Abstract
In the present study, we examined the pharmacological activity of the putative κ3-opioid receptor agonist naloxone benzoylhydrazone (NalBzoH) at recombinant human opioid receptors individually expressed in Chinese hamster ovary (CHO) cells and native opioid receptors present in rat striatum.
At the μ-opioid receptor (MOR), NalBzoH stimulated guanosine-5′-O-(3-[35S]thio)triphosphate ([35S]GTPγS) binding (pEC50=8.59) and inhibited cyclic AMP accumulation (pEC50=8.74) with maximal effects (Emax) corresponding to 55 and 65% of those obtained with the MOR agonist DAMGO, respectively. The MOR antagonist CTAP blocked the stimulatory effects of NalBzoH and DAMGO with similar potencies.
At the κ-opioid receptor (KOR), NalBzoH stimulated [35S]GTPγS binding (pEC50=9.70) and inhibited cyclic AMP formation (pEC50=9.45) as effectively as the selective KOR agonist (−)-U-50,488. The NalBzoH effect was blocked by the KOR antagonist nor-binaltorphimine (nor-BNI) (pKi=10.30).
In CHO cells expressing the δ-opioid receptor (DOR), NalBzoH increased [35S]GTPγS binding (pEC50=8.49) and inhibited cyclic AMP formation (pEC50=8.61) almost as effectively as the DOR agonist DPDPE. Naltrindole (NTI), a selective DOR antagonist, completely blocked the response to NalBzoH (pKi of 10.40).
In CHO cells expressing the nociceptin/orphanin FQ (N/OFQ) receptor (NOP), NalBzoH failed to exert agonist effects and antagonized the agonist-induced receptor activation.
When compared to other opioid receptor ligands, NalBzoH showed an efficacy that was lower than that of morphine at MOR, but higher at KOR and DOR.
In rat striatum, NalBzoH enhanced [35S]GTPγS binding and inhibited adenylyl cyclase activity. These effects were antagonized by either CTAP, nor-BNI or NTI, each antagonist blocking a fraction of the NalBzoH response.
These data demonstrate that NalBzoH displays agonist activity at MOR, DOR and KOR expressed either in a heterologous cell system or in a native environment.
Keywords: Naloxone benzoylhydrazone, recombinant human opioid receptors, rat striatum, [35S]GTPγS binding, receptor binding, cyclic AMP
Introduction
The naloxone derivative naloxone benzoylhydrazone (NalBzoH) is currently used as a pharmacological tool in opioid receptor research. The early observations that in rat and mouse NalBzoH caused analgesia independently of μ- (MOR), δ- (DOR) and κ- (KOR) opioid receptors led to the proposal that the compound behaved as an agonist of a novel subtype of KOR, termed κ3 (Gistrak et al., 1989; Paul et al., 1990). Radioligand binding studies demonstrated that [3H]NalbzoH bound with high affinity to κ3-opioid sites, which were pharmacologically characterized as being relatively insensitive to the KOR agonists U-50,488 and U-69,593 and the KOR antagonist nor-binaltorphimine (nor-BNI) (Clark et al., 1989). [3H]NalbzoH-labelled κ3-opioid receptors were identified in different neuronal cell systems, including calf striatum (Clark et al., 1989), mouse, rat and guinea-pig brain (Cheng et al., 1992; Berzetei-Gurske et al., 1995) and BE(2)-C and SH-SY5Y neuroblastoma cell lines (Standifer et al., 1994; Cheng et al., 1995). In addition, functional studies performed in these cell lines showed that NalBzoH inhibited cyclic AMP accumulation with a pharmacological profile consistent with the involvement of κ3 opioid receptors (Standifer et al., 1994; Cheng et al., 1995; Mathis et al., 2001).
Besides being considered as a selective agonist of the pharmacologically identified κ3-opioid receptor (Reisine & Pasternak, 1996; Pick et al., 1997; Mogil & Pasternak, 2001; Minowa et al., 2003), NalBzoH has also been shown to bind to other opioid receptors (Clark et al., 1989; Price et al., 1989; Standifer et al., 1991; Ciszewska et al., 1996; Cox et al., 2005), at which it was generally reported to display antagonist activity. For instance, NalBzoH was found to behave as a potent MOR antagonist both in vivo (Gistrak et al., 1989; Paul et al., 1990; France & Woods, 1992) and in vitro (Berzetei-Gurske et al., 1995; Brown & Pasternak, 1998). In addition, NalBzoH was reported to antagonize the analgesia elicited by DOR and KOR agonists in mice (Paul et al., 1990) and to act as a mixed antagonist/partial agonist at KOR in isolated preparations of peripheral tissues (Berzetei-Gurske et al., 1995). With the cloning of a new member of the opioid receptor family, the NOP receptor (previously termed ORL1) (for a review, see Meunier, 2000; Mogil & Pasternak, 2001) for the endogenous peptide nociceptin/orphanin FQ (N/OFQ) (Meunier et al., 1995; Reinscheid et al., 1995), it became apparent that, differently from many other opioid receptor ligands, NalBzoH acted also as an antagonist at this receptor and the compound has been subsequently employed by many investigators to demonstrate the involvement of the NOP receptor in the behavioural and cellular effects of N/OFQ (Abdulla & Smith, 1997; Nabeshima et al., 1999; Calo' et al., 2000; Chiou, 2001; Flau et al., 2002). Furthermore, Noda et al. (1998) observed that the NalBzoH-induced analgesia was absent in NOP receptor-deficient mice, indicating that blockade of the NOP receptor, rather than stimulation of the κ3-opioid receptors, might mediate the in vivo action of NalBzoH.
