Abstract
Related sphingolipids, sphingosine 1-phosphate (S1P) and sphingosylphosphorylcholine (SPC), have important effects on vascular smooth muscle. The aim of this study was to investigate the intracellular pathways regulated by S1P and SPC in rat cerebral artery.
In cerebral arteries, S1P increased extracellular signal-regulated kinase (ERK)1/2 phosphorylation (5.2±1.4-fold increase) but did not activate p38 mitogen-activated protein kinase (p38MAPK) as assessed by immunoblotting. In contrast, SPC increased p38MAPK phosphorylation (3.0±0.3-fold increase) but did not stimulate ERK1/2. This differential activation was confirmed by measuring activation of heat shock protein (HSP) 27, a known downstream target of p38MAPK. Only SPC, but not S1P, activated HSP27.
In enzymatically dispersed cerebral artery myocytes, SPC increased [Ca2+]i in a concentration-dependent manner (peak response at 10 μM: 0.4±0.02 ratio units) as determined using the Ca2+ indicator, Fura 2. In contrast to S1P, the SPC-induced [Ca2+]i increase did not involve intracellular release but was due to Ca2+ influx via L-type Ca2+ channels.
Despite differences in signalling, both S1P and SPC phosphorylated the transcription factor cAMP response element-binding protein (CREB). S1P-induced CREB activation was dependent on ERK1/2 and Ca2+-calmodulin-dependent protein kinase (CaMK) activation. CREB activation by SPC required both p38MAPK and CaMK activation, but not ERK1/2.
In conclusion, S1P and SPC activate distinct MAP kinase isoforms and increase [Ca2+]i via different mechanisms in rat cerebral artery. This does not affect the ability of S1P or SPC to activate CREB, although this occurs via different pathways.
Keywords: Vascular smooth muscle; cerebral; sphingolipids; intracellular, calcium; mitogen-activated protein kinase; transcription factor
Introduction
Sphingolipids, derived from sphingomyelin metabolism, activate intracellular signalling pathways in many cell types and are now known to have important physiological effects on the cardiovascular system (Lavade et al., 2001). Effects may vary depending upon the sphingolipid and/or blood vessel examined, for example vasoconstriction (Coussin et al., 2002) or vasorelaxation (Zheng et al., 2000a). In particular, two members of this family, sphingosine 1-phosphate (S1P) and sphingosylphosphorylcholine (SPC), may have a selective role in modulating several aspects of vascular function (Meyer zu Heringdorf et al., 2002; Alewijnse et al., 2004; Saba & Hla, 2004; Watterson et al., 2005). S1P is found throughout the cardiovascular system (Saba & Hla, 2004). It is stored in high concentrations in platelets that do not express S1P lyase (Yatomi et al., 2000). Activation of prothrombotic stimuli results in the release of S1P from platelets achieving high concentrations in serum (nM to μM range) (Yatomi et al., 1995; 2000). Owing to the lipophilic nature of S1P and the levels reached in vivo, vascular smooth muscle (VSM) cells are exposed to relatively high concentrations in pathophysiological conditions. It is now clear that S1P-induced cellular effects occur predominantly through activation of selective S1P receptors on the plasma membrane (Pyne & Pyne, 2000; Sanchez & Hla, 2004). These receptors belong to the G-protein-coupled receptor superfamily, originally known as endothelial differentiation gene (EDG) receptors (Lee et al., 1998). Several isoforms have now been cloned and S1P1, S1P2, S1P3, S1P4 and S1P5 have high affinities for S1P with EC50's in the nM range (Lee et al., 1998; Pyne & Pyne, 2000; Sanchez & Hla, 2004). Both cultured and freshly isolated VSM cells express at least S1P1, S1P2 and S1P3 (Kluk & Hla, 2001; Tamama et al., 2001; Coussin et al., 2002). Receptor activation can lead to increases in [Ca2+]i, activation of mitogen-activated protein kinases (MAPK), as well as other signalling pathways such as RhoA/Rho kinase (Wu et al., 1995; An et al., 1999; Kluk & Hla, 2001; Coussin et al., 2002; Lockman et al., 2004). Recent studies have begun to reveal possible physiological roles of S1P receptors in the cardiovascular system. A study with S1P1 receptor knockout mice has demonstrated the essential involvement of this receptor for full blood vessel development (Liu et al., 2000). In cultured VSM cells, S1P stimulates DNA synthesis (Kluk & Hla, 2001; Tamama et al., 2001) suggesting that S1P receptors could play important roles in regulating proliferation. We have recently demonstrated that S1P receptors are expressed in native VSMs and can activate several intracellular pathways (Coussin et al., 2002; 2003).
SPC has now also been identified as a lipid mediator with similarities to S1P (Meyer zu Heringdorf et al., 2002). It occurs naturally in normal plasma at a similar concentration to S1P and may be elevated in serum (Liliom et al., 2001). In many cultured cell types, SPC, similar to S1P, can increase DNA synthesis and can activate MAPK isoforms (Meyer zu Heringdorf et al., 2002). SPC can also increase [Ca2+]i in several different cells types (Meyer zu Heringdorf et al., 2002). Both S1P and SPC can produce vasoconstriction in some vascular beds (Bischoff et al., 2000), including cerebral artery (Coussin et al., 2002; Shirao et al., 2002). As both S1P and SPC have overlapping effects it may be anticipated that these sphingolipids act via similar mechanisms. Indeed, SPC can act as a low affinity agonist at S1P receptors (Gonda et al., 1999), although no sphingolipid receptors identified thus far have similar affinity for S1P and SPC. Potential candidates for SPC high-affinity receptors have been proposed (Xu et al., 2000), such receptors have yet to be conclusively identified (Bektas et al., 2003).
The ability of S1P and SPC to activate MAPK in VSM cells, at least in culture, suggests a potential role as mitogenic stimuli in vivo. The proliferation of VSM cells by mitogenic stimuli can be correlated with the selective increase/decrease in expression of a variety of genes, although the processes that lead to a switch in fully differentiated VSM cell phenotype are not well understood (Owens et al., 2004). The key to understanding these events will be to elucidate the chain of intracellular events activated by extrinsic factors, such as sphingolipids. Myogenic factors stimulate a variety of pathways such as protein kinases and/or increases in intracellular Ca2+ ([Ca2+]i). These in turn regulate transcription factors, leading to selective expression and/or repression of genes that encode for specific proteins required for VSM proliferation (Kumar & Owens, 2003). It is therefore important to assess the signalling pathways stimulated by sphingolipids in differentiated VSM, and to further examine the effects of this stimulation on transcription factor activity.
