Abstract
The in vivo effects of a selective partial agonist for neuronal nicotinic acetylcholine receptor (nAChRs) α4β2 subtype, TC-2559, characterised recently in in vitro preparations, have been profiled. The brain bioavailability of TC-2559 and its effects on the spontaneous firing and bursting properties of the dopaminergic (DAergic) neurones recorded extracellularly in the ventral tegmental area (VTA) were studied following systemic administration in anaesthetised rats.
Cumulative doses of TC-2559 (0.021–1.36 mg kg−1, i.v.) increased both the firing and bursting activities of VTA DA neurones. The effect of bolus doses of TC-2559 of 0.66 or 1.32 mg kg−1, i.v., was approximately equivalent to that of 0.0665 mg kg−1, i.v. nicotine.
The excitation evoked by both nicotine and TC-2559 was fully reversed by DHβE (0.39–0.77 mg kg−1, i.v.), an α4β2-subtype-preferring nicotinic antagonist, and application of nicotine after DHβE failed to evoke any excitation. MLA (0.23 mg kg−1, i.v.), an α7 selective antagonist, failed to alter TC-2559-evoked excitation and bursting activities, and a novel α7 agonist (PSAB-OFP; 0.23 mg kg−1, i.v.) was also without effect.
The present results indicated that TC-2559 fully mimics nicotine by increasing both the excitability and bursting behaviour of VTA DA neurones, effects that are predominantly due to activation of α4β2-like nAChRs.
TC-2559 has been demonstrated to be a useful in vivo pharmacological tool for studying the α4β2 subtype of nicotinic receptor.
Keywords: nAChR, nicotine, TC-2559, dopaminergic neurone, ventral tegmental area, rat
Introduction
Neuronal nicotinic acetylcholine receptors (nAChRs) are ion channels composed of five transmembrane subunits. To date, there are 12 types of neuronal nAChRs subunits, identified and classified as α2–α10 and β2–β4 (McGehee & Role, 1995). They form two types of nAChRs, hetero-pentameric structures assembled with various α and β subunits combinations, or homo-pentamers of α subunits, for example of the α7 type. In the mammalian brain, α4β2 and α7 subtype neuronal nAChRs are most highly represented and distributed, with other subtype receptors containing, for example, α3, α5, α6, β3 and β4 localised in distinct brain regions (see Picciotto et al., 2001).
The midbrain dopaminergic (DAergic) nuclei, including the ventral tegmental area (VTA), are important in reinforcement, motility and associative motor learning (Berke & Hyman, 2000). Neuronal nAChRs are densely distributed in the VTA (Clarke et al., 1984, 1985), indicating their possible regulation of dopamine (DA) function in both normal and disease conditions. Activation of nAChRs by nicotine increases VTA DA neuronal activity both in vivo (Grenhoff et al., 1986; Erhardt et al., 2002; Schilstrom et al., 2003) and in vitro (Calabresi et al., 1989; Pidoplichko et al., 1997; Fisher et al., 1998; Grillner & Svensson, 2000; Chen et al., 2003). Nicotine also induces an increased bursting discharge pattern of the VTA DA neurones (Grenhoff et al., 1986; Schilstrom et al., 2003). This pattern of neuronal activity per se is believed to be an important determinant of the extracellular levels of released dopamine (DA) (Gonon, 1988; Chergui et al., 1994, 1996; Kitai et al., 1999; see Balfour et al., 2000 for full discussion). Systemic application of nicotine indeed induced DA release in the striatum and nucleus accumbens (Nisell et al., 1994a, 1994b; Pontieri et al., 1996; Schilstrom et al., 1998; Fu et al., 2000), brain regions which are thought to contribute to the locomotor-stimulating and reinforcing actions of nicotine (see Stolerman et al., 1995; Balfour et al., 2000).
The identity of the subtype(s) of nAChRs involved in nicotine-evoked excitation of DAergic neurones in the VTA, however, remains unclear, largely due to lack of selective pharmacological tools for different subtypes of nAChRs. TC-2559, a neuronal nicotinic receptor agonist (Bencherif et al., 2000), has been recently profiled and characterised in vitro as a selective α4β2 subtype partial agonist (Chen et al., 2003). An in vitro electrophysiology study using brain slice preparations also showed that TC-2559 increased VTA DA neuronal activity in a dose-dependent manner (Chen et al., 2003). Recent evidence from studies using genetically modified mice has implicated both α4- and β2-containing nAChRs as a major mediator of the nicotinic action in the DAergic system (Picciotto et al., 1998; Klink et al., 2001; Marubio et al., 2003).
We have profiled TC-2559 along with other nicotinic agonists and antagonists in in vivo studies in anaesthetised rats. The data support its selectivity as an α4β2-like nAChR agonist (Chen et al., 2003), and we have used this property to study the role of such nAChRs in the excitation of VTA DA neurones in animals with intact neuronal circuitry. In particular, we examined the effect of TC-2559 on the bursting activities of DA neurones, which was not presented in the earlier in vitro brain slice study (Chen et al., 2003).
A preliminary report of some of the present data has been published (Wang et al., 2002).
Methods
All the experiments were carried out under the Animals (Scientific Procedures) Act, 1986 and approved by the Eli Lilly & Co. Ethics Committee. Stock animals were kept under normal animal house conditions within the Eli Lilly animal facility, with a 12 h light–dark light schedule. At the end of the experiment, the animals were killed by an overdose of anaesthetic and exsanguination.
Brain exposure experiments
A total of 36 male Sprague–Dawley rats (300–370 g) were used in these experiments and all animals were anaesthetised with urethane (1.2 g kg−1, i.p.). The level of anaesthesia was assessed by the absence of a withdrawal reflex and, if necessary, additional anaesthetic (urethane, 0.4 g kg−1, i.p.) was administered. The lateral tail vein was cannulated for administration of drugs. Rats were killed by rapid intravenous overdose of urethane at different time points (1.5, 3, 6, 9, 15 and 30 min) after drug administration. Brain tissues were then removed for in vitro measurements. Brain samples were prepared by the addition of twice the volume (ml) of water to weight (g) of brain cortex, followed by homogenisation to produce a smooth suspension. Homogenised brain cortex samples (500 μl) were then extracted with methyl-t-butyl ether and centrifuged. The solvent layer was transferred to clean tubes and evaporated under nitrogen at 40°C. The residue was dissolved in 50% methanol and analysed using liquid chromatography and a Sciex API 3+ Mass Spectrometer, with turbo-ionspray in positive ion MRM mode, for detection. Data were calculated using MacQuan 1.4. In order to estimate the total brain concentration, 1 g of the brain tissue was assumed to be equivalent to be 1 ml in volume.
Electrophysiology experiments
General preparation
Experiments were carried out on 69 male Sprague–Dawley rats (260–350 g), anaesthetised with choral hydrate (400 mg kg−1, i.p.). The level of anaesthesia was assessed by the absence of a withdrawal reflex and cardiovascular response to paw-pinch and the stability of resting blood pressure and heart rate. Additional anaesthetic (chloral hydrate, 100–150 mg kg−1, i.v.) was administered as necessary.
Rectal temperature was monitored and maintained between 37±0.5°C with a Harvard Homeothermic Blanket. When surgical anaesthesia was established, the femoral artery was cannulated for recording blood pressure using a pressure transducer (Gould) connected to a Grass Model 7D Polygraph (Grass Medical Instruments, Quincy, MA, U.S.A.) and a lateral tail vein was cannulated for administration of drugs/fluids. The animals were then placed in a stereotaxic frame. A hole was drilled in the skull above the VTA area approximately 5.8 mm caudal to the bregma and 0.6 mm lateral to the midline.
