid, disodium salt, 4.75 hydrate) was kindly provided by Novartis Pharma (Basel, Switzerland), and clodronate (dichloromethylene-1,1-bisphosphonate) by Leiras Pharmaceutical Co. (Turku, Finland). [8-14C]adenosine triphosphate (NH4 salt) was obtained from Amersham Biosciences U.K. Ltd. (Little Chalfont, Buckinghamshire, U.K.). Lovastatin was purchased from Sigma (St Louis, MO, U.S.A.), FTI-227 and GGTI-298 were from Calbiochem (Darmstadt, Germany). Clodronate metabolite (AppCCl2p) was kindly provided by Professor A. Azhayev (University of Kuopio, Finland). ApppI was synthesized in our laboratory as described above.
Statistical analysis
The apoptosis data were tested by using the Mann–Whitney U-test with Bonferroni correction.
Results
Identification of ApppI
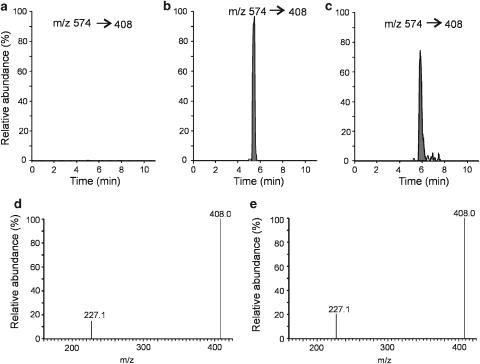
In this study, osteoclasts adhered to zoledronic acid-exposed bone were found to synthesize ApppI. The formation of ApppI was ascertained by using high-performance liquid chromatography-tandem mass spectrometry (HPLC/MS/MS) by monitoring the daughter fragments in the selective reaction monitoring (SRM) mode. Typical SRM chromatograms of untreated osteoclast extract, osteoclasts treated with zoledronic acid (N-BP) and ApppI standard are shown in Figure 2. It is notable that zoledronic acid was introduced to osteoclasts ‘indirectly', that is, in bone-bound form. This form of delivery suggests that the ApppI that was found is indeed synthesized in bone adhered osteoclasts, as the other cells present in the culture are not capable of dissolving and cannot endocytose the bone-bound reagent (Salo et al., 1997). The major component from the treated cells (Figure 2b) was not observed in the chromatogram of the untreated cell extract (Figure 2a), and the paucity of peaks demonstrates the high selectivity of the SRM method used for detection. The major peak in the chromatogram from zoledronic acid-treated cells was similar to that of the ApppI standard (Figure 2b and c). Also the MS/MS spectra of major peaks were identical, where the most intense fragment ions at m/z 227 and 408 are shown to confirm the existence of ApppI (Figure 2d and e). The full scan negative ion mass spectrum of ApppI shows a deprotonated molecular ion at m/z 574 (M-H)− as the base peak (Figure 3a). The corresponding sodium adduct ion (M-2H+Na)− can be seen at m/z 596 (Figure 3a). The MS/MS spectrum of ApppI is based on major fragment ions at m/z 227 and 408 (Figure 3b). The highest signal strength was obtained by using the fragment ion at m/z 408, which was used for the quantitation of ApppI.
Figure 2.
Identification of ApppI as an endogenous metabolic product in osteoclasts treated with zoledronic acid. Before to culture, bovine bone slices were coated with N-BP by rinsing with 2 mM free zoledronic acid for 1 min. Control bone slices were rinsed with 0.9% saline. Selective reaction monitoring chromatograms of the extract from untreated cells (a), extract from osteoclasts treated with zoledronic acid for 24 h (b), untreated cell extract spiked with 0.2 μM ApppI (c), MS/MS spectrum from the peak of Figure 2b (d), MS/MS spectrum from the peak of Figure 2c (e). The chromatograms are drawn on the same scale.
Figure 3.
Mass spectrometric identification of ApppI. Full scan negative-ion ESI mass spectrum (a) and MS/MS spectrum of ApppI (b).