In the present study, we have examined the S1P- and SPC-induced intracellular signalling in rat cerebral arteries. S1P and SPC can produce an increase in [Ca2+]i; however this occurs via different mechanisms. Additionally, S1P can activate extracellular signal-regulated kinase (ERK) 1/2 but not p38MAPK. In contrast, SPC activates p38MAPK but not ERK1/2. This differential signalling does not affect overall activation of the Ca2+-dependent transcription factor, cAMP response element-binding protein (CREB). However, S1P-dependent CREB activation requires both ERK1/2 and Ca2+-calmodulin-dependent protein kinase (CaMK) activation. SPC-induced CREB activation is dependent on activation of p38MAPK and CaMK but not ERK1/2.
Methods
Reagents
SPC and S1P were purchased from Sigma (Poole, U.K.). CREB, phospho-CREB polyclonal antibodies and both pan- and phospho-ERK antibodies were obtained from New England Biolabs (Herts, U.K.). Pan- and phospho-p38MAPK antibodies as well as pan- and phospho-heat shock protein 27 (HSP27) antibodies were purchased from Santa Cruz (CA, U.S.A.). Thapsigargin, KN-93 (2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine), PD98059 (2′-amino-3′-methoxyflavone), SB203580 (4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole) and nifedipine were obtained from Calbiochem (Nottingham, U.K.). Monoclonal (anti-mouse) and polyclonal (anti-goat) secondary antibodies conjugated to horseradish peroxidase were purchased from Dako Ltd (Cambridgeshire, U.K.). Polyclonal (anti-rabbit) secondary antibody was purchased from Sigma. FURA-2/AM was purchased from Molecular Probes, U.S.A. All other chemical reagents were from Sigma.
Tissue preparation
Male Sprague–Dawley rats (6 weeks old, 300–350 g) were euthanized by inhalation of CO2 followed by cervical dislocation. All procedures were in accordance with institutional guidelines. Cerebral arteries (middle and basilar) were immediately removed and placed into ice-cold physiological saline solution as previously described (Coussin et al., 2002). Arteries were cleaned of connective tissue and the endothelium removed by gentle rubbing of the lumen. Dissected arteries were preincubated at 37°C overnight, which gave a consistently low baseline level of activation for ERK1/2, p38MAPK and CREB. Inhibitors were added for a further 30 min, following which cerebral arteries were stimulated with either S1P or SPC.
Immunoblotting
Stimulated tissue was added to lysis buffer and homogenized at 4°C in a Braun homogenizing vessel as previously described (Coussin et al., 2002). Protein was measured using a Lowry protein assay to ensure equal protein loading. In addition, membranes were stained with Ponceau Red to confirm equal protein loading. Whole-tissue homogenates were used for immunoblotting with anti-CREB antibodies (phospho- and pan), anti-ERK1/2 antibodies (phospho- and pan-), anti-p38MAPK antibodies (phospho- and pan-) and anti-HSP27 antibodies (phospho- and pan-). Proteins were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Coussin et al., 2002). The membranes were immunoblotted with primary antibodies and immunoreactive bands were visualized using enhanced chemiluminescence quantified with an imaging densitometer (Biorad GS-690). All samples are run in simultaneously in parallel on two separate gels, and each is stained with either phospho- or pan antibody. Blots are developed at the same time. Any blot in which the pan-antibody reveals protein variation between samples of >5% is not analysed. Only blots that revealed entirely nonsaturated pixels (as determined using Multianalyst software, Biorad, Herts, U.K.) were quantified to ensure linearity of densitometric analysis.
Enzymatic dissociation of VSM myocytes
Smooth muscle cells from cerebral arteries were isolated by enzymatic dissociation as described previously (Coussin et al., 2002). Isolated smooth muscle cells were stored on glass-bottom dishes maintained at 4°C and used within 6 h. Only cells that had an elongated morphology were used for imaging.
Imaging of [Ca2+]i
Individual VSM myocytes were loaded with 2 μmol l−1 Fura-2 AM for 1 h in a physiological Krebs salt solution followed by a 20-min de-esterification period. A Zeiss Axiovert 200 inverted microscope, equipped with a cooled CCD camera (Photometrics) and a polychromatic illumination system (T.I.L.L. Photonics), was used to capture fluorescence images with excitations at 340 and 380 nm. The ratio of the fluorescence intensity between the pair of frames (FR340/380) was calculated after background subtraction. The Metafluor 4.6 software (Universal Imaging Corporation) controlled the illuminator and camera, and performed image ratioing and analysis. Results are expressed as F340/380 ratio. All experiments were performed at room temperature (22–24°C).
Analysis of data
Data are expressed as mean±s.e.m. Significance was tested by means of Student's t-test or ANOVA where required. A value of P<0.05 was considered significant.
Results
Effects of S1P and SPC on MAP kinase activation
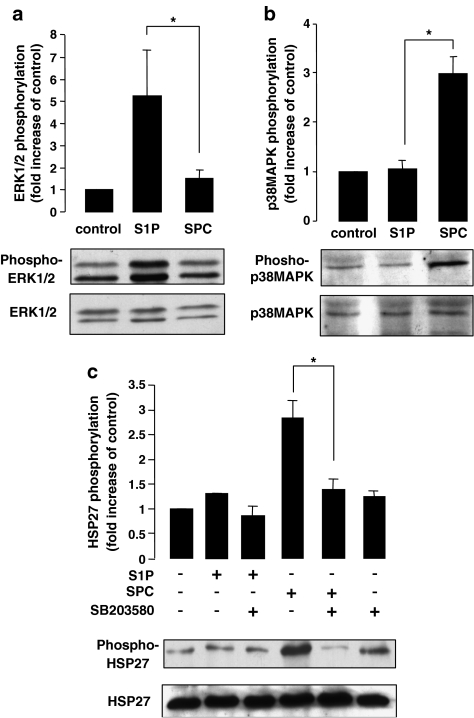
In order to investigate whether S1P or SPC can induce phosphorylation of ERK1/2 in cerebral arteries, immunoblot analysis using phospho-specific anti-ERK1/2 antibodies was performed. Rat cerebral arteries were stimulated with either S1P (5 μM) or SPC (10 μM) for 15 min at 37°C. ERK1/2 phosphorylation was increased approximately four-fold in S1P-stimulated arteries compared to unstimulated control arteries. However, no significant increase in ERK1/2 phosphorylation was observed in the SPC-treated arteries (Figure 1a). To determine whether S1P and SPC were activating other members of the MAP kinase family, immunoblots using an antibody specific to the phosphorylated form of p38MAPK were performed. An increase in phosphorylation of approximately three-fold was observed in SPC-stimulated arteries compared to unstimulated control arteries. No significant increase in phosphorylation of p38MAPK was observed, however, in the S1P-stimulated arteries (Figure 1b). Time course experiments indicated that for both S1P-induced ERK1/2 activation and for SPC-induced p38MAPK activation, 15 min was the peak activation level. The other major member of the MAPK family, c-Jun N-terminal kinase (JNK), was not activated by either S1P or SPC in rat cerebral arteries as determined by phospho-JNK antibodies (data not shown).