A single glass microelectrode pulled from starbore glass capillary (Radnoti Glass Technology, Inc., Monrovia, CA, U.S.A.) and filled with a solution containing 2% Pontamine Sky Blue in 2 M NaCl, with an in vitro impedance of ∼10 MΩ, was lowered into the brain using a Burleigh 6000ULN Controller (Burleigh Instrument, Burleigh Park, Fishers, NY, U.S.A.). Single-unit activity of VTA neurones was found within the co-ordinates: 5.6–6.0 mm posterior to the bregma, 0.5–0.8 mm lateral to the midline and 6.7–8.5 mm below the brain surface. DAergic VTA neurones were identified by their characteristic triphasic action potentials of more than 2 ms duration and firing rate of 1–8 Hz, as described in detail previously (Guyenet & Aghajanian, 1978, Grenhoff et al., 1986). At the end of some experiments, inhibition by apomorphine (15–60 μg kg−1, i.v.) was used to confirm the DAergic nature of the recorded neurone (Mereu et al., 1987). In some experiments, current deposition of Pontamine Sky Blue with subsequent histology was used to confirm the location of the recording site. Recording from only one cell was made in each animal and any cells outside the VTA, as marked histologically, were not included in the analysis.
Experimental protocol
Physiological saline of the same volume and concentration as for drug administration was injected i.v. following at least 3–5 min of stable neuronal recording. Then, 3 min after this saline vehicle, drugs were applied (i.v.) either cumulatively with an interdose interval of 3 min or as a bolus dose. At the end of the cumulative dose regimen, any neuronal response was challenged by i.v. injection of DHβE 5 min after the last agonist dose. For the bolus dosing study, the antagonist was injected at least 5 min after the testing drug or when the neuronal response had stabilised. In some experiments, a further dose of nicotine (or test compound) was injected 3–5 min after DHβE administration.
Iontophoresis study
Extracellular recordings were made from VTA neurones using seven-barrelled glass microelectrodes (tip diameter 5–7 μm; Clark Electromedical, GC 150F-10) as described previously (Wang & Ramage, 2001). The recording barrel contained 2 M sodium chloride, and the other barrels contained Pontamine Sky Blue (2% in 2 M NaCl) for current balancing and dye ejection, and the drugs TC-2559 (20 mM in 150 mM NaCl, pH 4.5) and DHβE (20 mM in 150 mM NaCl, pH 4.5). Drugs were administered in the vicinity of the neurones by iontophoresis (Neurophore, Medical System Inc., Great Neck, NY, U.S.A.). Drugs were ejected using positive currents (with a retaining current of 15–20 nA applied between ejection periods). Responses were classified as excitation or inhibition if, during the ejection period, activity was increased or decreased by at least 20% of the baseline (Wang & Ramage, 2001).
Data capture and analysis
Neuronal activity was amplified × 2000 and filtered (0.3–3 kHz; Dagan Corporation, Minneapolis, MN, U.S.A.). Arterial blood pressure (BP) and neuronal activity were displayed on a computer using an AD interface (CED 1401 micro, Cambridge) and Spike2 software (CED), and stored on the hard disk of a Pentium III computer and subsequently copied to CD discs. Off-line analysis of the recorded data was made using Spike2 software.
Burst firing of VTA DAergic neurones was analysed, according to Grace & Bunney (1984) and Grenhoff & Svensson (1993), using the burst analysis program of Spike2 software. The burst was defined to start when an interspike interval was shorter than 80 ms and to terminate when the interval became greater than 160 ms. The number of spikes in the bursts was then expressed as a % of the total number of spikes in 3-min epochs. If the number of spikes in the bursts was less than 5% of the total spikes in a given period (3 min), the neurone was defined as a nonbursting VTA neurone. Baseline values for neuronal firing were taken as the mean over 3 min before the administration of saline and/or drug and then standardised to 100%. After the drug administration, the firing rate and bursting rate were taken as the mean over 1-min epochs and then compared to the baseline value and expressed as a % of the pre-drug control.
All data are presented as mean±s.e.m., and all comparisons of the means were made using one-way ANOVA with post-hoc Dunnett's test and/or Student's t-test. Differences between means were taken as significant when P<0.05.
Localisation of recording sites
Recording sites were marked by iontophoretic ejection of Pontamine Sky Blue at the end of the recordings. Rat brains were then removed and fixed in 10% formol saline, and serial frozen sections (50 μm) were cut and counterstained with neutral red. The marked recording sites were displayed on standard sections of brain taken from the stereotaxic atlas of the rat brain (Paxinos & Watson, 1986).
Drugs
Drugs were obtained from the following sources: chloral hydrate, dihydro-β-erythroidine hydrobromide (DHβE) and apomorphine-HCl from Sigma Aldrich Chemical Co., Poole, Dorset, U.K.; Pontamine Sky Blue dye from BDH, Poole, Dorset, U.K.; nicotine tartrate from Research Biochemicals, Semat Technical Ltd, St Albans, Herts.; methyllycaconitine (MLA) from Tocris Cookson, Bristol; (E)-N-methyl-4-[3-(5-ethoxypyridin)y1]-3-buten-1-amine demi galactarate (TC-2559) and (R)-(−)-5′-phenylspiro[1-azabicyclo[2.2.2]octane-3,2′-(3′H)furo[2,3-b]pyridine (PSAB-OFP of Broad et al., 2002; Compound 35 of Astles et al., 2002) were synthesised at the Lilly Research Centre, Windlesham, Surrey, U.K.
All the drugs were dissolved in 0.9% saline and injected intravenously in a volume of 1 ml kg−1. The doses for the drugs are all related to their freebase.
Results
Brain exposure results
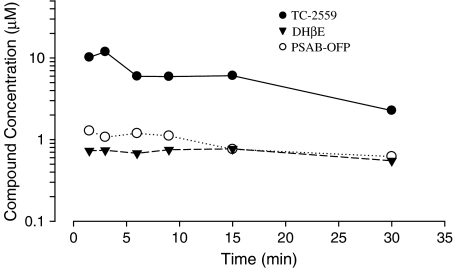
In order to identify the appropriate systemic dose for the in vivo electrophysiology studies, three of the drugs (TC-2559, DHβE and PSAB-OFP) used in present study were initially investigated for brain bioavailability. Bolus doses of TC-2559 (1.32 mg kg−1), DHβE (0.77 mg kg−1) or PSAB-OFP (0.23 mg kg−1) were injected intravenously and then the total brain concentrations were measured at 1.5, 3, 6, 9, 15 and 30 min after injection. TC-2559 (1.32 mg kg−1) had an initial brain concentration of ∼10 μM that peaked at 3 min, quickly declined and was then maintained at ∼6 μM for up to 15 min after drug administration. DHβE (0.77 mg kg−1) and PSAB-OFP (0.23 mg kg−1) had reasonably stable total brain concentrations of ∼0.8–0.7 and ∼1.2–0.7 μM, respectively, throughout the 1.5–30 min time range studied (Figure 1).
Figure 1.
Traces showing total brain concentration of TC-2559 (1.32 mg kg−1, i.v.), DHβE (0.77 mg kg−1, i.v.) and PSOB-OFP (0.23 mg kg−1, i.v.) from 1.5 to 30 min after i.v. bolus injection. (Each data point is an average from two animals.)
Electrophysiological results
A total of 66 VTA neurones from 66 rats were tested with systemic administration of drugs and three VTA neurones from three rats were studied with ionophoretic application of drugs. The mean arterial blood pressure during data acquisition for 66 rats was 70±2 mmHg (systolic: 92±2 mmHg and diastolic: 58±2 mmHg). In all, 23 recording sites within the VTA area were marked with Pontamine Sky Blue and recovered after histological processing. A further four marked sites were outside the VTA boundary, so that the data from these four neurones were discarded and not included in the 69 neurones reported here. In addition, 25 of the 66 VTA neurones (37%) were tested with intravenous administration of low doses of apomorphine (13–52 μg kg−1), which inhibited spontaneous firing of all the neurones tested by 76±5% (n=25) in firing. Given the nature of the location, firing pattern and the spike shape of the neurones, and that all of a random sample of 25 neurones were inhibited by apomorphine, we are confident that the majority of the neurones analysed were DAergic neurones of the VTA.