In addition to mass spectrometry, the formation of ApppI was also ascertained by using nuclear magnetic resonance (NMR). Typically, a good 1H NMR spectrum is obtained from a sample containing at least 300 μg of pure compound. C6 glioma cells were chosen for this purpose because these grow well and produce a sufficient amount of ApppI for the NMR detection. Osteoclasts were inappropriate for NMR detection of ApppI because of a difficulty in isolating an adequate number of cells. 1H NMR (D2O) spectrum of ApppI from C6 glioma cells after treatment with 100 μM risedronate for 24 h showed the following typical chemical shifts and coupling constants: 8.53 (1H, s), 8.27 (1H, s), 6.13 (1H, d, J=5.8 Hz), 4.55 (1H, m), 4.39 (1H, m), 4.27–4.23 (3H, m), 3.99 (2H, m), 2.22 (2H, t, J=7.4 Hz), 1.58 (3H, s), C=CH2 signals under HDO-line at 4.8. The chemical shifts of biological ApppI were similar to that of the synthetic one as described above.
ApppI production in J774 macrophages
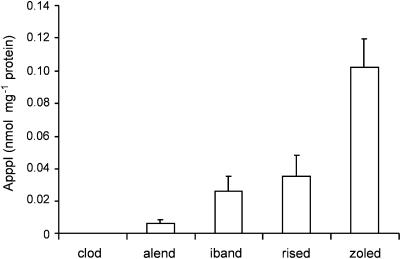
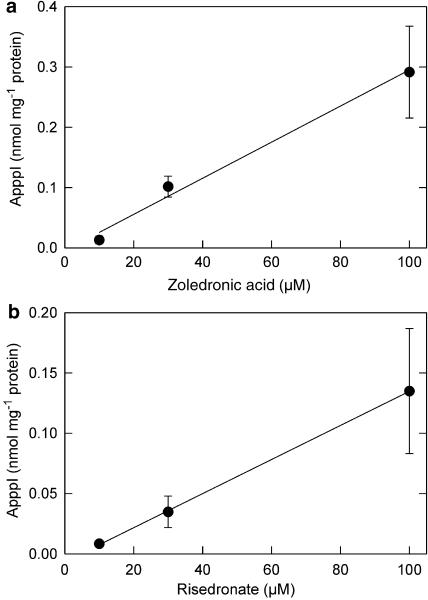
According to Dunford et al. (2001), the N-BPs inhibit FPP synthase in the following order of potency: zoledronic acid>risedronate>ibandronate>alendronate. Clodronate (non-N-BP) does not have any significant inhibitory effect on FPP synthase activity (Dunford et al., 2001). To determine the potency of BPs in promoting the production of ApppI, J774 macrophages were treated with various BPs for 24 h. Macrophages, instead of osteoclasts, were selected as a model for this study, because the macrophage assay is readily optimized for detailed analysis of bisphosphonate metabolism, as shown earlier (Mönkkönen et al., 2001). After the drug treatment, the ApppI contents from cell lysates were measured by using HPLC-ESI-MS. This experiment indicated that ApppI production correlated well with the capacity of N-BPs to inhibit FPP synthase. Clodronate did not induce ApppI production (Figure 4). Moreover, ApppI production was induced by zoledronic acid (Figure 5a) and risedronate (Figure 5b) in a dose dependent manner.
Figure 4.
The efficiency of ApppI production with different bisphosphonates. J774 macrophage cells were treated with 30 μM free bisphosphonate for 24 h. The molar amount of ApppI was determined in cell extracts by using HPLC-ESI-MS as described under Methods. clod=clodronate; alend=alendronate; iband=ibandronate; rised=risedronate; zoled=zoledronic acid. The columns and error bars represent means±s.d., n=6.
Figure 5.
Zoledronic acid and risedronate dependence of ApppI production. J774 macrophage cells were treated with 10–100 μM free zoledronic acid (a) or risedronate (b) for 24 h. ApppI analysis was performed as in Figure 4. The values represent means±s.d., n=4–6. R=0.94 for zoledronic acid and R=0.89 for risedronate.