Figure 1.
S1P- and SPC-induced phosphorylation of MAP kinases and HSP27 in rat cerebral arteries. (a) Representative immunoblots showing changes in phosphorylation of ERK1/2 by S1P and SPC in rat cerebral arteries. The bar graph represents fold increase in phosphorylation of ERK1 and ERK2 combined compared to basal levels. S1P (5 μM) significantly increased phosphorylation after 15 min stimulation (n=6). SPC (10 μM), however, did not significantly increase ERK1/2 phosphorylation after 15 min stimulation. (b) Representative immunoblots and bar graph demonstrating increased phosphorylation of p38MAPK by S1P and SPC in rat cerebral arteries. After 15 min of stimulation, SPC (10 μM) significantly increased phosphorylation (n=6) while S1P (5 μM) did not significantly increase p38MAPK phosphorylation (n=6). (c) Representative immunoblots and bar graph showing the fold increases in phosphorylation of HSP27 by S1P and SPC in the rat cerebral artery. Following 15 min stimulation, SPC (10 μM) significantly increased HSP27 phosphorylation; however, S1P (5 μM) did not increase HSP27 phosphorylation (n=5). Preincubation with the p38MAPK inhibitor, SB203580 (30 μM), for 30 min, the SPC (10 μM)-induced phosphorylation was significantly inhibited (n=5). Data shown are mean±s.e.m. Asterisk denotes P<0.05.
In order to confirm the divergent MAPK activation profile of these related sphingolipids, the ability of S1P and SPC to phosphorylate HSP27, a known substrate of p38MAPK, was determined (Larsen et al., 1997). Using an antibody specific to the phosphorylated form of HSP27, an increase of three-fold was observed in SPC stimulated arteries compared to unstimulated control arteries. No significant increase was observed, however, in the S1P-stimulated arteries when compared to controls (Figure 1c). To confirm the role of p38MAPK in the HSP27 phosphorylation, cerebral arteries were pretreated with SB203580. Preincubation significantly decreased the SPC-induced HSP27 phosphorylation. No effect on phosphorylation levels of HSP27 was observed in either the S1P alone or S1P in the presence of SB203580 arteries (Figure 1c).
S1P- and SPC-induced increase in [Ca2+]i
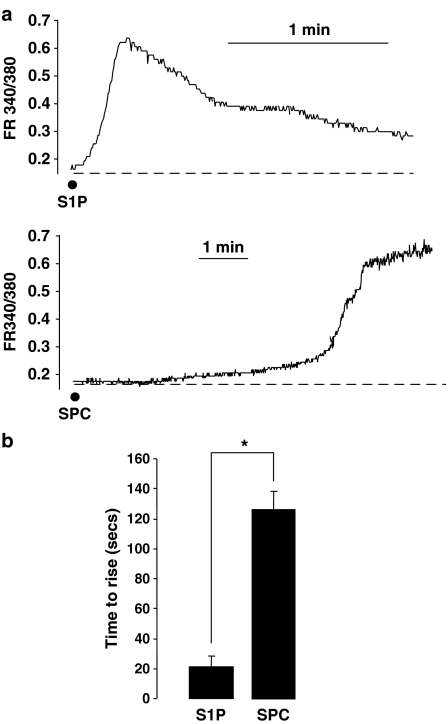
Intracellular Ca2+ was measured in freshly isolated cerebral artery myocytes using the fluorescent indicator dye Fura-2. Both S1P and SPC produced an increase in [Ca2+]i. The S1P-induced increase was characterized by a rapid Ca2+ spike followed by a slower decline to baseline (Figure 2a) as previously described (Coussin et al., 2002). In contrast, the SPC-induced increase revealed a longer time delay before the onset of the Ca2+ rise (Figure 2a and b). The slower increase consisted of an initial slow rise, followed by a more rapid Ca2+ increase that subsequently reached a plateau. The increase was maintained for at least 20 min (not shown).
Figure 2.
Sphingolipid-induced increases in [Ca2+]i from cerebral artery myocytes. (a) Representative Fura-2 fluorescence ratio traces (FR340/380) showing the changes in fluorescence produced upon application of S1P (5 μM, n=12) and SPC (10 μM, n=41) in freshly isolated myocytes from rat cerebral artery. (b) Bar graph showing the time taken for the fluorescence ratio to rise to peak after addition of S1P (5 μM, n=12) and SPC (10 μM, n=41). Data are mean±s.e.m. Asterisk denotes P<0.05.