We have recently found that in specific layers of the rat main olfactory bulb, NalbzoH caused G protein activation and modulated adenylyl cyclase activity, inducing either stimulation or inhibition of cyclic AMP formation (Onali & Olianas, 2004). By using selective agonists and antagonists, we demonstrated that NalBzoH acted independently of KOR and NOP receptors and exerted its agonist activity through the stimulation of both MOR and DOR. Because these observations were in contrast with the pharmacological profile of NalBzoH reported in the literature, we decided to further investigate the action of the compound on opioid receptors expressed in well-defined cell systems. In the present study, we characterized the activity of the compound at the four cloned human opioid receptors heterologously expressed in Chinese hamster ovary (CHO) cells. Moreover, we also examined the action of the compound at the opioid receptors endogenously expressed in rat striatal membranes.
Methods
Cell culture
CHO-K1 cells stably expressing the human MOR-1 (CHO/MOR), DOR (CHO/DOR), KOR (CHO/KOR) and NOP receptor (CHO/NOP) were grown as a monolayer culture in tissue culture flasks (Falcon), which were incubated at 37°C in a humidified atmosphere (5% CO2) in Ham's F12 medium (GIBCO BRL) containing L-glutamine and sodium bicarbonate and supplemented with 10% heat inactivated foetal calf serum (GIBCO BRL), 0.5% penicillin/streptomycin (GIBCO BRL) and either 350 μg ml−1 hygromycin (GIBCO BRL) for CHO/DOR or 400 μg ml−1 geneticin (GIBCO BRL) for the other recombinant cell lines.
Cell membrane preparation
Cells were grown in 100 mm plastic Petri dishes (Falcon), the culture medium was removed and the cells were washed with ice-cold phosphate-buffered saline. Thereafter, the cells were scraped into an ice-cold buffer containing 10 mM HEPES/NaOH (pH 7.4) and 1 mM EDTA and lysed with a Dounce tissue grinder. The cell lysate was centrifuged at 1000 × g for 2 min at 4°C. The supernatant was collected and centrifuged at 32,000 × g for 20 min at 4°C. The pellet was resuspended in the homogenization buffer at a protein concentration of 1.0–1.5 mg ml−1 and stored in aliquots at −80°C.
Dissection of rat striatum and membrane preparation
Male Sprague–Dawley rats (200–300 g) were used. Animals were maintained in a 12 h light/dark cycle with food and water ad libitum. Experiments were performed according to the principles of laboratory animal care (Law on animal experiments in Italy, D.L. 116/92). Rats were killed by decapitation. The brain was rapidly removed from the skull placed in ice-cold phosphate-buffered saline and the meninges and major blood vessels were peeled off. The brain was then placed on its dorsal surface, exposing the ventral surface of the forebrain. Using a razor blade, the first transverse cut was made through the forebrain anterior at the level of the optic chiasm followed by a second transverse cut at the level of the hypothalamus. The tissue block was placed on a tissue slicer with the dorsal surface down. 300 μm-thick sections were cut, one at a time. Each section was immediately placed in ice-cold phosphate buffered saline until it was dissected. Individual sections were transferred to a glass slide and, by using a dissecting microscope with a diascopic illuminator base, the dorsal striatum was dissected with small knifes. The tissue fragments from individual slices were pooled and homogenized in an ice-cold buffer containing 10 mM HEPES/NaOH, 1 mM EGTA, 1 mM MgCl2, 1 mM dithiothreitol, 0.1 mM phenylmethylsulphonyl fluoride, 50 kallikrein inhibitor units (KIU) ml−1 of aprotinin and 10 μg ml−1 of soyabean trypsin inhibitor (pH 7.4), using a Teflon/glass tissue grinder. The homogenate was centrifuged at 27,000 × g for 20 min at 4°C. The pellet was resuspended in the same buffer and centrifuged again. The final pellet was resuspended to a protein concentration of 0.8–1.0 mg ml−1 and either used immediately for the adenylyl cyclase assay or stored at −80°C for the binding assays.
Assay of [35S]GTPγS binding
Cell and tissue membranes were diluted 10-fold in an ice-cold buffer containing 10 mM HEPES/NaOH and 1 mM EDTA (pH 7.4), centrifuged at 32,500 × g for 30 min at 4°C and resuspended in the same buffer containing 0.1% bovine serum albumin (BSA). The binding of [35S]GTPγS was assayed in a reaction mixture (final volume 100 μl) containing 25 mM HEPES/NaOH (pH 7.4), 10 mM MgCl2, 1 mM EDTA, 150 mM KCl, 10 KIU of aprotinin, 10 μM leupeptin, 10 μM bestatin, 1.0 nM [35S]GTPγS. GDP was added at the concentration of 5 μM for CHO/NOP, 10 μM for CHO/MOR, 30 μM for CHO/KOR and CHO/DOR and 50 μM for rat striatal membranes. Membranes (3–4 μg protein) were preincubated for 20 min at 30°C with the test compounds. For each compound, control samples received an equal volume (10 μl) of vehicle to determine the basal values. The vehicle used was either 0.1% BSA for peptide agonists, 1% dimethyl sulfoxide (DMSO) for NalBzoH, or H2O for the other compounds. The reaction was started by the addition of [35S]GTPγS and continued for 40 min at 30°C. The incubation was terminated by the addition of 5 ml of ice-cold buffer containing 10 mM HEPES/NaOH (pH 7.4) and 1 mM MgCl2, immediately followed by rapid filtration on glass fibre filters (Whatman GF/C) presoaked in the same buffer. The filters were washed twice with 5 ml of buffer and the radioactivity trapped was determined by liquid scintillation spectrometry. Nonspecific binding was determined in the presence of 100 μM GTPγS. Assays were performed in duplicate.