The mean firing frequency for 66 VTA neurones, tested with systemic drug administration, was 4.3±0.2 Hz. Among them, 47 neurones were classified as bursting VTA neurones (see Methods), with a firing frequency of 4.8±0.2 Hz and a bursting activity of 36±3%. The remaining 19 neurones were classified as nonbursting neurones, with a mean firing frequency of 3.2±0.3 Hz and a bursting rate of 2.0±0.5%.
Effect of broad-spectrum nAChR agonist, nicotine
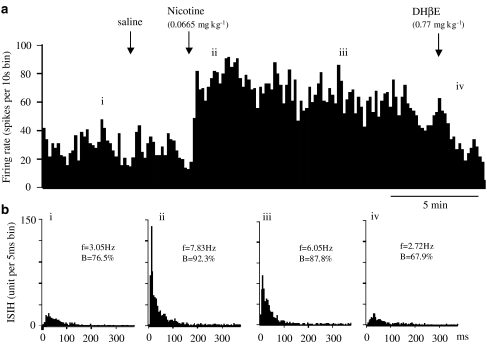
As a standard for comparison with the studies of the selective agonists, we examined the effect of nicotine on 15 VTA DA neurones, eight of which were classified as bursting neurones, and largely confirmed previous findings (Grenhoff et al., 1986; Erhardt et al., 2002). A single intravenous dose of nicotine (0.0665 mg kg−1) evoked a sustained excitation for more than 5 min in 12 of the 15 neurones tested (Figure 2). Of the remaining three, nicotine evoked biphasic excitation-inhibition on one, inhibition-excitation on another and no effect on the remaining neurone. As a whole group (n=15), nicotine increased firing rate peaked in the second minute at 147±15% of the baseline rate (P<0.01), whereas the saline vehicle was without effect (Figure 2).
Figure 2.
Effect of nicotine and subsequent doses of DHβE on the firing and bursting rates of a VTA DA neurone in choral hydrate anaesthetised rat. (a) Rate histogram showing that bolus dose of nicotine (0.0665 mg kg−1) increased neuronal firing. Arrows indicate the time when saline, nicotine or DHβE was injected intravenously. ‘i–iv' above the rate histogram indicate the periods when the inter-spike interval histograms (ISIHs) in (b) were recorded. (b) ISIH showing nicotine also increased bursting activity. (i) control; (ii, iii) after nicotine injection; (iv) after DHβE injection. Both the rate and bursting change evoked by nicotine were reversed by DHβE (0.77 mg kg−1, i.v.). (In (b), f=firing frequency, B=% of spikes in burst.)
Nicotine (0.0665 mg kg−1, i.v.) also increased the bursting rate in seven of the eight bursting VTA DA neurones. As a group, the increases of bursting rate at the fifth minute after nicotine injection was 154±25% of the baseline control, which is significantly higher than saline controls (90±9% n=5, P<0.05). Nicotine also induced bursting in five of seven nonbursting VTA DA neurones tested.
Nicotine (0.0665 mg kg−1, i.v.) affected the bursting and nonbursting neurones to a similar extent. Thus, the peak firing at 2 min after nicotine for the bursting and nonbursting groups was 150±18% (n=8) and 144±26% (n=7) of baseline values, respectively.
Effect of selective α4β2 agonist, TC-2559
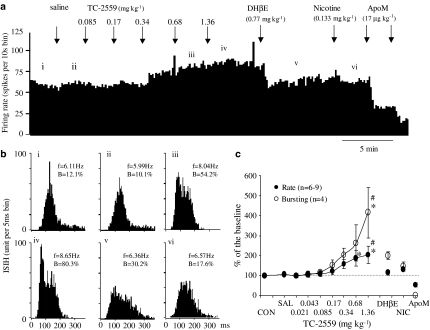
(a) Cumulative dosing: The initial study of the α4β2 agonist, TC-2559, was carried out to study the dose–response relationship for TC-2559 on VTA neurones. Cumulative doses of TC-2559 (0.021–1.36 mg kg−1, i.v.) dose-dependently resulted in a sustained increase in the firing rate (P<0.05) of the nine VTA DAergic neurones tested. The firing rate increases at cumulative doses of approximately 0.68 and 1.36 mg kg−1 (i.v.) were 185±48% (n=5) and 206±51% of the base firing rate, respectively (Figure 3). This suggests that the 0.68 and 1.36 mg kg−1 doses are similar and near maximal.
Figure 3.
Effect of cumulative doses of TC-2559 and subsequent doses of DHβE and nicotine on the firing and bursting rates of a VTA DA neurone in choral hydrate anaesthetised rat. (a) Rate histogram showing that cumulative doses of TC-2559 (0.085, 0.17, 0.34, 0.68 and 1.36 mg kg−1, intravenously at arrows) dose dependently increased neuronal firing. ‘i–vi' above the rate histogram indicate the periods (3 min) when the ISIHs in (b) were recorded. (b) ISIHs showing cumulative doses of TC-2559 also increased the bursting activity. (i) Control; (ii) after saline injection, (iii, iv) after TC-2559 injection; (v) after DHβE injection and (vi) after nicotine injection. Both the rate and bursting changes evoked by TC-2559 were reversed by a single dose of DHβE (0.77 mg kg−1, i.v.). Subsequent application of nicotine failed to induce changes in either rate or bursting. (c) Group data showing TC-2559 significantly (*P<0.05) increased the VTA DA neurone activity, which was subsequently reversed by DHβE (#P<0.05). Neuronal activity inhibited by apomorphine (17–52 μg kg−1 (a, c)) confirmed that the recorded cells were DAergic neurones. (In (b), f=firing frequency, B=% of spikes in burst.)
Five of the above nine VTA neurones were characterised as bursting neurones, and, on these, cumulative dosing of TC-2559 was found to increase the bursting rate dose-dependently (P<0.05), whereas saline was without effect. The bursting rate increase, after a cumulative dose of approximately 0.68 and 1.36 mg kg−1 TC-2559, was 263±91% (n=5) and 415±125% (n=4) of the control bursting rate, respectively (Figure 3). TC-2559 also induced bursting in one of four nonbursting neurones.
(b) Bolus dosing: Bolus application of TC-2559 (0.66 or 1.32 mg kg−1, i.v.) mimicked the effect of nicotine, by evoking a sustained increase in both the spontaneous and burst firing.
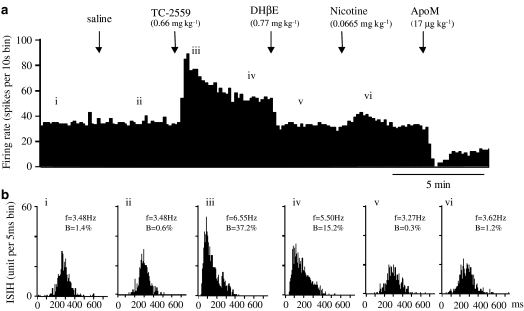
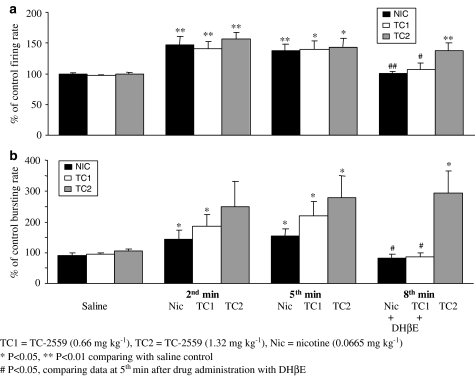
At 0.66 mg kg−1 bolus dose, TC-2559 (n=14) evoked a significant increase in VTA DA neuronal firing, throughout the 5-min testing period, compared with either control (P<0.05, n=14) or saline (P<0.01, n=11, Figure 4). The peak increase (141±12% of the base firing rate) occurred at the second minute in 14 VTA DA neurones tested (Figure 6). Similarly, in 12 bursting VTA DA neurones in this group, TC-2559 (0.66 mg kg−1) evoked a significant increase in bursting rate from the second minute after TC-2559 injection, which was maintained at least to the fifth minute compared to that either of control bursting rate (P<0.05, n=12) or bursting rate after saline injection (P<0.05, n=9). The peak increase in bursting activity occurred at the fifth minute to 220±44% of the baseline control (P<0.05, n=9, Figure 6). TC-2559 (0.66 mg kg−1) also induced bursting in one of two nonbursting VTA DA neurones tested. In this group of experiments, nicotinic receptor antagonist, DHβE, was administered 5 min after TC-2559 (see data below), so that the long-lasting effect of TC-2559 was not tested.