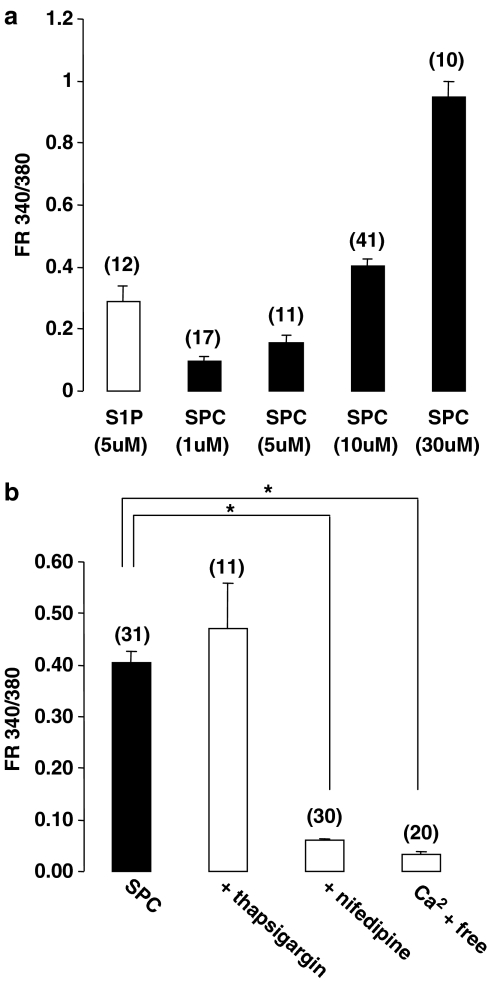
S1P-induced increases in intracellular Ca2+ have been previously shown to be concentration dependent in rat cerebral artery myocytes (Coussin et al., 2002). The SPC-induced increase in [Ca2+]i is also concentration dependent (Figure 3a), although a maximum response at the highest concentration (30 μM) was not achieved. Higher concentrations were not attempted due to solubility problems and therefore the EC50 was not calculated. The concentration of 10 μM SPC used in all other experiments produced approximately 50% of the maximal Ca2+ increase measured. The intracellular mechanisms responsible for the SPC-induced [Ca2+]i increase were further examined. Preincubation with thapsigargin, which blocks the sarcoplasmic reticulum Ca2+ ATPase, did not prevent the SPC-induced Ca2+ increase (Figure 3b). To clarify the potential role of extracellular calcium influx, Ca2+ myocytes were either pretreated with the dihydropyridine inhibitor of L-type Ca2+ channels, nifedipine or stimulated following removal of extracellular Ca2+. Both pretreatment with nifedipine and removal of extracellular Ca2+ significantly decreased the SPC-induced increase in [Ca2+]i back to near resting levels (Figure 3b).
Figure 3.
Characteristics of the SPC-induced [Ca2+]i increase in rat cerebral artery myocytes. (a) Mean data showing the increase in fluorescence ratio (peak) relative to resting levels induced by increasing concentrations of SPC. EC50 could not be calculated as the curve was not saturable at soluble concentrations of SPC. (b) Mean data showing the increase in fluorescence ratio (relative to resting levels) induced by SPC (10 μM) following pretreatment with either 1 μM thapsigargin, 2 μM nifedipine or in the presence of no added extracellular Ca2+. Thapsigargin had no effect on SPC-induced [Ca2+]i. However, both nifedipine and the removal of extracellular Ca2+ significantly decreased the Ca2+ increase induced by SPC. Resting Ca2+ levels at 10 μM SPC are 0.30±0.01 FR340/380 (n=83). Data are mean±s.e.m. with numbers of cells in parentheses. Asterisk denotes P<0.05.
S1P- and SPC-induced activation of CREB
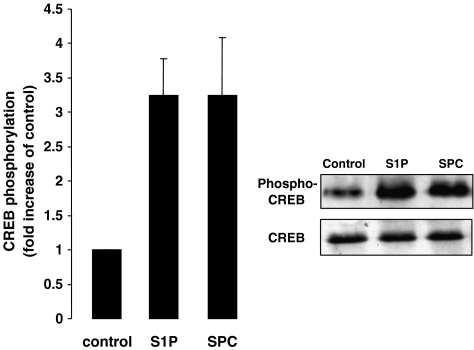
Activation of the transcription factor CREB has been shown to be dependent on ERK1/2 and increases in [Ca2+]i (Wu et al., 2001). In order to investigate whether S1P and SPC can activate CREB in cerebral arteries, immunoblots using an antibody specific for phosphorylated (serine 133)-CREB was used. An increase in CREB phosphorylation of approximately three-fold was observed in S1P-stimulated arteries when compared to unstimulated arteries (Figure 4). Similarly, a three-fold increase was also observed in SPC-stimulated arteries when compared to control arteries (Figure 4).
Figure 4.
Phosphorylation of CREB by S1P and SPC in rat cerebral arteries. Representative immunoblot and bar graph showing changes in CREB phosphorylation by S1P and SPC. After 15 min of stimulation, both S1P (5 μM) and SPC (10 μM) significantly increased CREB phosphorylation (n=5). Data shown are mean±s.e.m. Asterisk denotes P<0.05.
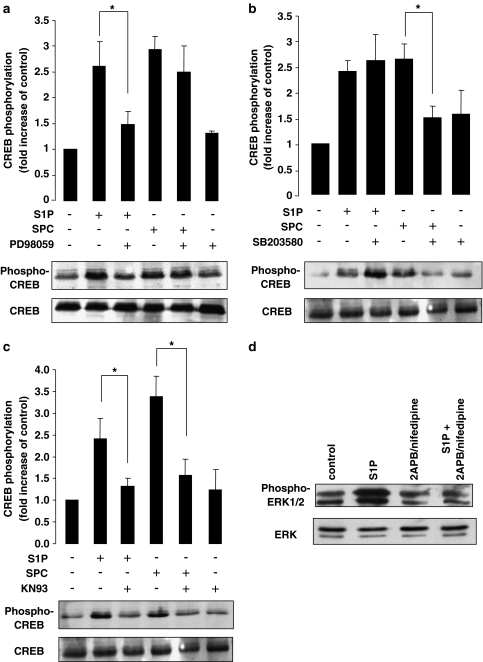
The intracellular pathways involved in S1P- and SPC-induced CREB phosphorylation were investigated. Several kinases have been implicated in CREB phosphorylation including both ERK1/2 and p38MAPK. Inhibitors were used to assess their potential role in S1P- and SPC-induced CREB phosphorylation. As S1P was shown to phosphorylate ERK1/2, arteries were treated with the selective MEK inhibitor PD98059 prior to S1P or SPC stimulation. Preincubation of the arteries with PD98059 significantly decreased the S1P-induced CREB phosphorylation; however, had no significant effect on the SPC-induced CREB activation (Figure 5a). When arteries were preincubated with the selective p38MAPK inhibitor SB203580, a significant decrease in SPC-induced CREB activation was observed. However, there was no significant effect of SB203580 on the S1P-induced CREB activation (Figure 5b). As well as ERK1/2 and p38MAPK, other kinases have been implicated in the signalling pathways resulting in CREB phosphorylation. One such kinase is CaMK activated via an increase in [Ca2+]i. As both S1P and SPC stimulation produce increases in intracellular calcium in cerebral artery myocytes (albeit via different mechanisms), the involvement of CaMK in CREB activation was examined. Preincubation of cerebral arteries with KN-93, a selective CaMK inhibitor, significantly inhibited both the S1P- and SPC-induced CREB activation (Figure 5c).
Figure 5.