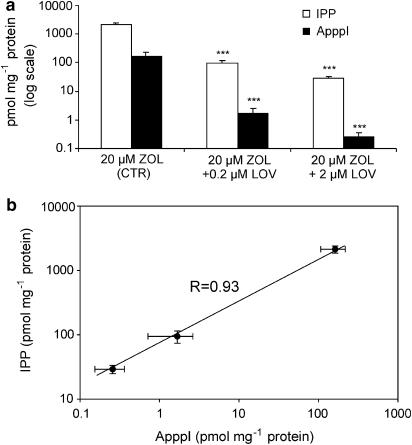
The question whether ApppI production is a result of specific inhibition of FPP synthase by N-BPs was addressed by studying whether other inhibitors of the mevalonate pathway, such as lovastatin (for HMG-CoA reductase), FTI-227 (for farnesyl transferase), and GGTI-298 (for geranylgeranyl transferase I), could induce production of ApppI in J774 macrophages. These inhibitors did not cause any ApppI production in J774 cells (data not shown). Gober et al. (2003) recently reported that N-BPs induce IPP accumulation in tumor cells via the inhibition of FPP synthase. This finding raised a further question, that is, is there any link between IPP accumulation and ApppI production induced by N-BPs? To answer this question, we cotreated J774 cells with lovastatin (an inhibitor of HMG-CoA reductase) and zoledronic acid. At 0.2–2 μM concentrations, lovastatin inhibited the IPP accumulation and the ApppI production (P<0.001) in a dose dependent manner after cotreatment with 20 μM zoledronic acid (Figure 6a). Furthermore, ApppI production correlated well with the intracellular concentration of IPP (R=0.93) (Figure 6b).
Figure 6.
The effect of lovastatin on the accumulation of IPP and the production of ApppI induced by free zoledronic acid after 24 h treatment in J774 macrophages (a), IPP accumulation correlates with the ApppI production (b). The molar amounts of IPP and ApppI were determined in cell extracts by using HPLC-ESI-MS as described under Methods. Mean±s.d., n=7–9. ***P<0.001 compared with controls.
The formation of AppCp-type metabolites of non-N-BPs (such as clodronate) is catalyzed by aminoacyl-tRNA synthetases. As non-N-BPs with short side chains resemble pyrophosphate (PPi) in structure, the reverse reaction can occur with a non-N-BP in place of PPi, leading to the formation of an analog of ATP (AppCp) containing the bisphosphonate (Rogers et al., 1996). Since IPP also resembles PPi in structure, we hypothesized that the N-BP-induced ApppI production could be catalyzed by aminoacyl-tRNA synthetases, similarly to the metabolism of non-N-BPs to AppCp-metabolite. If this was the case, then one could predict that IPP and non-N-BP would compete for the binding site for PPi in this reverse reaction (Figure 7). To clarify the influence of ApppI production on the metabolism of clodronate and vice versa, J774 macrophages were coexposed to various concentrations of clodronate and risedronate. At 10–100 μM concentrations, clodronate did not affect the risedronate induced ApppI production, but 300 μM clodronate decreased the ApppI production induced by risedronate (P<0.05) (Figure 8a). Conversely, 30–100 μM risedronate decreased the metabolism of clodronate to AppCCl2p-metabolite (P<0.05) after cotreatment of the J774 macrophages (Figure 8b).
Figure 7.
The metabolism of non-N-BP to AppCp-metabolite and the formation of ApppI induced by N-BPs is catalyzed by aminoacyl-tRNA synthetases.
Figure 8.
Effect of clodronate on the ApppI production evoked by risedronate (a), and risedronate on the metabolism of clodronate to AppCCl2p (b) in J774 macrophages. BPs were in free form. The molar amounts of ApppI and AppCCl2p were determined in cell extracts by using HPLC-ESI-MS as described under Methods. Mean±s.d., n=4–6. *P<0.05 compared with controls.