Assay of [3H]cyclic AMP accumulation
CHO cells grown in 36-mm plastic dishes were incubated in Ham's F-12 containing 10 μCi ml−1 of [3H]adenine for 1 h at 37°C in an incubator. Thereafter, the medium was removed, and the cells were incubated in an oxygenated Krebs–HEPES buffer containing 1 mM 3-isobutyl-1-methylxanthine (IBMX) for 10 min at 37°C. Forskolin (FSK) (10 μM) and the various test compounds were then added and the incubation was continued for 10 min. Control samples were incubated in the presence of an equal volume of vehicle. When DMSO was used, the final concentration was 0.1%. The incubation was stopped by the aspiration of the medium and the addition of an ice-cold solution containing 6% (w v−1) perchloric acid and 0.1 mM [14C]cyclic AMP (∼4000 c.p.m.). After 30 min at ice-bath temperature, the solution was neutralized by the addition of ice-cold 0.6 M KOH and left on ice for additional 30 min. Following centrifugation at 20,000 × g for 5 min, the supernatant was collected and [3H]cyclic AMP was isolated by sequential chromatography on Dowex and alumina columns as described by Salomon et al. (1974). The recovery of [3H]cyclic AMP from each sample was corrected on the basis of the recovery of [14C]cyclic AMP.
Assay of adenylyl cyclase
The adenylyl cyclase activity was assayed in a reaction mixture (final volume 100 μl) containing 50 mM HEPES/NaOH (pH 7.4), 2.0 mM MgCl2, 0.3 mM EGTA, 0.05 mM [α-32P]ATP (150 c.p.m. pmol−1), 0.5 mM [3H]cyclic AMP (80 c.p.m. nmol−1), 1 mM IBMX, 5 mM phosphocreatine, 50 U ml−1 of creatine phosphokinase, 100 μM GTP, 50 μg of BSA, 10 μg of bacitracin, 10 KIU of aprotinin and 10 μM FSK. The reaction was started by the addition of the tissue preparation (20–25 μg protein) and was carried out at 25°C for 20 min. The reaction was stopped by the addition of 200 μl of a stopping solution containing 2% (w v−1) sodium dodecyl sulphate, 45 mM ATP, 1.3 mM cyclic AMP (pH 7.5). Cyclic AMP was isolated by sequential chromatography on Dowex and alumina columns as described above. The recovery of [32P]cyclic AMP from each sample was calculated on the basis of the recovery of [3H]cyclic AMP. Assays were carried out in duplicate.
Receptor binding assays
Radioligands used for receptor binding assays were [3H]diprenorphine in the experiments using CHO/MOR and KOR cell membranes, [3H]NTI in the experiments using CHO/DOR cell membranes and [3H]N/OFQ in those using CHO/NOP cell membranes.
For saturation binding assays, membrane preparations of CHO/MOR (60 μg protein), KOR (15 μg protein) and DOR (30 μg protein) were incubated at 30°C for 120 min in a buffer containing 25 mM HEPES/NaOH (pH 7.4), 10 mM MgCl2, 1 mM EDTA and 150 mM KCl. The concentrations of [3H]diprenorphine and [3H]NTI ranged from 20 pM to 3.0 nM and from 8 pM to 1.5 nM, respectively. The assay volume was 1.0 ml for [3H]diprenorphine binding and 2.0 ml for [3H]NTI binding. Nonspecific binding was determined in the presence of 10 μM naloxone. For [3H]N/OFQ binding, CHO/NOP cell membranes (50–60 μg protein) were incubated at 25°C for 90 min in a medium (final volume 0.5 ml) containing 50 mM Tris/HCl (pH 7.4), 2 mM EDTA, 0.2% BSA, 50 KIU of aprotinin, 10 μM bestatin, 100 μM bacitracin, 0.1 mM phenylmethylsulfonyl fluoride and [3H]N/OFQ (5 pM–1.5 nM). Nonspecific binding was determined in the presence of 5 μM N/OFQ.
For competition binding assays, membrane preparations of CHO/MOR (70–80 μg protein), KOR (15–20 μg protein) and DOR (40–50 μg protein) were incubated at 30°C for 60 min in a medium containing 25 mM HEPES/NaOH (pH 7.4), 10 mM MgCl2, 1 mM EDTA, 150 mM KCl, 10 KIU of aprotinin, 10 μM leupeptin and 10 μM bestatin. To keep the conditions similar to those used in the [35S]GTPγS binding assays, the incubation medium contained 1.0 nM GTPγS and GDP at the concentration of 10 μM for CHO/MOR and 30 μM for CHO/KOR and DOR. The concentrations of [3H]diprenorphine and [3H]NTI were 0.2 and 0.16 nM, respectively. The assay volume was 1.0 ml for [3H]diprenorphine binding and 2.0 ml for [3H]NTI binding. Nonspecific binding was determined in the presence of 10 μM naloxone. Compounds were studied at 8–10 different concentrations. For each compound, control samples received an equal volume of vehicle.