Intracellular mechanism of CREB phosphorylation by S1P and SPC. (a) Representative immunoblot and bar graph showing the fold increases in CREB phosphorylation by S1P and SPC in the rat cerebral artery in the presence of the ERK1/2 inhibitor, PD98059 (30 μM). The S1P-induced phosphorylation was significantly inhibited in the presence of PD98059 while the SPC-induced phosphorylation was unaffected (n=4). (b) Representative immunoblot and bar graph showing the fold increases in CREB phosphorylation by S1P and SPC in the presence of the p38MAPK inhibitor, SB203580 (30μM). The SPC-induced phosphorylation was significantly inhibited in the presence of SB203580 while the S1P-induced phosphorylation was unaffected (n=6). (c) Representative immunoblot and bar graph showing the fold increases in CREB phosphorylation by S1P and SPC in the presence of the CaM kinase inhibitor, KN93 (30 μM). Both the S1P- and SPC-induced CREB phosphorylation was significantly inhibited by KN93 (n=4). (d) Typical immunoblot showing S1P-induced ERK1/2 activation following pretreatment with 2-APB and nifedipine. ERK1/2 phosphorylation was significantly decreased in the presence of inhibitors (S1P stimulation 5.6±0.5-fold increase of control; S1P stimulation following 2-APB/nifedipine pretreatment – 0.7±0.1-fold increase of control, n=3, P<0.05). Data shown are mean±s.e.m. Asterisk denotes P<0.05.
To determine whether the activation of MAPK isoforms is also dependent on an increase in [Ca2+]i, cerebral arteries were stimulated with either S1P or SPC. For S1P-stimulated arteries, tissue was preincubated simultaneously with 2-aminoethoxydiphenylborate (2-APB, 30 μM) and 1 μM nifedipine for 30 min as previously described (Coussin et al., 2002) and ERK1/2 activation was assessed. Both 2-APB (an inhibitor of InsP3-induced Ca2+ release) and nifedipine were used as we have previously shown in this preparation that both InsP3-induced intracellular Ca2+ release and a minor component of Ca2+-influx from L-type Ca2+ channels are involved in S1P-induced increase in [Ca2+]i (Coussin et al., 2002; 2003). For SPC-stimulated arteries, tissue was preincubated with only 1 μM nifedipine for 30 min (see Figure 3) and p38MAPK activation measured. In S1P-stimulated arteries pretreated with 2-APB and nifedipine, ERK1/2 activation was significantly inhibited compared to controls (Figure 5d). However, in SPC-stimulated arteries pretreated with nifedipine, p38MAPK was unaffected (data not shown).
Discussion
On the basis of structural similarities between S1P and SPC (Meyer zu Heringdorf et al., 2002), it would be expected that some functional overlap exists. Although SPC can bind to the S1P receptors, its lower affinity suggests that effects will not be pharmacologically comparable. Some actions that S1P and SPC have in common (in some cell types) are possibly the result of binding to S1P receptors. In this study, we provide evidence that S1P and SPC activate different signalling pathways in rat cerebral arteries, and are therefore unlikely to be acting via the same receptors. Of the three main members of the MAPK family (ERK1/2, p38MAPK and JNK), S1P only stimulates ERK1/2 phosphorylation whereas SPC only stimulates p38MAPK. In addition, while both sphingolipids produce an increase in [Ca2+]i, the S1P-induced Ca2+ rise is predominantly via release from intracellular stores as previously shown (Coussin et al., 2002) whereas the SPC-induced increase is completely dependent on Ca2+ influx (via L-type Ca2+ channels). Interestingly, although different intracellular pathways are activated, both S1P and SPC can phosphorylate the transcription factor CREB to a similar extent.
The MAPK activation profile of S1P and SPC has been documented in several cell types. At least in cultured cells, S1P can activate ERK1/2, p38MAPK and JNK (Gonda et al., 1999). In cultured VSM cells, S1P stimulation leads to phosphorylation of ERK1/2 (Kluk & Hla, 2001), as well as p38MAPK and JNK (Usui et al., 2004). In native arteries, S1P can stimulate ERK1/2 activation (Coussin et al., 2002). Likewise, in some cultured cell types including VSM cells, SPC can activate ERK1/2, although its ability to activate other MAPKs, particularly in vascular tissue, has not previously been determined. In the present study, we show that S1P can activate ERK1/2 but not p38MAPK in cerebral arteries. This suggests that differences exist between cultured and native VSM cells and may reflect differential expression of S1P receptor subtypes. It also points towards a mitogenic, but not inflammatory, role for S1P in native arteries as p38MAPK is often associated with inflammatory processes (Saklatvala, 2004). In contrast to S1P, SPC activated p38MAPK but did not activate ERK1/2 in rat cerebral arteries. This suggests that SPC may induce inflammatory pathways in this tissue but does not appear to stimulate the classic mitogenic pathways. This differs from SPC effects in cultured VSM cells and may represent further important variations between models. It also indicates, at least in native arteries, that SPC and S1P cannot be considered mitogens with similar properties. The nature of the S1P and SPC with regard to their vascular mitogenic properties in vivo is unclear. However, the results of this study suggest that their respective roles may be different, perhaps even divergent.
Diverse sphingolipids can produce increases in [Ca2+]i in several cell types, including native VSM (Alewijnse et al., 2004) and in particular, cerebral artery VSM (Zheng et al., 2000b). The mechanism of this increase varies, probably depending on the vascular bed examined. S1P- and SPC-induced increases in [Ca2+]i can be due to either intracellular Ca2+ release from stores, extracellular Ca2+ influx, or both. We have previously shown in rat native cerebral arteries that S1P increases [Ca2+]i predominantly via intracellular Ca2+ release (Coussin et al., 2002) although a minor component occurs via delayed Ca2+ influx (Coussin et al., 2003). However, in rat renal arteries, the S1P-induced [Ca2+]i increase is almost completely blocked by an L-type Ca2+ channel blocker (Bischoff et al., 2001). S1P may utilize different mechanisms, depending on the vascular bed examined. Similarly, SPC has been shown to produce an increase in [Ca2+]i in cultured VSM cells, via a release from intracellular stores (Chin & Chueh, 2000). In mesenteric arteries, SPC increased contractility via phospholipase C (Altmann et al., 2003), presumably releasing Ca2+ from intracellular stores, while in pulmonary arteries, SPC-induced Ca2+ entry is observed (Thomas et al., 2005). In the present study, SPC increased [Ca2+]i in rat cerebral artery myocytes but via a different mechanism from S1P. The SPC-induced Ca2+ increase was entirely dependent on Ca2+ influx from activation of L-type Ca2+ channels. This is in agreement with SPC effects in bovine cerebral artery (Todoroki-Ikeda et al., 2000) although the increase in [Ca2+]i is of a much greater magnitude in the rat. The differences in Ca2+ signalling between SPC and S1P have several implications. It further indicates that SPC is not acting via the same receptor as S1P in this cell type. Whether these SPC effects on [Ca2+]i are dependent on a plasma membrane receptor (Bischoff et al., 2001), or occur independently of receptor activation is not yet known. Regardless, it is unlikely that SPC is binding to the S1P receptors responsible for intracellular Ca2+ release. The difference in Ca2+ homeostasis could also have consequences for downstream physiological effects. For example, Ca2+-dependent transcription factors important in mitogenic responses can be activated differentially, depending on the source, magnitude and time of Ca2+ rise (Dolmetsch et al., 1998). This has also been demonstrated in native VSM (Stevenson et al., 2001). The findings of our study, including the differences in MAPK activation as well as differences in Ca2+ signalling, suggest that S1P and SPC could have distinct effects on subsets of transcription factors. Depending on which subsets are activated would dictate the potential effects on VSM cell phenotype.