ApppI inhibits mitochondrial adenine nucleotide translocase (ANT)
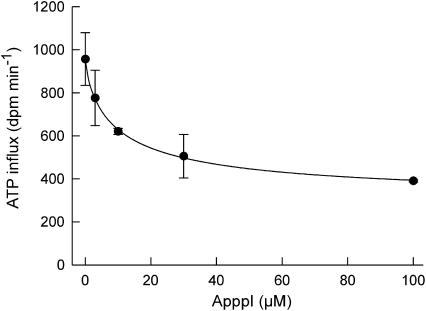
To further analyze the molecular mechanisms of action of ApppI, we next studied its effect in the isolated rat liver mitochondria. It has been previously shown that clodronate metabolite (AppCCl2p) inhibits mitochondrial ADP/ATP translocase (Lehenkari et al., 2002). To determine whether ApppI could modify the action of ANT, isolated rat liver mitochondria were treated with 0–100 μM ApppI for 10–60 s. The dose-dependent inhibition of ADP/ATP translocation in rat liver mitochondria after treatment with ApppI is depicted in Figure 9. The inhibition occurred rapidly and was detectable within 10 s (data not shown). Zoledronic acid at 100 μM concentration did not have an effect on ADP/ATP translocase (n=2, data not shown), indicating that N-BPs themselves do not affect it.
Figure 9.
ApppI dose dependently inhibits ADP/ATP translocation in rat liver mitochondria. Reaction time 60 s and 14C-ATP concentration 150 μM. The ADP/ATP translocator activity was assayed in the ‘forward' direction as described by Paulson & Shug (1984). The symbols and error bars represent means±s.d., n=2.
ApppI causes apoptosis
It has been demonstrated earlier that clodronate-mediated apoptosis is caused by intracellular accumulation of the clodronate ATP analog, AppCCl2p (Frith et al., 2001; Lehenkari et al., 2002), which inhibits mitochondrial adenine nucleotide translocase (ANT) (Lehenkari et al., 2002). As ApppI also inhibits ANT translocation in the mitochondria (Figure 9), we hypothesized that it should also cause apoptosis in a similar manner to AppCCl2p. To investigate this possibility, the apoptotic effects of zoledronic acid, clodronate, synthesized ApppI, or synthesized AppCCl2p were examined in isolated rat osteoclasts. Liposome-encapsulated delivery was purposefully selected to ensure controlled and similar transportation of the compound into the target cells. Additionally, liposome encapsulation is known to slow down the degradation of ApppI and AppCCl2p, and to enhance and control the cellular uptake of these compounds (Mönkkönen et al., 1994b; Frith et al., 2001). Compared with control cultures, zoledronic acid and clodronate, ApppI and AppCCl2p significantly induced apoptosis (P<0.001) in isolated osteoclasts cultured on bone slices after 24 h treatment (Figure 10). The present data demonstrate that ApppI, similarly to AppCCl2p, can induce apoptosis, probably by inhibiting mitochondrial ADP/ATP translocase.
Figure 10.
Induction of apoptosis in osteoclasts by zoledronic acid (ZOL), ApppI, clodronate (CLOD) or AppCCl2p. The cells were cultured on bone slices for 24 h in the presence of nontreated liposomes (CTR), 0.1 μM to 1 μM liposome-encapsulated ZOL, ApppI, AppCCl2p or 1 μM to 10 μM liposome-encapsulated CLOD. The number of apoptotic osteoclasts was determined as described under Methods. Mean±s.e.m., n=6. ***P<0.001 compared with controls.
Discussion
FPP synthase is the major pharmacologic target of N-BPs. Inhibition of this enzyme in osteoclasts prevents the biosynthesis of isoprenoid lipids (FPP and GGPP), which are essential for the post-translational farnesylation and geranylgeranylation of small GTPase signaling proteins. Loss of bone-resorptive activity and osteoclast apoptosis primarily result from the lack of these geranylgeranylated small GTPases (Rogers, 2003). Furthermore, there is a clear correlation between the ability of N-BPs to inhibit FPP synthase and protein prenylation in vitro and their abilities to prevent bone resorption in vivo (Dunford et al., 2001).