Figure 4.
Effect of TC-2559 and subsequent doses of DHβE, nicotine and apomorphine on the firing and bursting rates of a VTA DA neurone in choral hydrate anaesthetised rat. (a) Rate histogram showing that bolus dose of TC-2559 (0.66 mg kg−1) increased neuronal firing. Arrows indicate the time when saline or drugs were injected intravenously. ‘i–vi' above the rate histogram indicate the periods (3 min) when the ISIHs in (b) were recorded. (b) ISIH showing TC-2559 also increased the bursting activity. (i) Control; (ii) after saline; (iii, iv) after TC-2559; (v) after DHβE; and (vi) after nicotine injection. Both the rate and bursting changes evoked by TC-2559 were reversed by a single dose of DHβE (0.77 mg kg−1, i.v.). Subsequent application of nicotine (0.0665 mg kg−1) failed to induce changes in either rate or bursting. Neuronal activity inhibited by apomorphine (17 μg kg−1, i.v.; (a) confirmed that the recorded cell was DAergic neurone). (In (b), f=firing frequency, B=% of spikes in burst.)
Figure 6.
Group data of the effects of single doses of drugs on the firing rate (a) and the bursting rate (b) of the VTA DA neurones. Bar histograms represent the group data (mean±s.e.m.) of nicotine (0.0665 mg kg−1, n=15 in (a) and n=8 in (b)), TC-2559 (0.66 mg kg−1, n=14 in (a) and n=12 in (b)) and TC-2559 (1.32 mg kg−1, n=12 in (a) and n=9 in (b)). Statistics: *P<0.05 compared with saline control, and #P<0.05 of the fifth minute data after drug injection compared with the data taken after DHβE (0.77 mg kg−1) injection.
The long-lasting action of TC-2559 on the VTA DAergic neurones was tested with the bolus dose at 1.32 mg kg−1. In all 12 VTA DA neurones tested, TC-2559 (1.32 mg kg−1) evoked a significant increase in both spontaneous and burst firing for up to 15 min of the testing period. At this dose, TC-2559 significantly (P<0.05) evoked increases both in basal firing rate from the second minute and in the bursting rate from the fifth min after i.v. administration until at least to the 10th minute. Thus, at the second min after administration, the firing rate and bursting rate were 156±11% (n=12, P<0.05) and 250±82% (n=9, P=0.1) of pre-drug values, respectively, and at the 10th minute these values were 132±10% (n=11, P<0.05) and 290±86% (n=8, P<0.05), respectively (data not shown). TC-2559 at 1.32 mg kg−1 dose did not induce bursting in any of the three nonbursting neurones tested.
Bursting and nonbursting neurons were affected to the same extent by TC-2559. Thus, at 2 min after TC-2559 (1.32 mg kg−1, i.v.), the peak firing rate was 151±29% for three nonbursting neurones and 158±12% for nine bursting neurones.
By comparing the data above for bolus and cumulative dosing, it can be seen that there are only minor differences between the two modes of administration, and similarly, the effects of TC-2559 were long-lasting (see Figure 6). Together, these results suggest that there is minimal desensitisation of this excitatory response.
Effect of α4β2 preferring antagonist, DHβE
The α4β2 preferring antagonist, DHβE (Harvey et al., 1996; Chavez-Noriega et al., 1997; Holladay et al., 1997), was used in this study to confirm that agonist-evoked excitation was due to activation of α4β2 receptors. DHβE at either 0.39 or 0.77 mg kg−1 (i.v.) doses reversed the effects of both agonists (nicotine, n=10; cumulative TC-2559, n=5; 0.66 mg kg−1 TC-2559, n=11; 1.32 mg kg−1 TC-2559, n=4) on firing rate increases from 149±6 to 100±4% (P<0.001) of the baseline firing rate. On 21 bursting neurones, DHβE reversed the effects of both agonists (nicotine, n=6; cumulative TC-2559, n=3; 0.66 mg kg−1 TC-2559, n=8; 1.32 mg kg−1 TC-2559, n=4) on bursting rate increases from 265±39 to 104±11% (P<0.001) of the baseline bursting rate (see Figures 2, 3, 4, 5 and 6).
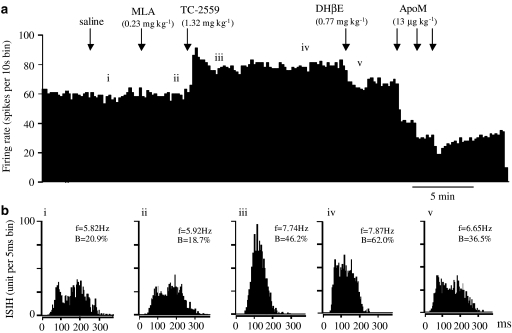
Figure 5.
Effect of MLA on the excitatory action of TC-2559 on the firing and bursting rates of a VTA DA neurone in choral hydrate anaesthetised rat. (a) Rate histogram showing with MLA (0.23 mg kg−1) pre-treatment, bolus dose of TC-2559 (1.32 mg kg−1) increased neuronal firing. Arrows indicate the time when saline, MLA, TC-2559 and DHβE was injected intravenously (note the long-lasting effect, >10 min, of the TC-2559). ‘i–v' above the rate histogram indicate the periods (3 min) when the ISIHs in (b) were recorded. (b) ISIHs showing TC-2559 also increased the bursting activity, despite the pre-treatment of the MLA. (i) saline injection; (ii) after MLA injection; (iii, iv) after TC-2559 injection; (v) after DHβE injection. Both the rate and bursting changes evoked by TC-2559 were reversed by a single dose of DHβE (0.77 mg kg−1). Neuronal activity inhibited by apomorphine (13 × 3 μg kg−1 (a)) confirmed that the recorded cell was DAergic neurone. (In (b), f=firing frequency, B=% of spikes in burst.)
There was no difference in terms of two different doses of DHβE used (0.39 or 0.77 mg kg−1), both fully reversing agonist(s)-evoked excitation to the baseline value. Thus, DHβE at 0.39 mg kg−1 reversed agonist-evoked firing rate increases from 138±7 to 97±3% (n=9), and the bursting rate increases from 150±21 to 106±10% in four bursting neurones. Similarly, DHβE at 0.77 mg kg−1 reversed agonist-evoked firing rate increases from 153±8 to 101±5% (n=21) and the bursting rate increases from 292±46 to 103±14% in 17 bursting neurones.
Effect of nicotine after DHβE
In order to establish whether any other nAChR subtypes, in addition to α4β2, are involved in nicotine-induced excitation of VTA DA neurones, nicotine was administered after DHβE (0.39 or 0.77 mg kg−1, i.v.). In nine VTA DA neurones tested, nicotine at either 0.0665 (n=5) or 0.133 (n=4) mg kg−1 applied 5 min after DHβE failed to evoke any changes in either firing rate (108±7%, n=9) or bursting rate (121±22%, n=6 bursting neurones; Figures 3, 4 and 6).