Reactions were terminated by the addition of 5 ml of ice-cold buffer containing either 10 mM HEPES/NaOH (pH 7.4) and 1 mM MgCl2 for [3H]diprenorphine and [3H]NTI binding or 50 mM Tris/HCl and 1 mM EDTA for [3H]N/OFQ binding, immediately followed by rapid filtration through glass fibre filters (Whatman GF/C) presoaked with polyethylenimine at the concentration of 0.1% for [3H]diprenorphine and [3H]NTI binding and 0.2% for [3H]N/OFQ binding. The filters were washed twice with 5 ml of buffer and the radioactivity trapped was determined by liquid scintillation spectrometry. Assays were performed in triplicate.
Protein content was determined by the method of Bradford (1976) using BSA as a standard.
Materials
[α-32P]ATP (30–40 Ci mmol−1), [2,8-3H]cyclic AMP (25 Ci mmol−1), [8-14C]cyclic AMP (45.1 mCi mmol−1), [2,8-3H]adenine (28.8 Ci mmol−1), [35S]GTPγS (1306 Ci mmol−1), [15,16-3H]diprenorphine (53 Ci mmol−1), [5′,7′-3H]naltrindole ([3H]NTI) (20 Ci mmol−1) were obtained from Perkin Elmer (Boston, MA, U.S.A.). [leucyl-3H]N/OFQ ([3H]N/OFQ) (167 Ci mmol−1) was from Amersham (Buckinghamshire, U.K.). FSK and GTPγS were from Calbiochem (San Diego, CA, U.S.A.) and Boehringer (Mannheim, Germany), respectively. N/OFQ was purchased from Neosystem (Strasbourg, France). (−)-U-50,488 hydrochloride (trans-(−)3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl] benzeneacetamide hydrochloride), nor-BNI dihydrochloride, endomorphin-1 and CTAP were from Tocris Cookson Ltd (Avonmouth, U.K.). DPDPE ((2-D-penicillamine, 5-D-penicillamine)-enkephalin) was purchased from Bachem AG (Bubendorf, Switzerland). Morphine hydrochloride and naloxone hydrochloride were from Salars (Como, Italy). NalBzoH, NTI, DAMGO ((D-Ala2-N-methyl-Phe-Gly-ol5)-enkephalin), nalorphine hydrochloride, pentazocine hydrochloride, bestatin, aprotinin, bacitracin, leupeptin and the other reagents were from Sigma RBI (St Louis, MO, U.S.A.).
Statistical analysis
Results are reported as means±s.e.m. Data from concentration–response curves were analysed by the program Graph Pad Prism (San Diego, CA, U.S.A.), which yielded agonist concentration producing half-maximal effect (EC50 values) and maximal effects (Emax). For statistical analysis, the EC50 values were converted to the logarithmic form (pEC50=negative logarithm of EC50) as they are log-normally distributed (Fleming et al., 1972). The percent of maximal effect (% Emax) by an agonist was calculated as net maximal effect of the agonist/net maximal effect elicited by either DAMGO (10 μM), (−)-U-50,488 (10 nM) or DPDPE (100 nM) in CHO/MOR, KOR and DOR, respectively, X100. Saturation binding data were analysed by the LIGAND program (Munson & Rodbard, 1980), which provided the ligand dissociation constant (KD) and maximal binding capacity (Bmax). Antagonist potencies were determined in experiments where the compounds were examined for their ability to reverse the agonist effect or to displace the radioligand in a concentration-dependent manner. The data were analysed as competition curves by nonlinear regression analysis and the antagonist inhibitory constant (Ki) was calculated according to the Cheng & Prusoff (1973) equation. Ki values were converted to the logarithmic form (pKi). The Ki/EC50 ratio was determined to be significantly different from one when the corresponding pKi and pEC50 values were significantly different. When the difference was not significant, the ratio was considered equal to 1. The efficacy of the various opioid receptor ligands in stimulating [35S]GTPγS binding was calculated according to the method of Ehlert (1985), in which efficacy=(Emax-A/Emax-B)×(Ki/EC50+1) × 0.5, where Emax-A is the maximal effect elicited by the test compound, Emax-B is the maximal effect elicited by either DAMGO, (−)-U-50,488 or DPDPE in CHO/MOR, KOR and DOR, respectively, Ki is the inhibition constant of the compound obtained from competition binding experiments and EC50 is the concentration of the compound producing half-maximal effect. Statistical significance of the difference between means was determined by Student's t-test.
Results
Radioligand KD and Bmax values
Saturation binding experiments yielded the following KD and Bmax values: [3H]diprenorphine in CHO/MOR cell membranes 80±15 pM and 350±30 fmol mg−1 protein, respectively (n=3); [3H]diprenorphine in CHO/KOR cell membranes 97±18 pM and 2800±150 fmol mg−1 protein, respectively (n=3); [3H]NTI in CHO/DOR cell membranes 54±8 pM and 2260±107 fmol mg−1 protein, respectively (n=3); [3H]N/OFQ in CHO/NOP cell membranes 40±8 pM and 400±50 fmol mg−1 protein, respectively (n=3).