In this study we show that N-BPs can induce the production of a novel intracellular ATP analog (ApppI) in mammalian cells, including osteoclasts. Other inhibitors of the mevalonate pathway, such as lovastatin, FTI-227, and GGTI-298, did not evoke ApppI production in J774 macrophage cells. The order of potency of N-BPs to induce ApppI production was alendronate<ibandronate<risedronate<zoledronic acid (Figure 4), which is identical to their order of inhibitor potency against FPP synthase (Dunford et al., 2001). Thus, ApppI production correlates with the capacity of N-BPs to inhibit FPP synthase. In our study, the HMG-CoA reductase inhibitor, lovastatin, decreased the IPP accumulation and the ApppI production induced by zoledronic acid (P<0.001) (Figure 6a). In addition, ApppI production correlated well with the increase in the IPP concentration in J774 cells (R=0.93) (Figure 6b). Taken together, these data strongly suggest that ApppI production is a unique effect of N-BPs and results from inhibition of FPP synthase in the mevalonate pathway and the subsequent accumulation of IPP (Figure 1). Further, our data show that clodronate decreased the ApppI production (Figure 8a), and conversely, risedronate decreased the metabolism of clodronate to the AppCCl2p-metabolite after their cotreatment in J774 macrophages (Figure 8b). This finding suggests that aminoacyl-tRNA-synthetases, which catalyze the formation of AppCp-type metabolites from non-N-BPs (Rogers et al., 1996), also appear to be involved in the ApppI production induced by N-BPs. ApppI does not contain BP in its structure, and in that way differs from the AppCp-type metabolites of non-N-BPs. Instead of BP, ApppI contains IPP in its structure (Figure 7). Potentially, N-BPs may also induce the accumulation of dimethylallyl pyrophosphate (DMAPP) in the mevalonate pathway, and thus lead to production also of the ATP analog containing DMAPP. Further studies are in progress to address this question.
Mitochondria are potent integrators and coordinators of apoptosis. Dissipation of mitochondrial membrane potential, an increase in mitochondrial Ca2+ levels, extensive oxidation of mitochondrial NADPH, a decrease in cellular ATP levels and a burst of reactive oxygen species precede opening of the permeability transition pore (PT), leading to release of cytochrome c and other apoptogenic proteins (Kroemer & Reed, 2000; Mayer & Oberbauer, 2003). The main components of the PT pore are VDAC and ANT, which are adjacent in the outer mitochondrial membrane and inner mitochondrial membrane, respectively (Mayer & Oberbauer, 2003). Inhibitors of ANT are known to influence mitochondrial permeability transition and apoptosis, and these inhibitors can be divided in two classes, PT pore-closing (as bongkrekic acid) and PT pore-opening (as atractyloside) (Zamzami et al., 1996; Chavez et al., 1999). On the other hand, Kokoszka et al. (2004) using cells and mitochondria from livers of ANT knockout mice have reported that ANT is not an essential structural component of the PT pore, although ANT does contribute to its regulation. However, in that study, ANT was only indirectly estimated so that presence of recidual ANT activity could not be ruled out. Thus, the study does not unequivocally prove that ANT is not involved in PT pore function, as recently pointed out by Halestrap (2004). The present experiments on the exchange of radiolabeled ATP in isolated mitochondria (Figure 9) confirmed that ANT is a target of ApppI. N-BPs themselves did not have any effect on ADP/ATP translocase. It has been reported that anoxic damage of the mitochondria can be prevented by ATP, ADP and nonhydrolyzable AppCp, which is translocated by ANT (Watanabe et al., 1985). Also, a nonhydrolyzable metabolite of non-N-BP (clodronate), AppCCl2p, can inhibit ANT (Lehenkari et al., 2002). Thus, the proapoptotic effects of ApppI seem to be similar to those of other ANT inhibitors, such as AppCCl2p (Lehenkari et al., 2002). This was further confirmed in the experiments where the apoptotic effects of zoledronic acid, ApppI, clodronate, or AppCCl2p were examined in isolated rat osteoclasts. All of the tested compounds were able to induce apoptosis in osteoclasts (Figure 10). It is notable that the concentrations of ApppI used in the ANT (0–100 μM) and apoptosis assay (0.1 and 1 μM) (Figures 9 and 10) cannot be directly compared. ANT assay was carried out by using free ApppI in cell free system and osteoclast apoptosis assay by using liposome-encapsulated ApppI. Cellular uptake of liposome occurs by endocytosis and osteoclasts as highly endocytic cells avidly take up liposomal compounds. Endocytosis is an active mechanism of cellular uptake, which allows higher intracellular concentrations of compound to be achieved compared to extracellular medium. For example, after 24 h exposure to 30 μM liposome-encapsulated clodronate in macrophages, the intracellular concentration of clodronate was about 4 mM (Mönkkönen et al., 2001). Therefore, the intracellular concentration of ApppI in osteoclasts would be much higher than the extracellular concentrations in liposomal treatments (0.1 and 1 μM).