Effect of a selective α7 antagonist, methyllycaconitine (MLA)
As α7 nAChR receptors have also been implicated in VTA excitability (see Discussion for references), we examined whether TC-2559 has any activity for α7 nAChR receptors on the VTA DAergic neurones by using the selective α7 antagonist methyllycaconitine (MLA), at a dose of 0.23 mg kg−1 (i.v.), which is known to reverse the inhibitory effect of the α7 agonist, PSAB-OFP, on the blink reflex in Sprague–Dawley rats in vivo (Kulla & Lodge, unpublished data).
MLA (0.23 mg kg−1, i.v.) for 3 min, before the agonist challenge, had no effect on base firing and bursting rate, 103% (n=9) and 100% (n=7) comparing with the control baseline, respectively (Figure 5). TC-2559 at 1.32 mg kg−1 (i.v.), 3 min after MLA, evoked firing rate increase in all nine and bursting rate increase in six of seven bursting neurons tested. TC-2559 increased the firing rate to 147±6% (n=9, P<0.01) peaked at the first min and the bursting rate to 501±115% (n=6, P<0.05) peaked at the eighth minute (Figure 5). These values are not statistically different from those evoked by TC-2559 at 1.32 mg kg−1 (i.v.) alone (156±11% (n=12) for rate and 290±86% (n=8) for bursting).
Effect of a selective α7 agonist, PSAB-OFP
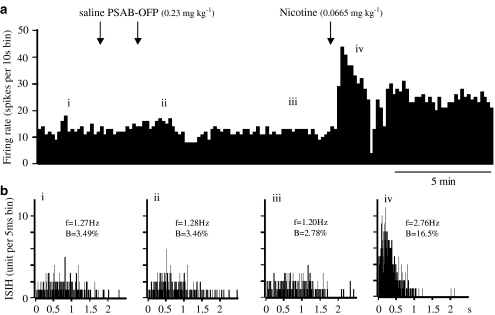
This apparent and surprising lack of involvement of α7 nAChRs on the excitability of VTA DA neurones was further tested by administering a selective α7 receptor agonist, PSAB-OFP (Astles et al., 2002; Broad et al., 2002) to seven rats, while recording from VTA neurones as characterised in the Methods section. Intravenous application of PSAB-OFP at 0.23 mg kg−1 evoked a profound short-lasting bradycardia and hypotension (data not shown), but had no effect on the neuronal firing rate and bursting rate in any of the seven neurones tested up to 15 min after administration (Figure 7). Subsequent application of nicotine (0.0665 mg kg−1, i.v.) evoked increases in both firing rate and bursting rate of 128±7% (n=4) and 143±40% (n=3), respectively, in all four neurones tested (Figure 7), not significantly different from the effect of nicotine in untreated rats, in which the respective values were 147±15% (n=15) and 154±25% (n=8).
Figure 7.
Effect of PSAB-OFP and subsequent doses of nicotine on the firing and bursting rates of a VTA DA neurone. (a) Rate histogram showing that bolus dose of PSAB-OFP (0.23 mg kg−1) had no effect on firing rate, but nicotine (0.0665 mg kg−1) increased neuronal firing. Arrows indicate the time when saline, PSAB-OFP or nicotine was injected intravenously. ‘i–iv' above the rate histogram indicate the periods (3 min) when the inter spike interval histograms (ISIHs) in (b) were recorded. (b) ISIHs showing that PSAB-OFP had no effect but nicotine increased the bursting activity. (i) Control; (ii, iii) after PSAB-OFP injection; (iv) after nicotine injection. (In (b), f=firing frequency, B=% of spikes in burst.)
Effect of iontophoretic application of TC-2559 on the activity of VTA DA neurones
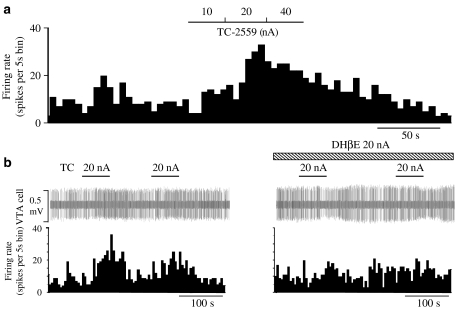
To investigate whether the excitation of VTA DA neurones is due to a direct activation of α4β2 nAChRs subtype within the VTA itself, TC-2559 and DHβE were administered into the vicinity of single VTA neurones by means of microiontophoresis. Iontophoretic application of TC-2559 (20–40 nA) evoked excitation (>20%) in all three VTA DA neurones tested (Figure 8). Co-application of DHβE (20 nA) blocked the TC-2559-evoked excitation in both neurones tested (Figure 8b).
Figure 8.
Effects of iontophoretic application of TC-2559 on the VTA DA neurone activity. Rate histogram showing that: (a) TC-2559 (10-40 nA) dose-dependently increased the firing rate and (b) TC-2559-evoked excitation was attenuated by co-application of DHβE (20 nA). The bars above the rate histogram indicate the time when drugs were ejected by iontophoresis.
Discussion
The major conclusion of this study is that TC-2559 is a useful tool for selectively activating α4β2 nAChRs in vivo. Following a single i.v. dose of 1.32 mg kg−1, TC-2559 achieves micromolar brain levels, and mimics the action of nicotine by increasing both the tonic firing and burst firing of VTA DA neurones. These actions of TC-2559 were blocked by DHβE, but not by MLA. An additional conclusion from the present study is that an α4β2-like receptor plays a dominant role in mediating nicotine-evoked excitation of these DAergic neurones.
These conclusions, however, depend on the effectiveness and selectivity of the compounds used and hence require some discussion. The drug doses used in this study were carefully chosen, with the aid of pharmacokinetic studies, so as to be (a) high enough to stimulate the receptors of interest, but (b) low enough not to affect other receptor subtypes. TC-2559 has been recently developed and characterised as a neuronal nAChR agonist (Bencherif et al., 2000) and selective for α4β2 subtype (Astles et al., 2002; Chen et al., 2003). Thus, the EC50 for TC-2559 to evoke excitation in VTA slice was found to be of 0.6 and 0.1 μM on α4β2-transfected HEK cells. On the other hand, TC-2559 is ineffective on α7 and α3β2 receptors up to 10 μM, and weakly effective on α2β4, α4β4 and α3β4 receptors, with EC50's in the range of 10–30 μM. In addition, the present brain exposure study demonstrated that TC-2559 at 1.32 mg kg−1 (i.v.) produced an initial peak whole brain concentration of about 12 μM (1.5–3 min after injection), which quickly declined to a maintained level of about 3–6 μM. Thus, at the 0.66 and 1.32 mg kg−1 i.v. doses used in this study, TC-2559 is likely to be selective for the α4β2 nicotinic receptor subtype.
Similarly, the initial brain exposure study showed that the whole brain concentration of DHβE, after 0.77 mg kg−1 i.v., reached a maximum of about 0.8 μM within 1.5 min, and was sustained for at least 30 min. This submicromolar concentration of DHβE is selective for α4β2 nAChRs (Holladay et al., 1997) or α4β2, α4β4 and α3β2 nAChRs (Harvey et al., 1996; Chavez-Noriega et al., 1997). Thus, the dose of DHβE used in the present study (0.39 and 0.77 mg kg−1, i.v.) was adequate for selective blockade of these α4 and β2 subunit-containing receptors. The α3β2 and α4β4 subtypes can, however, be excluded because TC-2559 is inactive on this subtype (Chen et al., 2003). Similarly, α3β4 heteromers can be excluded because concentrations of greater than 10 μM of TC-2559 (Chen et al., 2003) and DHβE (Harvey et al., 1996; Chavez-Noriega et al., 1997) are required for activity.