Effects of NalBzoH in CHO/MOR cells
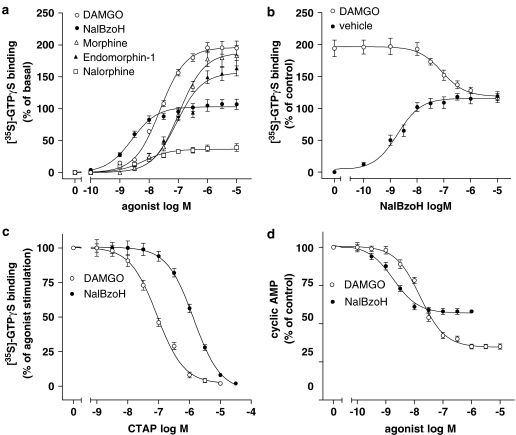
In CHO/MOR cell membranes, the selective MOR agonist DAMGO maximally stimulated [35S]GTPγS binding to membrane G proteins by 195±3% (P<0.001) (Figure 1a and Table 1). NalBzoH caused a concentration-dependent stimulation of the [35S]GTPγS binding with a potency about 10-fold higher than that of DAMGO and an Emax value corresponding to 107±4% increase of basal value (P<0.001) (Figure 1a and Table 1). Under the same experimental conditions, other opioid receptor ligands, such as morphine, endomorphin-1 and nalorphine maximally stimulated [35S]GTPγS binding by 180±9% (P<0.001), 165±7% (P<0.001), and 39±7% (P<0.001), respectively, and displayed potencies lower than that of NalBzoH (Figure 1a and Table 1). When NalBzoH was combined with DAMGO (1 μM), it reduced the stimulation of [35S]GTPγS binding elicited by the peptide to a level equal to its maximal effect with a pKi value of 8.68±0.07 (n=3) (Figure 1b). Moreover, the responses to DAMGO and NalBzoH were completely blocked by the MOR antagonist CTAP (Pelton et al., 1986) with pKi values of 8.68±0.06 and 8.52±0.08, respectively (Figure 1c). In intact CHO/MOR cells, both DAMGO and NalBzoH significantly inhibited FSK-stimulated cyclic AMP accumulation with different pEC50 and Emax values (Figure 1d and Table 1).
Figure 1.
Effects of NalBzoH in CHO/MOR cells. (a) Concentration-dependent stimulation of [35S]GTPγS binding by NalBzoH and other opioid receptor ligands. Values are reported as percent of basal activity and are the mean±s.e.m. of three to 10 determinations. (b) Antagonism of DAMGO-stimulated [35S]GTPγS binding by NalBzoH. [35S]GTPγS binding was determined at the indicated concentrations of NalBzoH in the presence of either vehicle (0.1% BSA) or 1 μM DAMGO. Values are reported as percent of basal activity and are the mean±s.e.m. of three determinations. (c) Antagonism of NalBzoH- and DAMGO-stimulated [35S]GTPγS binding by CTAP. Values indicate the percent of the stimulatory effect induced by either DAMGO (1 μM) or NalBzoH (1 μM) at each concentration of CTAP and are the mean±s.e.m. of three determinations. (d) Concentration-dependent inhibition of FSK-stimulated cyclic AMP accumulation by NalBzoH and DAMGO. Values are reported as percent of control activity (vehicle) and are the mean±s.e.m. of three determinations.
Table 1.
Potencies and efficacies of NalBzoH and other opioid receptor ligands in CHO/MOR, KOR and DOR cells
| Cyclic AMP assay | [35S]GTPγS binding assay | ||||||
| Ligand | Emaxa | pEC50 (n) | % Emaxb | pEC50 (n) | Kic (n) | Ki/EC50 | Efficacye |
| CHO/MOR | |||||||
| NalBzoH | 40±2* | 8.74±0.04 (3) | 55±6 | 8.59±0.05 (6) | 8.53±0.09 (3) | 1.1d | 0.55 |
| DAMGO | 62±1* | 7.81±0.03 (3) | 100 | 7.62±0.03 (10) | 7.06±0.04 (3) | 3.6 | 2.30 |
| Morphine | 94±3 | 6.97±0.05 (3) | 6.82±0.09 (3) | 1.4d | 0.94 | ||
| Endomorphin-1 | 83±4 | 7.05±0.06 (3) | 6.96±0.05 (3) | 1.2d | 0.83 | ||
| Nalorphine | 20±4 | 8.16±0.04 (3) | 8.13±0.06 (3) | 1.0d | 0.20 | ||
| CHO/KOR | |||||||
| NalBzoH | 82±3* | 9.45±0.06 (3) | 102±5 | 9.70±0.09 (3) | 8.64±0.10 (4) | 11.8 | 6.43 |
| (−)-U-50,488 | 83±2* | 9.60±0.06 (3) | 100 | 9.40±0.02 (6) | 7.95±0.09 (4) | 28.6 | 14.80 |
| Nalorphine | 92±3 | 8.76±0.03 (3) | 7.66±0.08 (3) | 12.7 | 6.31 | ||
| Morphine | 80±4 | 6.81±0.06 (3) | 5.94±0.12 (3) | 7.4 | 3.35 | ||
| Pentazocine | 57±6 | 7.67±0.05 (3) | 7.58±0.09 (3) | 1.2d | 0.57 | ||
| CHO/DOR | |||||||
| NalBzoH | 82±4* | 8.61±0.05 (3) | 92±4 | 8.49±0.04 (3) | 7.24±0.08 (3) | 17.9 | 8.69 |
| DPDPE | 88±2* | 9.74±0.09 (3) | 100 | 8.76±0.05 (6) | 7.09±0.07 (3) | 46.7 | 23.85 |
| Morphine | 60±5 | 6.38±0.06 (3) | 5.25±0.11 (3) | 13.7 | 4.41 | ||
| Nalorphine | 50±3 | 7.29±0.03 (3) | 6.53±0.05 (3) | 5.7 | 1.67 | ||
Percent of inhibition of the forskolin-stimulated response in the presence of vehicle alone.