The role of the ANT inhibition as the mechanisms of action of BPs is dependent on the intracellular concentration of ApppI or AppCCl2p. We have previously shown that the cytoplasmic concentration of AppCCl2p can reach 1000 μM in clodronate-treated macrophages. The intracellular concentration of AppCCl2p was estimated assuming a cellular volume of 0.408 mm3 per million cells (Mönkkönen et al., 2001). Similar calculation indicates that cytoplasmic ApppI can reach about 130 μM concentration in the N-BP-treated J774 macrophages. Concentration of 30 μM ApppI approximately halved ANT activity (47% inhibition) in isolated mitochondria (Figure 9). Therefore, ApppI should reach intracellular concentration high enough to inhibit ANT activity in cells. Further, osteoclasts can become exposed to the very high concentrations of BPs in vivo, as has been shown for alendronate (N-BP), which reaches about 1 mM concentration in the resorption space beneath an osteoclast (Sato et al., 1991). The high concentration of BP achieved in the resorption lacunae and the high endocytic capacity of osteoclasts make it likely that osteoclasts are also able to produce high concentrations of ApppI in vivo. The cellular uptake of N-BP and the activity of the mevalonate pathway are probably the critical points for ApppI production and thus for the inhibition of ANT. More detailed studies on the kinetics of ApppI formation, and its relation to ANT inhibition and apoptosis are in progress.
In conclusion, we have shown that, in addition to preventing protein prenylation, N-BP-induced inhibition of FPP synthase leads to formation of a novel endogenous ATP analog (ApppI), and this compound inhibits mitochondrial ANT, which then evokes apoptosis. This finding provides a new plausible mechanism of action for N-BPs, a finding that may also account for part of the anti-tumor effects of N-BPs (Green, 2003; Neville-Webbe et al., 2005). Further, this finding also introduces a new interesting metabolic concept; as far as we are aware, there are no other drugs that are able to induce the synthesis of new molecules which are neither metabolites of the drug nor endogenous molecules naturally occurring in cells.
Acknowledgments
This work was supported by the Academy of Finland, Finnish Cultural Foundation of Northern Savo, and Research and Science Foundation of Farmos. We thank Dr Jonathan Green (Novartis Pharma, Switzerland) and Dr Paavo Honkakoski (University of Kuopio, Finland) for valuable comments on the manuscript.