One potential issue with the design of the present studies is the role of desensitisation (Pidoplichko et al., 1997), which could underestimate the potency of agonists in cumulative dose–response curves and overestimate the potency of antagonists when given after the agonist. Indeed, peak responses to TC-2559 did decline and this may partly have been due to desensitisation, but also partly due to redistribution from the brain (see Figure 1). This decline was, however, not seen in the group data (see the right-hand columns of Figure 6). All the pharmacological data analysis in the present was taken from at least 3 min after agonist injection, when firing rates had essentially stabilised. Interestingly, the effect of a bolus dose of TC-2559 on a naïve preparation was not very different from the same dose given cumulatively (compare the effects of TC-2559 on % increase in firing in Figures 3, 4 and 5). Hence, although desensitisation cannot be disregarded, measuring effects at 3 min or more after agonist injection should allow a meaningful pharmacological analysis.
Our conclusion regarding the importance of α4β2-containing nAChRs in nicotine-evoked excitation of VTA DA neurones in vivo is supported by other published studies. Both α4 and β2 subunit proteins are strongly expressed in midbrain DAergic neurones (Goldner et al., 1997; Sorenson et al., 1998); mice lacking the α4 subunit have reduced nicotine-evoked currents in VTA neurones (Champtiaux et al., 2003; Marubio et al., 2003), and the effects of nicotine on the stress-induced cortical DA response are blocked by DHβE (George et al., 2000). Similarly, in β2 mutant mice, nicotine does not stimulate DA release in the ventral striatum nor evoke currents in DA neurones (Picciotto et al., 1998). However, a role for more complex receptors, such as α4α6α5(β2)2 and (α4)2α5(β2)2, proposed to be present in most VTA DA neurones (Klink et al., 2001), cannot be ruled out. The involvement of α6 would, however, appear minimal, because MLA, a brain penetrant (Turek et al., 1995), a 30–40 nM potent α6 antagonist (Mogg et al., 2002; Zoli et al., 2002), was without effect on the TC-2559 increased excitability of VTA somata in vivo (this study) and both MLA and α-conotoxin MII were inactive in vitro (Chen et al., 2003). Furthermore, α6−/− knockout animals showed robust DA release in the striatum to systemically applied nicotine (Champtiaux et al., 2003). Similarly, the involvement of β3 subunits is likely to be minimal in the direct excitation of VTA cell bodies, because these receptors are also sensitive to α-conotoxin MII and are largely located on the DAergic terminals within the striatum (Cui et al., 2003).
Perhaps most surprisingly, our data do not provide any evidence for an important role for α7 nAChRs in the nicotinic excitation of DAergic VTA neurones, for which there is strong literature support. Firstly, 40% of VTA DA neurones express α7 subunit mRNA (Klink et al., 2001; Azam et al., 2002). Secondly, intra-VTA injection/infusion of MLA blocks nicotine-evoked increases of DA release in the nucleus accumbens (Schilstrom et al., 1998; Nomikos et al, 1999), nicotine-induced locomotion (Nomikos et al., 1999) and nicotine-induced potentiation of brain stimulation reward (Panagis et al., 2000). Thirdly, Schilstrom et al. (2003) reported that MLA blocked nicotine-evoked bursting activity of VTA DA neurones, while the firing rate increase by nicotine was sensitive to DHβE. Fourthly, α7 activation leads to glutamate release in the VTA (McGehee et al., 1995), leading to NMDA-receptor mediated excitation and burst firing of VTA neurones (Chergui et al., 1993) and NMDA receptor-dependent DA release in the nucleus accumbens (Balfour et al., 1996). However, in the present study, TC-2559, an ineffective ligand at α7 nAChRs, fully reproduced the effects of nicotine on VTA firing, implying that α7 nAChRs play only a minor role in this direct effect of nicotine on VTA neuronal excitability. Furthermore, MLA, at a dose known to inhibit α7 nAChRs in similar experimental conditions (Kulla & Lodge, unpublished data; see also Turek et al., 1995; Freir & Herron, 2003), had no effect on nicotine-evoked excitation and, at a brain concentration of less than 1 μM, DHβE, a nicotinic antagonist with little or no action on α7 nAChRs (Astles et al., 2002), fully reversed nicotine-evoked excitation. Finally, PSAB-OFP, a potent α7 agonist (Broad et al., 2002), at doses known to be centrally effective to inhibit blink reflex (Kulla & Lodge, unpublished data), did not alter the firing pattern of VTA neurones. Taken together, these data imply a minimal role for α7 nAChRs, at least under the present experimental conditions. Among possible explanations for the lack of α7 receptor-mediated excitation is that these receptors, including those in the VTA, undergo rapid desensitisation (Pidoplichko et al., 1997) and that, in the present experiments, there was only limited spontaneous glutamatergic input. Further characterisation of the role of α7 nicotinic receptors in mediating nicotinic function in midbrain DAergic neurones is needed.
Whether these excitatory actions of nicotine and TC-2559 are directly on DAergic neurones within the VTA cannot be ascertained from the present and previous systemic administration studies. Data from brain slice electrophysiology studies (Calabresi et al., 1989; Picciotto et al., 1998, 2001; Chen et al., 2003), and from in vivo intra-VTA injection of nicotinic antagonists which prevented DA release (Nisell et al., 1994b) as well as increased locomotion and Fos-like immunoreactivity in the nucleus accumbens (Panagis et al., 1996; Schilstrom et al., 2000), are in favour of a site of action within VTA. Our microiontophoretic results, in which all three DA neurones in VTA were excited by iontophoretic application of TC-2559, support this conclusion, but cannot rule out the indirect effects of nicotine and TC-2559, for example, via presynaptic actions on terminals synapsing on VTA DA neurones. Thus, GABAergic interneurones within the VTA express α3–α7 and β2–β4 subunit mRNAs (Klink et al., 2001; Azam et al., 2002) and putative α4β2 and α7 nAChRs (Klink et al., 2001). Previous in vivo studies, however, have demonstrated that nicotine increases GABA (and glutamate) release in the VTA (Erhardt et al., 2002) and hence the excitation observed on VTA DA neurones is unlikely to be due to the effects on GABAergic neurones. Indeed, anaesthesia is likely to facilitate the GABAergic activity in the VTA and may underestimate the nicotinic excitatory action on DAergic neurons in the present experiments. In support of a direct action on midbrain DA neurones are the observations that in vitro the excitatory effects of nicotine were tetrodotoxin insensitive and persisted in a low calcium buffer (Sorenson et al., 1998) and that the α4 and β2 subunits are indeed localised to the dendrites of these neurones (Sorenson et al., 1998).
In summary, the results of the present in vivo neuropharmacology study show that systemic or local application of selective nicotinic α4β2 receptor agonist TC-2559 mimics nicotine-evoked excitation of VTA DAergic neurones. Excitation evoked by either nicotine or TC-2559 is fully blocked by the same dose of DHβE, a selective α4β2 receptor antagonist. This implies, for the first time using pharmacological tools, that α4β2- or α4β2-like nAChRs are the dominant nAChRs mediating nicotinic excitation of VTA DAergic neurones in vivo, and hence make a major contribution to nicotinic modulation of the midbrain DAergic reward system (see Balfour et al., 2000). Thus, TC-2559 is a useful pharmacological tool for both in vivo (present data) and in vitro (Chen et al., 2003) study of nicotinic α4β2 receptor pharmacology. Future development of potent and selective α4β2 receptor ligands may lead to therapies for diseases in which abnormalities of the mesolimbic DA system are implicated.
Acknowledgments
We would like to thank Ann Bond and Jayne Hacker for technical assistance, and Eli Lilly Ltd for supplying TC-2559 and PSAB-OFP.