Percent of maximal stimulation of [35S]GTPγS binding with respect to that obtained with either DAMGO, (−)-U-50,488 or DPDPE in CHO/MOR, KOR and DOR cells, respectively, which was set at 100%.
Determined in competition binding experiments under the same conditions used in [35S]GTPγS binding assays.
Not significantly different from 1.
determined according to the equation described in Statistical Analysis section;
P<0.001 versus control; (n), number of experiments.
Effects of NalBzoH in CHO/KOR cells
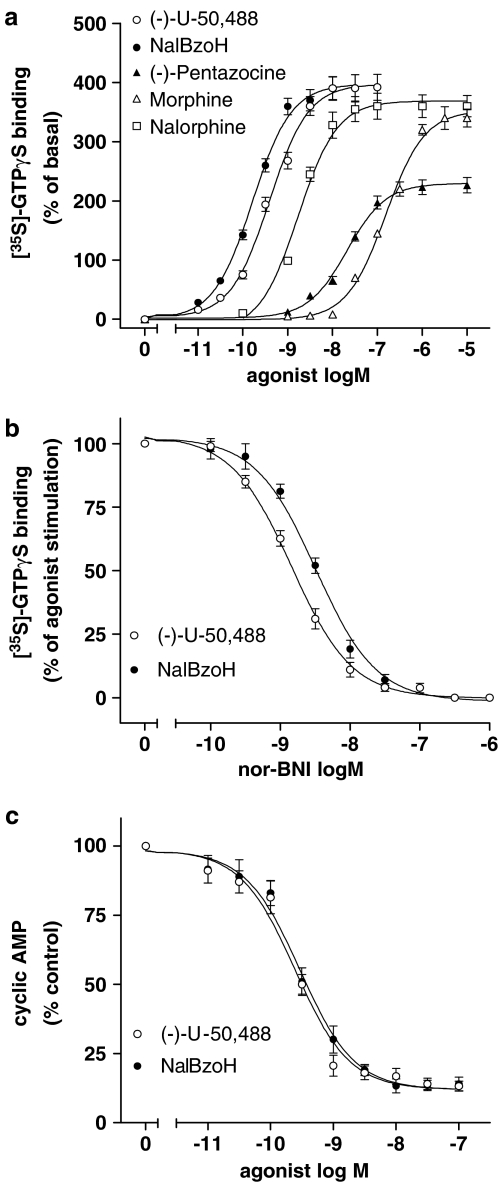
In these cells, the KOR agonist (−)-U-50,488 maximally enhanced the [35S]GTPγS binding by 4.9-fold (P<0.001) (Figure 2a and Table 1). NalBzoH stimulated [35S]GTPγS binding with pEC50 and Emax values similar to those of (−)-U-50,488. For comparison, the stimulatory effects of morphine, nalorphine and pentazocine were measured (Figure 2a). The corresponding Emax and pEC50 values are reported in Table 1. Nor-BNI counteracted the (−)-U-50,488 and NalBzoH stimulatory effects with pKi values of 10.10±0.04 and 10.30±0.06, respectively (Figure 2b). In intact CHO/KOR cells, (−)-U-50,488 and NalBzoH inhibited FSK-stimulated cyclic AMP accumulation with similar potencies and maximal effects (Figure 2c and Table 1).
Figure 2.
Effects of NalBzoH in CHO/KOR cells. (a) Concentration-dependent stimulation of [35S]GTPγS binding by NalBzoH and other opioid receptor ligands. Values are reported as percent of basal activity and are the mean±s.e.m. of three to six determinations. (b) Antagonism of NalBzoH- and (−)-U-50,488-stimulated [35S]GTPγS binding by nor-BNI. Values indicate the percent of the stimulatory effect induced by either (−)-U-50,488 (10 nM) or NalBzoH (10 nM) at each concentration of nor-BNI and are the mean±s.e.m. of three determinations. (c) Concentration-dependent inhibition of FSK-stimulated cyclic AMP accumulation by NalBzoH and (−)-U-50,488. Values are reported as percent of control activity (vehicle) and are the mean±s.e.m. of three determinations.