Abbreviations
- ANT
adenine nucleotide translocase
- AppCCl2p
adenosine 5′(β,γ-dichloromethylene) triphosphate
- ApppI
triphosphoric acid 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester
- BP
bisphosphonate
- ESI
electrospray ionization
- FPP
farnesyl pyrophosphate
- GGPP
geranylgeranyl pyrophosphate
- IPP
isopentenyl pyrophosphate
- MS
mass spectrometry
- NMR
nuclear magnetic resonance
- VDAC
voltage-dependent anion channel
References
- AURIOLA S., FRITH J.C., ROGERS M.J., KOIVUNIEMI A., MÖNKKÖNEN J. Identification of adenine nucleotide-containing metabolites of bisphosphonate drugs using ion-pair liquid chromatography-electrospray mass spectrometry. J. Chromatogr. B. 1997;704:187–195. doi: 10.1016/s0378-4347(97)00490-8. [DOI] [PubMed] [Google Scholar]
- BENFORD H.L., FRITH J.C., AURIOLA S., MÖNKKÖNEN J., ROGERS M.J. Farnesol and geranylgeraniol prevent activation of caspases by aminobisphosphonates: biochemical evidence for two distinct pharmacological classes of bisphosphonate drugs. Mol. Pharmacol. 1999;56:131–140. doi: 10.1124/mol.56.1.131. [DOI] [PubMed] [Google Scholar]
- BOYDE A., ALI N.N., JONES S.J. Resorption of dentine by isolated osteoclasts in vitro. Br. Dent. J. 1984;156:216–220. doi: 10.1038/sj.bdj.4805313. [DOI] [PubMed] [Google Scholar]
- CHAMBERS T.J., THOMSON B.M., FULLER K. Effect of substrate composition on bone resorption by rabbit osteoclasts. J. Cell Sci. 1984;70:61–71. doi: 10.1242/jcs.70.1.61. [DOI] [PubMed] [Google Scholar]
- CHAVEZ E., ZAZUETA C., GARCIA N. Carboxyatractyloside increases the effect of oleate on mitochondrial permeability transition. FEBS Lett. 1999;445:189–191. doi: 10.1016/s0014-5793(99)00128-3. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- DELMAS P.D. Treatment of postmenopausal osteoporosis. Lancet. 2002;359:2018–2026. doi: 10.1016/S0140-6736(02)08827-X. [DOI] [PubMed] [Google Scholar]
- DUNFORD J.E., THOMPSON K., COXON F.P., LUCKMAN S.P., HAHN F.M., POULTER C.D., EBETINO F.H., ROGERS M.J. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J. Pharmacol. Exp. Ther. 2001;296:235–242. [PubMed] [Google Scholar]
- EGGERER H., LYNEN F. Biosynthesis of terpenes. VIII. Synthesis of 3-methyl-buten-3-yl 1-pyrophosphate. Ann. Chem. 1960;630:58–70. [Google Scholar]
- FRITH J.C., MÖNKKÖNEN J., AURIOLA S., MÖNKKÖNEN H., ROGERS M.J. The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum. 2001;44:2201–2210. doi: 10.1002/1529-0131(200109)44:9<2201::aid-art374>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- GOBER H.J., KISTOWSKA M., ANGMAN L., JENO P., MORI L., DE LIBERO G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN J.R. Antitumor effects of bisphosphonates. Cancer. 2003;97:840–847. doi: 10.1002/cncr.11128. [DOI] [PubMed] [Google Scholar]
- HALESTRAP A.P. Mitochondrial permeability: dual role for the ADP/ATP translocator. Nature. 2004;430:984. doi: 10.1038/nature02816. [DOI] [PubMed] [Google Scholar]
- HUGHES D.E., WRIGHT K.R., UY H.L., SASAKI A., YONEDA T., ROODMAN G.D., MUNDY G.R., BOYCE B.F. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Miner. Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- KOKOSZKA J.E., WAYMIRE K.G., LEVY S.E., SLIGH J.E., CAI J., JONES D.P., MACGREGOR G.R., WALLACE D.C. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROEMER G., REED J.C. Mitochondrial control of cell death. Nat. Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- LEHENKARI P.P., KELLINSALMI M., NÄPANKANGAS J.P., YLITALO K.V., MÖNKKÖNEN J., ROGERS M.J., AZHAYEV A., VÄÄNÄNEN H.K., HASSINEN I.E. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol. Pharmacol. 2002;61:1255–1262. doi: 10.1124/mol.61.5.1255. [DOI] [PubMed] [Google Scholar]