CREB is a transcription factor that has been implicated in the regulation of vascular cell phenotype, although its role in this process is still unclear. It has been suggested that CREB activation by mitogenic stimuli is essential for proliferation (Tokunou et al., 2001). In addition, a dominant negative form of CREB inhibited neointimal growth in balloon-injured arteries of rabbits (Tokunou et al., 2003). However, other studies have suggested that an increased expression of CREB is essential to prevent VSM cell proliferation, and that a decrease in CREB expression acts as a switch to allow the initiation of proliferation (Klemm et al., 2001). These apparently divergent findings have yet to be reconciled. Despite an unclear role in vascular function, a variety of stimuli can result in the phosphorylation and subsequent activation of CREB. A number of kinases involved in this phosphorylation have now been identified, including MAPK and CaMK (Wu et al., 2001). It has been shown that the initial CREB phosphorylation is rapidly induced by a rise in [Ca2+]i and subsequent activation of CaMK, which is followed by a more prolonged CREB activation via a MAPK-dependent pathway (Wu et al., 2001). This complex and interacting array of signalling pathways suggests that regulation of CREB can vary depending on many different factors. We have previously shown that S1P-induced CREB activation is partly dependent on a rise in intracellular Ca2+ and CaMK activation (Coussin et al., 2003). In the present study, we further demonstrate that this is also dependent on ERK1/2 activation. This is in agreement with stimulation by other agonists (Egan & Nixon, 2004). We also further demonstrate that S1P-induced ERK1/2 is also dependent on an increase in [Ca2+]i as previously shown (Egan & Nixon, 2004) and may partly explain the interdependence on both signals. Interestingly, despite differences in SPC and S1P intracellular signalling, SPC also activated CREB to a similar extent. This was similarly dependent on a rise in intracellular Ca2+ and involved CaMK activation. However, an inhibitor of p38MAPK significantly decreased SPC-induced CREB activation. p38MAPK has been implicated as a potential kinase involved in CREB activation in VSM (Ono et al., 2004). Activation of p38MAPK by S1P was not dependent on a rise in [Ca2+]i. Therefore, both S1P and SPC can activate CREB via different mechanisms, and in this case will not have different effects on downstream gene expression. However, activation of other transcription factors may not be similar following stimulation with S1P and SPC. For example, as also shown here only SPC activates HSP27 (via p38MAPK) as expected. HSP27 can have effects on gene expression via transcription factor activation, such as increasing nuclear factor-κB activity (Parcellier et al., 2003). This may lead to differences between S1P and SPC on gene expression. Further experiments are currently determining which transcription factors are differentially regulated by S1P, compared to SPC.
In conclusion, S1P and SPC activate different intracellular signalling pathways in native VSM. This relates particularly to the increase in [Ca2+]i and activation of MAPK isoforms. Although both S1P and SPC can induce CREB activation to a similar extent, this occurred via different mechanisms. As both MAPK and [Ca2+]i are important regulatory pathways in transcription factor activation, it is likely that S1P and SPC will have distinct functional effects on cerebral artery VSM phenotype.
Acknowledgments
This study was supported by the British Heart Foundation and The Wellcome Trust. We thank Dr R.H. Scott for assistance with this study.
Abbreviations
- CaMK
Ca2+-calmodulin-dependent protein kinase
- CREB
cAMP response element-binding protein
- EDG
endothelial differentiation gene
- ERK
extracellular signal-regulated kinase
- HSP
heat shock protein
- JNK
c-Jun N-terminal kinase
- KN-93
2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine
- p38MAPK
p38 mitogen-activated protein kinase
- PD98059
2′-amino-3′-methoxyflavone
- S1P
sphingosine 1-phosphate
- SB203580
4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole
- SPC
sphingosylphosphorylcholine
- VSM
vascular smooth muscle
References
- ALEWIJNSE A.E., PETERS S.L.M., MICHEL M.C. Cardiovascular effects of sphingosine 1-phosphate and other sphingomyelin metabolites. Br. J. Pharmacol. 2004;143:666–684. doi: 10.1038/sj.bjp.0705934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALTMANN C., STEENPASS V., CZYBORRA P., HEIN P., MICHEL M.C. Comparison of signalling mechanisms involved in rat mesenteric microvessel contraction by noradrenaline and sphingosylphosphorylcholine. Br. J. Pharmacol. 2003;138:261–271. doi: 10.1038/sj.bjp.0705028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AN S., BLEU T., ZHENG Y. Transduction of intracellular calcium signals through G-protein medicated activation of phospholipase C by recombinant sphingosine 1-phosphate receptors. Mol. Pharmacol. 1999;55:787–794. [PubMed] [Google Scholar]
- BEKTAS M., BARAK L.S., JOLLY P.S., LIU H., LYNCH K.R., LACANA E., SUHR K.B., MILSTEIN S., SPIEGEL S. The G protein-coupled receptor GPR4 suppresses ERK activation in a ligand-independent manner. Biochemistry. 2003;42:12181–12189. doi: 10.1021/bi035051y. [DOI] [PubMed] [Google Scholar]