Effects of NalBzoH in CHO/DOR cells
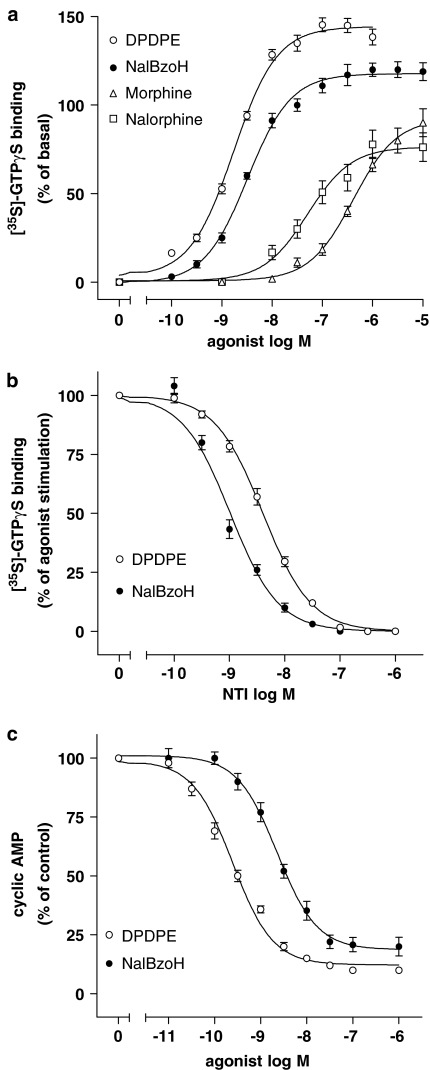
In cell membrane preparations, the DOR agonist DPDPE maximally stimulated [35S]GTPγS binding by 142±4% (P<0.001) (Figure 3a and Table 1). NalBzoH stimulated the binding of the radioligand almost as effectively as DPDPE, whereas morphine and nalorphine were less efficacious (Figure 3a, Table 1). The DOR antagonist NTI completely blocked the stimulations elicited by DPDPE and NalBzoH with pKi values of 10.30±0.04 and 10.40±0.08, respectively (Figure 3b). In intact cells, DPDPE and NalBzoH inhibited cyclic AMP accumulation by a similar degree (Figure 3c and Table 1).
Figure 3.
(a) Effects of NalBzoH in CHO/DOR cells. Concentration-dependent stimulation of [35S]GTPγS binding by NalBzoH and other opioid receptor ligands. Values are reported as percent of basal activity and are the mean±s.e.m. of 3–6 determinations. (b) Antagonism of NalBzoH- and DPDPE-stimulated [35S]GTPγS binding by NTI. Values indicate the percent of the stimulatory effect induced by either NalBzoH (100 nM) or DPDPE (100 nM) at each concentration of NTI and are the mean±s.e.m. of three determinations. (c) Concentration-dependent inhibition of FSK-stimulated cyclic AMP accumulation by NalBzoH and DPDPE. Values are reported as percent of control activity (vehicle) and are the mean±s.e.m. of three determinations.
Effects of NalBzoH in CHO/NOP cells
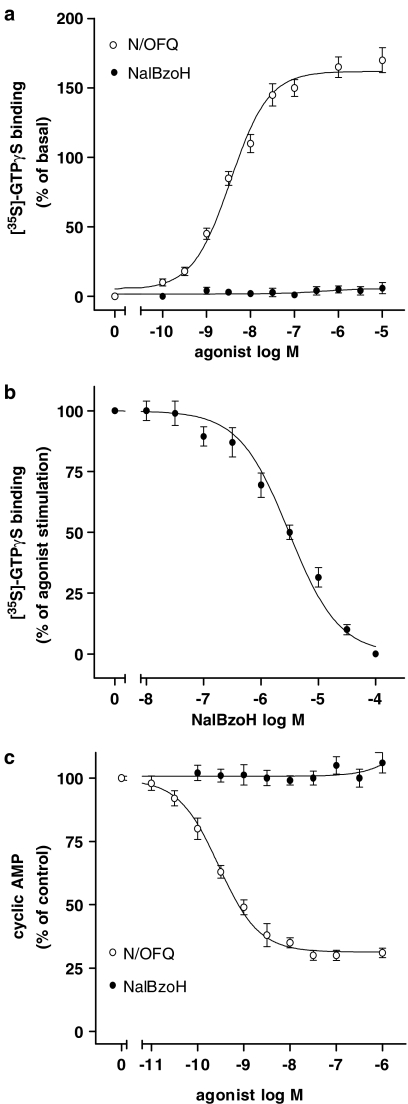
In membrane preparations, the NOP agonist N/OFQ stimulated the [35S]GTPγS binding by 155±5% (P<0.001, n=3) with a pEC50 of 8.46±0.07 (Figure 4a). NalBzoH failed to affect basal [35S]GTPγS binding at concentrations up to 10 μM (Figure 4a), and, when combined with N/OFQ (100 nM), it inhibited the stimulation elicited by the peptide with a pKi value of 7.01±0.06 (Figure 4b). In intact cells, N/OFQ inhibited cyclic AMP accumulation by 70±4% (P<0.001, n=3) with a pEC50 of 9.55±0.06, whereas NalBzoH had no effect at concentrations up to 10 μM (Figure 4c). NalBzoH (10 nM–100 μM) antagonized the N/OFQ (20 nM) inhibition of cyclic AMP accumulation with a pKi value of 7.15±0.04 (n=3) (results not shown).
Figure 4.
(a) Effects of NalBzoH and N/OFQ on [35S]GTPγS binding in CHO/NOP cells. Values indicate the percent of basal activity and are the mean±s.e.m. of three determinations. (b) Antagonism of N/OFQ-stimulated [35S]GTPγS binding by NalBzoH. Values are reported as percent of the stimulatory effect induced by N/OFQ (100 nM) at each concentration of NalBzoH and are the mean±s.e.m. of three determinations. (c) Effects of N/OFQ and NalBzoH on FSK-stimulated cyclic AMP accumulation in intact CHO/NOP cells. Values are reported as percent of control activity (vehicle) and are the mean±s.e.m. of three determinations.