- MAYER B., OBERBAUER R. Mitochondrial regulation of apoptosis. News Physiol. Sci. 2003;18:89–94. doi: 10.1152/nips.01433.2002. [DOI] [PubMed] [Google Scholar]
- MÖNKKÖNEN H., MOILANEN P., MÖNKKÖNEN J., FRITH J.C., ROGERS M.J., AURIOLA S. Analysis of an adenine nucleotide-containing metabolite of clodronate using ion pair high-performance liquid chromatography electrospray ionization mass spectrometry. J. Chromatogr. B. 2000;738:395–403. doi: 10.1016/s0378-4347(99)00559-9. [DOI] [PubMed] [Google Scholar]
- MÖNKKÖNEN H., ROGERS M.J., MAKKONEN N., NIVA S., AURIOLA A., MÖNKKÖNEN J. The cellular uptake and metabolism of clodronate in RAW 264 macrophages. Pharm. Res. 2001;18:1550–1555. doi: 10.1023/a:1013026313647. [DOI] [PubMed] [Google Scholar]
- MÖNKKÖNEN J., TASKINEN M., AURIOLA S.O., URTTI A. Growth inhibition of macrophage-like and other cell types by liposome-encapsulated, calcium-bound, and free bisphosphonates in vitro. J. Drug Target. 1994a;2:299–308. doi: 10.3109/10611869409015910. [DOI] [PubMed] [Google Scholar]
- MÖNKKÖNEN J., VALJAKKA R., HAKASALO M., URTTI A. The effects of liposome surface charge and size on the intracellular delivery of clodronate and gallium in vitro. Int. J. Pharm. 1994b;107:189–197. [Google Scholar]
- NEVILLE-WEBBE H.L., ROSTAMI-HODJEGAN A., EVANS C.A., COLEMAN R.E., HOLEN I. Sequence- and schedule-dependent enhancement of zoledronic acid induced apoptosis by doxorubicin in breast and prostate cancer cells. Int. J. Cancer. 2005;113:364–371. doi: 10.1002/ijc.20602. [DOI] [PubMed] [Google Scholar]
- PAULSON D.J., SHUG A.L. Inhibition of the adenine nucleotide translocator by matrix-localized palmityl-CoA in rat heart mitochondria. Biochim. Biophys. Acta. 1984;766:70–76. doi: 10.1016/0005-2728(84)90218-4. [DOI] [PubMed] [Google Scholar]
- ROGERS M.J. New insights into the molecular mechanisms of action of bisphosphonates. Curr. Pharm. Des. 2003;9:2643–2658. doi: 10.2174/1381612033453640. [DOI] [PubMed] [Google Scholar]
- ROGERS M.J., BROWN R.J., HODKIN V., BLACKBURN G.M., RUSSELL R.G., WATTS D.J. Bisphosphonates are incorporated into adenine nucleotides by human aminoacyl-tRNA synthetase enzymes. Biochem. Biophys. Res. Commun. 1996;224:863–869. doi: 10.1006/bbrc.1996.1113. [DOI] [PubMed] [Google Scholar]
- ROUX C., DOUGADOS M. Treatment of patients with Paget's disease of bone. Drugs. 1999;58:823–830. doi: 10.2165/00003495-199958050-00005. [DOI] [PubMed] [Google Scholar]
- SALO J., LEHENKARI P., MULARI M., METSIKKÖ K., VÄÄNÄNEN H.K. Removal of osteoclast bone resorption products by transcytosis. Science. 1997;11:270–273. doi: 10.1126/science.276.5310.270. [DOI] [PubMed] [Google Scholar]
- SATO M., GRASSER W., ENDO N., AKINS R., SIMMONS H., THOMPSON D.D., GOLUB E., RODAN G.A. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J. Clin. Invest. 1991;88:2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE F., HASHIMOTO T., TAGAWA K. Energy-independent protection of the oxidative phosphorylation capacity of mitochondria against anoxic damage by ATP and its non-metabolizable analogs. J. Biochem. 1985;97:1229–1234. doi: 10.1093/oxfordjournals.jbchem.a135168. [DOI] [PubMed] [Google Scholar]
- ZAMZAMI N., MARCHETTI P., CASTEDO M., HIRSCH T., SUSIN S.A., MASSE B., KROEMER G. Inhibitors of permeability transition interfere with the disruption of the mitochondrial transmembrane potential during apoptosis. FEBS Lett. 1996;384:53–57. doi: 10.1016/0014-5793(96)00280-3. [DOI] [PubMed] [Google Scholar]