Abbreviations
- DA
dopamine
- DAergic
dopaminergic
- nAChR
nicotinic acetylcholinergic receptor
- VTA
ventral tegmental area
References
- ASTLES P.C., BAKER S.R., BOOT J.R., BROAD L.M., DELL C.P., KEENAN M. Recent progress in the development of subtype selective nicotinic acetylcholine receptor ligands. Curr. Drug Targets CNS & Neurol. Disord. 2002;1:337–348. doi: 10.2174/1568007023339256. [DOI] [PubMed] [Google Scholar]
- AZAM L., WINZER-SERHAN U.H., CHEN Y., LESLIE F.M. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J. Comp. Neurol. 2002;444:260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- BALFOUR D.J.K., BIRRELL C.E., MORAN R.J., BENWELL E.M. Effects of D-CPPene on mesoaccumbens dopamine responses to nicotine in the rat. Eur. J. Pharmacol. 1996;316:153–156. doi: 10.1016/s0014-2999(96)00792-3. [DOI] [PubMed] [Google Scholar]
- BALFOUR D.J.K., WRIGHT A.E., BENWELL E.M., BIRRELL C.E. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav. Brain Res. 2000;113:73–83. doi: 10.1016/s0166-4328(00)00202-3. [DOI] [PubMed] [Google Scholar]
- BENCHERIF M., BANE A.J., MILLER C.H., DULL G.M., GATTO G.J. TC-2559: A novel orally active ligand selective at neuronal acetylcholine receptors. Eur. J. Pharmacol. 2000;409:45–55. doi: 10.1016/s0014-2999(00)00807-4. [DOI] [PubMed] [Google Scholar]
- BERKE J.D., HYMAN S.E. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- BROAD L.M., FELTHOUSE C., ZWART R., MCPHIE G.I., PEARSON K.H., CRAIG P.J., WALLACE L., BROADMORE R.J., BOOT J.R., KEENAN M., BAKER S.R., SHER E. PSAB-OFP, a selective α7 nicotinic receptor agonist, is also a potent agonist of the 5-HT3 receptor. Eur. J. Pharmacol. 2002;452:137–144. doi: 10.1016/s0014-2999(02)02273-2. [DOI] [PubMed] [Google Scholar]
- CALABRESI P., LACEY M.G., NORTH R.A. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br. J. Pharmaol. 1989;98:135–140. doi: 10.1111/j.1476-5381.1989.tb16873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMPTIAUX N., GOTTI C., CORDERO-ERAUSQUIN M., DAVID D.J., PRZYBYLSKI C., LÉNA C., CLEMENTI F., MORETTI M., ROSSI F.M., LE NOVÉRE N., MCINTOSH J.M., GARDIER A.M., CHANGEUX J.P. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J. Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAVEZ-NORIEGA L.E., CRONA J.H., WASHBURN M.S., URRUTIA A., ELLIOTT K.J., JOHNSON E.C. Pharmacological characterisation of recombinant human neuronal nicotinic acetylcholine receptors hα2β2, hα2β4, hα3β2, hα3β4, hα4β2, hα4β4 and hα7 expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 1997;280:346–356. [PubMed] [Google Scholar]
- CHEN Y., SHARPLES T.J.W., PHILLIPS K.G., BENEDETTI G., BROAD L.M., ZWART R., SHER E. The nicotinic α4β2 receptor selective agonist TC-2559 increases dopamine neuronal activity in the ventral tegmental area of rat midbrain slices. Neuropharmacology. 2003;45:334–344. doi: 10.1016/s0028-3908(03)00189-8. [DOI] [PubMed] [Google Scholar]
- CHERGUI K., CHARLETY P.J., AKAOKA H., SAUNIER C.F., BRUNET J.L., BUDA M., SVENSSON T.H., CHAUVET G. Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurones in vivo. Eur. J. Neurosci. 1993;5:137–144. doi: 10.1111/j.1460-9568.1993.tb00479.x. [DOI] [PubMed] [Google Scholar]
- CHERGUI K., NOMIKOS G.G., MATHÉ J.M., GONON F., SVENSSON T.H. Burst stimulation of the medial forebrain bundle selectively increase Fos-like immunoreactivity in the limbic forebrain of the rat. Neuroscience. 1996;72:141–156. doi: 10.1016/0306-4522(95)00513-7. [DOI] [PubMed] [Google Scholar]
- CHERGUI K., SUAUD-CHAGNY M.F., GONON F. Nonlinear relationship between impulse flow, dopamine release and dopamine elimination in the rat brain in vivo. Neuroscience. 1994;62:641–645. doi: 10.1016/0306-4522(94)90465-0. [DOI] [PubMed] [Google Scholar]
- CLARKE P.B.S., PERT C.B., PERT A. Autoradiographic distribution of nicotine receptors in rat brain. Brain Res. 1984;323:390–395. doi: 10.1016/0006-8993(84)90320-2. [DOI] [PubMed] [Google Scholar]
- CLARKE P.B.S., SCHWARTZ R.D., PAUL S.M., PERT C.B., PERT A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J. Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUI C., BOOKER T.K., ALLEN R.S., GRADY S.R., WHITEAKER P., MARKS M.J., SALMINEN O., TRITTO T., BUTT C.M., ALLEN W.R., STITZEL J.A., MCINTOSH J.M., BOULTER J., COLLINS A.C., HEINEMANN S.F. The β3 nicotinic receptor subunit: A component of α-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J. Neurosci. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERHARDT S., SCHWIELER L., ENGBERG G. Excitatory and inhibitory responses of dopamine neurons in the ventral tegmental area to nicotine. Synapse. 2002;43:227–237. doi: 10.1002/syn.10044. [DOI] [PubMed] [Google Scholar]
- FISHER J.L., PIDOPLICHKO V.I., DANI J.A. Nicotine modifies the activity of ventral tegmental area dopaminergic neurons and hippocampal GABAergic neurons. J. Physiol. (Paris) 1998;92:209–213. doi: 10.1016/s0928-4257(98)80012-0. [DOI] [PubMed] [Google Scholar]
- FREIR D.B., HERRON C.E. Nicotine enhances the depressive actions of Aβ1-40 on long-term potentiation in the rat hippocampal CA1 region in vivo. J. Neurophysiol. 2003;89:2917–2922. doi: 10.1152/jn.00996.2002. [DOI] [PubMed] [Google Scholar]
- FU Y., MATTA S.G., GAO W., BROWER V.G., SHARP B.M. Systemic nicotine stimulates dopamine release in nucleus accumbens: re-evaluation of the role of N-methyl-D-aspartate receptors in the ventral tegmental area. J. Pharmacol. Exp. Ther. 2000;294:458–465. [PubMed] [Google Scholar]
- GEORGE T.P., VERRICO C.D., PICCIOTTO M.R., ROTH R.H. Nicotinic modulation of mesoprefrontal dopamine neurons: pharmacologic and neuroanatomic characterization. J. Pharmacol. Exp. Ther. 2000;295:58–66. [PubMed] [Google Scholar]
- GOLDNER F.M., DINELEY K.T., PATRICK J.W. Immunohistochemical localization of the nicotinic acetylcholine receptor subunit α6 to dopaminergic neurones in the substantial nigra and ventral tegmental area. Neuroreport. 1997;8:2739–2742. doi: 10.1097/00001756-199708180-00019. [DOI] [PubMed] [Google Scholar]
- GONON F.G. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- GRACE A.A., BUNNEY B.S. The control of firing pattern in nigral dopamine neurons: burst firing. J. Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRENHOFF J., ASTON-JONES G., SVENSSON T.H. Nicotine effects on the firing pattern of midbrain dopamine neurons. Acta. Physiol. Scand. 1986;128:351–358. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- GRENHOFF J., SVENSSON T.H. Prozosin modulates the firing pattern of dopamine neurons in rat ventral tegmental area. Eur. J. Pharmacol. 1993;233:79–84. doi: 10.1016/0014-2999(93)90351-h. [DOI] [PubMed] [Google Scholar]