- BISCHOFF A., CZYBORRA P., FETSCHER C., MEYER ZU HERINGDORF D., JAKOBS K.H., MICHEL M.C. Sphingosine 1-phosphate and sphingosylphosphorylcholine constrict renal and mesenteric microvessels in vitro. Br. J. Pharmacol. 2000;130:1871–1878. doi: 10.1038/sj.bjp.0703515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISCHOFF A., FINGER J., MICHEL M.C. Nifedipine inhibits sphinogosine-1-phosphate-induced renovascular contraction in vitro and in vivo. Naunyn. Schmiedebergs Arch. Pharmacol. 2001;364:179–182. doi: 10.1007/s002100100446. [DOI] [PubMed] [Google Scholar]
- CHIN T.Y., CHUEH S.H. Distinct signalling mechanisms induced by ATP and sphingosylphosphorylcholine in porcine aortic smooth muscle cells. Br. J. Pharmacol. 2000;129:1365–1374. doi: 10.1038/sj.bjp.0703190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUSSIN F., SCOTT R.H., NIXON G.F. Sphingosine 1-phosphate induces CREB activation in rat cerebral artery via a protein kinase C-mediated inhibition of voltage-gated K+ channels. Biochem. Pharmacol. 2003;66:1861–1870. doi: 10.1016/s0006-2952(03)00546-x. [DOI] [PubMed] [Google Scholar]
- COUSSIN F., SCOTT R.H., WISE A., NIXON G.F. Comparison of sphingosine 1-phosphate-induced intracellular signalling pathways in vascular smooth muscles: differential role in vasoconstriction. Circ. Res. 2002;91:151–157. doi: 10.1161/01.res.0000028150.51130.36. [DOI] [PubMed] [Google Scholar]
- DOLMETSCH R.E., XU K., LEWIS R.S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- EGAN C.E., NIXON G.F. Endothelin-1 and depolarization-induced differential regulation of cAMP response element-binding protein in proliferating and developed vascular smooth muscle. Cell. Signal. 2004;16:1387–1396. doi: 10.1016/j.cellsig.2004.04.008. [DOI] [PubMed] [Google Scholar]
- GONDA K., OKAMOTO H., TAKUWA N., YATOMI Y., OKAZAKI H., SAKURAI T., KIMURA S., SILLARD R., HARII K., TAKUWA Y. The novel sphingosine 1-phosphate receptor AGR16 is coupled via pertussis toxin-sensitive and -insensitive G-proteins to multiple signalling pathways. Biochem. J. 1999;337:67–75. [PMC free article] [PubMed] [Google Scholar]
- HERINGDORF D.M.Z., HIMMEL H.M., JAKOBS K.H. Sphingosylphosphorylcholine – biological functions and mechanisms of action. Biochem. Biophys. Acta. 2002;1582:178–189. doi: 10.1016/s1388-1981(02)00154-3. [DOI] [PubMed] [Google Scholar]
- KLEMM D.J., WATSON P.A., FRID M.G., DEMPSEY E.C., SCHAAK J., COLTON L.A., NESTEROVA A., STENMARK K.R., REUSCH J.E.B. cAMP response element-binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J. Biol. Chem. 2001;276:46132–46141. doi: 10.1074/jbc.M104769200. [DOI] [PubMed] [Google Scholar]
- KLUK M.J., HLA T. Role of the sphingosine 1-phosphate receptor EDG-1 in vascular smooth muscle cell proliferation and migration. Circ. Res. 2001;89:496–502. doi: 10.1161/hh1801.096338. [DOI] [PubMed] [Google Scholar]
- KUMAR M.S., OWENS G.K. Combinatorial control of smooth muscle-specific gene expression. Arterioscler. Thromb. Vasc. Biol. 2003;23:543–553. doi: 10.1161/01.ATV.0000065197.07635.BA. [DOI] [PubMed] [Google Scholar]
- LARSEN J.K., YAMBOLIEV I.A., WEBER L.A., GERTHOFFER W.T. Phosphorylation of the 27-kDa heat shock protein via p38 MAP kinase and MAPKAP kinase in smooth muscle. Am. J. Physiol. 1997;273:L930–L940. doi: 10.1152/ajplung.1997.273.5.L930. [DOI] [PubMed] [Google Scholar]
- LAVADE T., AUGE N., VELDMAN R.J., CUVILLIER O., NEGRE-SALVAYRE A., SALVAYRE R. Sphingolipid mediators in cardiovascular cell biology and pathology. Circ. Res. 2001;89:957–968. doi: 10.1161/hh2301.100350. [DOI] [PubMed] [Google Scholar]
- LEE M.J., VAN BROCKLYN J.R., THANGADA S., LIU C.H., HAND A.R., MENZELEEV R., SPIEGEL S., HLA T. Sphingosine-1-phosphate as a ligand for the G protein coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- LILIOM K., SUN G., BUNEMANN M., VIRAG T., NUSSER N., BAKER D.L., WANG D.A., FABIAN M.J., BRANDTS B., BENDER K., EICKEL A., MALIK K.U., MILLER D.D., DESIDERIO D.M., TIGYI G., POTT L. Sphingosylphosphocholine is a naturally occurring lipid mediator in blood plasma: a possible role in regulating cardiac function via sphingolipid receptors. Biochem. J. 2001;355:189–197. doi: 10.1042/0264-6021:3550189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y., WADA R., YAMASHITA T., MI Y., DENG C.X., HOBSON J.P., ROSENFELDT H.M., NAVA V.E., CHAE S.S., LEE M.J., LIU C.H., HLA T., SPIEGEL S., PROIA R.L. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOCKMAN K., HINSON J.S., MEDLIN M.D., MORRIS D., TAYLOR J.M., MACK C.P. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response co-factors. J. Biol. Chem. 2004;279:42422–42430. doi: 10.1074/jbc.M405432200. [DOI] [PubMed] [Google Scholar]
- ONO H., ICHIKI T., FUKUYAMA K., IINO N., MASUDA S., EGASHIRA K., TAKESHITA A. cAMP-response element-binding protein mediates tumor necrosis factor-alpha-induced vascular smooth muscle cell migration. Arterioscler. Thromb. Vasc. Biol. 2004;24:1634–1639. doi: 10.1161/01.ATV.0000138052.86051.0d. [DOI] [PubMed] [Google Scholar]