- GRILLNER P., SVENSSON T.H. Nicotine-induced excitation of midbrain dopamine neurons in vitro involves ionotropic glutamate receptor activation. Synapse. 2000;38:1–9. doi: 10.1002/1098-2396(200010)38:1<1::AID-SYN1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- GUYENET P.G., AGHAJANIAN G.K. Antidromic identification of dopaminergic and other output neurons of the rat substantia nigra. Brain Res. 1978;150:69–84. doi: 10.1016/0006-8993(78)90654-6. [DOI] [PubMed] [Google Scholar]
- HARVEY S.C., MADDOX F.N., LUETJE C.W. Multiple determinants of Dihydro-β-Erythroidine sensitivity on rat neuronal nicotinic receptor α subunits. J. Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- HOLLADAY M.W., DART M.J., LYNCH J.K. Neuronal nicotinic acetylcholine receptors as targets for drug discovery. J. Med. Chem. 1997;40:4169–4194. doi: 10.1021/jm970377o. [DOI] [PubMed] [Google Scholar]
- KITAI S.T., SHEPARD P.D., CALLAWAY J.C., SCROGGS R. Afferent modulation of dopamine neuron firing patterns. Curr. Op. Neurobiol. 1999;9:690–697. doi: 10.1016/s0959-4388(99)00040-9. [DOI] [PubMed] [Google Scholar]
- KLINK R., D'EXAERDE A.D., ZOLI M., CHANGEUX J.P. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J. Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARUBIO L.M., GARDIER A.M., DURIER S., DAVID D., KLINK R., ARROYO-JIMENEZ M.M., MCINTOSH J.M., ROSSI F., CHAMPTIAUX N., ZOLI M., CHANGEUX J.P. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur. J. Neurosci. 2003;17:1329–1337. doi: 10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- MCGEHEE D.S., HEATH M.J.S., GELBER S., DEVAY P., ROLE L.W. Nicotine enhancement of fast excitatory transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- MCGEHEE D.S., ROLE L.W. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu. Rev. Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- MEREU G., YOON K.W.P., BOI V., GESSA G.L., NAES L., WESTFALL T.C. Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. Eur. J. Pharmacol. 1987;141:395–399. doi: 10.1016/0014-2999(87)90556-5. [DOI] [PubMed] [Google Scholar]
- MOGG A.J., WHITEAKER P., MCINTOSH J.M., MARKS M., COLLINS A.C., WONNACOTT S. Methyllycaconitine is a potent antagonist of α-conotoxin-MII-sensitive presynaptic nicotinic acetylcholine receptors in rat striatum. J. Pharmacol. Exp. Ther. 2002;302:197–204. doi: 10.1124/jpet.302.1.197. [DOI] [PubMed] [Google Scholar]
- NISELL M., NOMIKOS G.G., SVENSSON T.H. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994a;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- NISELL M., NOMIKOS G.G., SVENSSON T.H. Infusion of nicotine in the ventral tegmental area or the nucleus accumbens of the rat differentially affects accumbal dopamine release. Pharmacol. Toxicol. 1994b;75:348–352. doi: 10.1111/j.1600-0773.1994.tb00373.x. [DOI] [PubMed] [Google Scholar]
- NOMIKOS G.G., HILDEBRAND B.E., PANAGIS G., SVENSSON T.H. Nicotine withdrawal in the rat: role of α7 nicotinic receptors in the ventral tegmental area. Neuroreport. 1999;10:697–702. doi: 10.1097/00001756-199903170-00007. [DOI] [PubMed] [Google Scholar]
- PANAGIS G., KASTELLAKIS A., SPYRAKI C., NOMIKOS G. Effects of methyllycaconitine (MLA), an α7 nicotinic receptor antagonist, on nicotine- and cocaine-induced potentiation of brain stimulation reward. Psychopharmacology (Berlin) 2000;149:388–396. doi: 10.1007/s002130000384. [DOI] [PubMed] [Google Scholar]
- PANAGIS G., NISELL M., NOMIKOS G.G., CHERGUI K., SVENSSON T.H. Nicotine injections into the ventral tegmental area increase locomotion and Fos-like immunoreactivity in the nucleus accumbens of the rat. Brain Res. 1996;730:133–142. doi: 10.1016/0006-8993(96)00432-5. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates 1986New York: Academic Press; 2nd edn [Google Scholar]
- PICCIOTTO M.R., CALDARONE B.J., BRUNZELL D.H., ZACHARIOU V., STEVENS T.R., KING S.L. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol. Ther. 2001;92:89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- PICCIOTTO M.R., ZOLI M., RIMONDINI R., LÉNA C., MARUBIO L.M., PICH E.M., FUXE K., CHANGEUX J.P. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- PIDOPLICHKO V.I., DEBIASI M., WILLIAMS J.T., DANI J.A. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- PONTIERI F.E., TANDA G., ORZI F., DI CHIARA G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- SCHILSTROM B., DE VILLIERS S., MALMERFELT A., SVENSSON T.H., NOMIKOS G.G. Nicotine-induced Fos expression in the nucleus accumbens and the medial prefrontal cortex of the rat: role of nicotinic and NMDA receptors in the ventral tegmental area. Synapse. 2000;36:314–321. doi: 10.1002/(SICI)1098-2396(20000615)36:4<314::AID-SYN8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- SCHILSTROM B., RAWAL N., MAMELI-ENGVALL M., NOMIKOS G.G., SVENSSON T.H. Dual effects of nicotine on dopamine neurons mediated by different nicotinic receptor subtypes. In. J. Neuropsychopharmacol. 2003;6:1–11. doi: 10.1017/S1461145702003188. [DOI] [PubMed] [Google Scholar]
- SCHILSTROM B., SVENSSON H.M., SVENSSON T.H., NOMIKOS G.G. Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of α7 nicotinic receptors in the ventral tegmental area. Neuroscience. 1998;85:1005–1009. doi: 10.1016/s0306-4522(98)00114-6. [DOI] [PubMed] [Google Scholar]
- SORENSON E.M., SHIROYAMA T., KITAI S.T. Postsynaptic nicotinic receptors on dopaminergic neurones in the substantia nigra pars compacta of the rat. Neuroscience. 1998;87:659–673. doi: 10.1016/s0306-4522(98)00064-5. [DOI] [PubMed] [Google Scholar]
- STOLERMAN I.P., MIRZA N.R., SHOAIB M. Nicotine psychopharmacology: addiction, cognition and neuroadaptation. Med. Res. Rev. 1995;15:47–72. doi: 10.1002/med.2610150105. [DOI] [PubMed] [Google Scholar]
- TUREK J.W., KANG C.-H., CAMPBELL J.E., ARNERIC S.P., SULLIVAN J.P. A sensitive technique for the detection of the α7 neuronal nicotinic acetylcholine receptor antagonist, methyllycaconitine, in rat plasma and brain. J. Neurosci. Methods. 1995;61:113–118. doi: 10.1016/0165-0270(95)00032-p. [DOI] [PubMed] [Google Scholar]
- WANG Y., RAMAGE A.G. The role of central 5-HT1A receptors in the control of B-fibre cardiac and bronchoconstrictor vagal preganglionic neurones in anaesthetized cats. J. Physiol. 2001;536:753–767. doi: 10.1111/j.1469-7793.2001.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y., SHERWOOD J.L., LODGE D.Activation of α4β2 subtype of nicotinic acetycholine receptors (nAChR) excites dopaminergic neurones in the ventral tegmental area in anaesthetised rats FENS Abstr. 20021P143.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOLI M., MORETTI M., ZANARDI A., MCINTOSH J.M., CLEMENTI F., GOTTI C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J. Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]