- OWENS G.K., KUMAR M.S., WAMHOFF B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- PARCELLIER A., SCHMITT E., GURBUXANI S., SEIGNEURIN-BERNY D., PANCE A., CHANTOME A., PLENCHETTE S., KHOCHBIN S., SOLARY E., GARRIDO C. HSP27 is a ubiquitin-binding protein involved in I-κBα proteasomal degradation. Mol. Cell. Biol. 2003;23:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PYNE S., PYNE N. Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors. Pharmacol. Ther. 2000;88:115–131. doi: 10.1016/s0163-7258(00)00084-x. [DOI] [PubMed] [Google Scholar]
- SABA J.D., HLA T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ. Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- SAKLATVALA J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr. Opin. Pharmacol. 2004;4:372–377. doi: 10.1016/j.coph.2004.03.009. [DOI] [PubMed] [Google Scholar]
- SANCHEZ T., HLA T. Structural and functional characteristics of S1P receptors. J. Cell. Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- SHIRAO S., KASHIWAGI S., SATO M., MIWA S., NAKAO F., KUROKAWA T., TODOROKI-IKEDA N., MOGAMI K., MIZUKAMI Y., KURIYAMA S., HAZE K., SUZUKI M., KOBAYASHI S. Sphingosylphosphorylcholine is a novel messenger for Rho-kinase-mediated Ca2+ sensitization in the bovine cerebral artery: unimportant role for protein kinase C. Circ. Res. 2002;91:112–119. doi: 10.1161/01.res.0000026057.13161.42. [DOI] [PubMed] [Google Scholar]
- STEVENSON A.S., GOMEZ M.F., HILL-EUBANKS D.C., NELSON M.T. NFAT4 movement in native smooth muscle: a role for differential Ca2+ signalling. J. Biol. Chem. 2001;276:15018–15024. doi: 10.1074/jbc.M011684200. [DOI] [PubMed] [Google Scholar]
- TAMAMA K., KON J., SATO K., TOMURA H., KUWABARA A., KIMURA T., KANDA T., OHTA H., UI M., KOBAYASHI I., OKAJIMA F. Extracellular mechanism through the Edg family of receptors might be responsible for sphingosine-1-phosphate-induced regulation of DNA synthesis and migration of rat aortic smooth-muscle cells. Biochem. J. 2001;353:139–146. [PMC free article] [PubMed] [Google Scholar]
- THOMAS G.D., SNETKOV V.A., PATEL R., LEACH R.M., AARONSON P.I., WARD J.P.T. Sphingosylphosphorylcholine-induced vasoconstriction of pulmonary artery: activation of non-store-operated Ca2+ entry. Cardiovasc. Res. 2005;68:56–64. doi: 10.1016/j.cardiores.2005.05.013. [DOI] [PubMed] [Google Scholar]
- TODOROKI-IKEDA N., MIZUKAMI Y., MOGAMI K., KUSUDA T., YAMAMOTO K., MIYAKE T., SATO M., SUZUKI S., YAMAGATA H., HOKAZONO Y., KOBAYASHI S. Sphingosylphosphorylcholine induces Ca2+-sensitization of vascular smooth muscle contraction: possible involvement of rho-kinase. FEBS Lett. 2000;482:85–90. doi: 10.1016/s0014-5793(00)02046-9. [DOI] [PubMed] [Google Scholar]
- TOKUNOU T., ICHIKI T., TAKEDA K., FUNAKOSHI Y., IINO N., TAKESHITA A. cAMP response element-binding protein mediates thrombin-induced proliferation of vascular smooth muscle. Aterioscler. Thromb. Vasc. Biol. 2001;21:1764–1769. doi: 10.1161/hq2112.098770. [DOI] [PubMed] [Google Scholar]
- TOKUNOU T., SHIBATA R., KAI H., ICHIKI T., MORISAKI T., FUKUYAMA K., ONO H., IINO N., MASUDA S., SHIMOKAWA H., EGASHIRA K., IMAIZUMI T., TAKESHITA A. Apoptosis induced by inhibition of cyclic AMP response element-binding protein in vascular smooth muscle cells. Circulation. 2003;108:1246–1252. doi: 10.1161/01.CIR.0000085164.13439.89. [DOI] [PubMed] [Google Scholar]
- USUI S., SUGIMOTO N., TAKUWA N., SAKAGAMI S., TAKATA S., KANEKO S., TAKUWA Y. Blood lipid mediator sphingosine 1-phosphate potently stimulates platelet-derived growth factor-A and -B chain expression through S1P1-Gi-Ras-MAPK-dependent induction of Kruppel-like factor 5. J. Biol. Chem. 2004;279:12300–12311. doi: 10.1074/jbc.M305025200. [DOI] [PubMed] [Google Scholar]
- WATTERSON K.R., RATZ P.H., SPIEGEL S. The role of sphingosine-1-phosphate in smooth muscle contraction. Cell. Signal. 2005;17:289–298. doi: 10.1016/j.cellsig.2004.09.013. [DOI] [PubMed] [Google Scholar]
- WU G.Y., DEISSEROTH K., TSIEN R.W. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2808–2813. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU J., SPEIGEL S., STURGILL T.W. Sphingosine 1-phosphate rapidly activates the mitogen-activated protein kinase pathway by a G protein dependent mechanism. J. Biol. Chem. 1995;270:11484–11488. doi: 10.1074/jbc.270.19.11484. [DOI] [PubMed] [Google Scholar]
- XU Y., ZHU K., HONG G., WU W., BAUDHUIN L.M., XIAO Y., DAMRON D.S. Sphingosylphosphorylcholine is a ligand for ovarian cancer G-protein-coupled receptor 1. Nat. Cell. Biol. 2000;2:261–267. doi: 10.1038/35010529. [DOI] [PubMed] [Google Scholar]
- YATOMI Y., OHMORI T., GR R.L., KAZAMA F., OKAMOTO H., SANO T., SATOH K., KUME S., TIGYI G., IGARASHI Y., OZAKI Y. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood. 2000;96:3431–3438. [PubMed] [Google Scholar]
- YATOMI Y., RUAN F., HAKOMORI S., IGARASHI Y. Sphingosine 1-phosphate: a platelet-activating sphingolipid released from stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- ZHENG T., LI W., WANG J., ALTURA B.T., ALTURA B.M. Effects of neutral sphingomyelinase on phenylephrine-induced vasoconstriction and Ca2+ mobilization in rat aortic smooth muscle. Eur. J. Pharmacol. 2000a;391:127–135. doi: 10.1016/s0014-2999(00)00045-5. [DOI] [PubMed] [Google Scholar]
- ZHENG T., LI W., WANG J., ALTURA B.T., ALTURA B.M. Sphingomyelinase and ceramide analogues induce contraction and rises in [Ca2+]i in canine cerebral vascular muscle. Am. J. Physiol. 2000b;278:H1421–H1428. doi: 10.1152/ajpheart.2000.278.5.H1421. [DOI] [PubMed] [Google Scholar]