Determination of NalBzoH efficacies
To determine the efficacies of NalBzoH and other opioid receptor ligands in stimulating [35S]GTPγS binding, competition binding assays were performed under experimental conditions similar to those used in [35S]GTPγS binding assays. The resultant pKi values, together with the percent of maximal stimulation (%Emax) and pEC50 values are reported in Table 1. The agonist efficacy was then calculated taking into consideration both the magnitude of the agonist effect and the extent of receptor occupancy at which half-maximal effect occurred (Ki/EC50 ratio) (Ehlert, 1985). At MOR, NalBzoH displayed a Ki/EC50 ratio equal to 1, indicating that receptor activation closely followed the receptor occupancy. The calculated efficacy value was low, corresponding to about one fourth of that of the full agonist DAMGO. The efficacies of morphine and endomorphin-1, which produced near-maximal responses, also resulted lower than that of DAMGO but higher than that of NalBzoH, whereas nalorphine was the least efficacious agonist tested. Although at KOR and DOR NalBzoH elicited full stimulation of [35S]GTPγS binding and showed Ki/EC50 ratios significantly higher than 1, the calculated efficacy values were lower than those of the full agonists (−)-U,50,488 and DPDPE, respectively, but about two-fold higher than those of morphine.
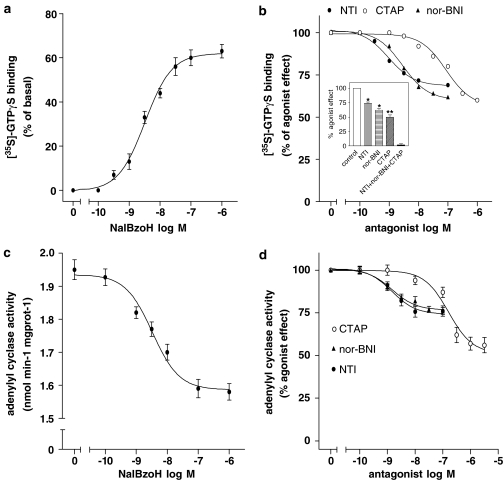
Effects of NalBzoH in rat striatum
In rat striatal membranes, NalBzoH caused a concentration-dependent stimulation of [35S]GTPγS binding with a pEC50 of 8.48±0.03 and a Emax corresponding to 60±4% increase of basal activity (P<0.001, n=3) (Figure 5a). Competition curves using selective opioid receptor antagonists showed that CTAP, nor-BNI and NTI maximally blocked the NalBzoH effect by 43±5 (P<0.001), 39±3 (P<0.01) and 29±4% (P<0.01), respectively (n=4) (Figure 5b). The estimated pKi values were 8.63±0.05, 10.04±0.09 and 10.30±0.06, respectively. The combined addition of CTAP (1 μM), nor-BNI (100 nM) and NTI (100 nM) caused a complete blockade of the NalBzoH stimulatory effect (Figure 5b, inset). In the same membrane preparations, NalBzoH inhibited FSK-stimulated adenylyl cyclase activity by 20±3% (P<0.05, n=4) with a pEC50 of 8.49±0.05 (Figure 5c). CTAP, nor-BNI and NTI maximally reduced the NalBzoH inhibition by 52±4% (P<0.001), 26±3% (P<0.01) and 24±4% (P<0.01) (n=4) with pKi values of 8.28±0.08, 10.30±0.05 and 10.44±0.07, respectively (Figure 5d).
Figure 5.
Agonist effects of NalBzoH in membranes of rat striatum. (a) Concentration-dependent stimulation of [35S]GTPγS binding. Values indicate the percent of basal activity and are the mean±s.e.m. of four determinations. (b) Antagonism of NalBzoH-stimulated [35S]GTPγS binding by CTAP, NTI and nor-BNI. Values are reported as percent of the stimulatory effect induced by NalBzoH (100 nM) at the indicated concentrations of each antagonist and are the mean±s.e.m. of four determinations. Inset: Blockade of NalBzoH (100 nM)-stimulated [35S]GTPγS binding by CTAP (1 μM), NTI (100 nM) and nor-BNI (100 nM) added alone and in combination. Values are reported as percent of the NalBzoH effect and are the mean±s.e.m. of three experiments. *P<0.01, **P<0.001 vs control. (c) Effects of NalBzoH on FSK-stimulated adenylyl cyclase activity. Values are the mean±s.e.m. of four determinations. (d) Antagonism of NalBzoH-induced inhibition of rat striatal adenylyl cyclase activity by CTAP, NTI and nor-BNI. Values are reported as percent of the inhibitory effect induced by NalBzoH (100 nM) at the indicated concentrations of each antagonist and are the mean±s.e.m. of four determinations.
Discussion
In the present study, the pharmacological activity of NalBzoH at the four cloned human opioid receptors individually expressed in CHO cells and at the endogenous opioid receptors present in rat striatum was investigated.
In CHO/MOR cells, NalBzoH was more potent than DAMGO in stimulating [35S]GTPγS binding and inhibiting cyclic AMP accumulation. However, in the [35S]GTPγS binding and cyclic AMP assays, the Emax values of NalbzoH were approximately 50 and 30% lower than those of DAMGO, respectively, indicating that NalBzoH behaved as a partial agonist. Indeed, when combined with DAMGO, NalBzoH antagonized the [35S]GTPγS response to the full agonist with a pKi value that was close to its affinity for MOR. Moreover, as expected for a partial agonist, NalBzoH showed a Ki/EC50 ratio close to 1 and the estimated efficacy value was lower than that of DAMGO and other MOR agonists, such as morphine and endomorphin-1. Also, the latter agonists displayed efficacies lower than that of DAMGO, a finding in agreement with that previously obtained in transfected cell lines (Emmerson et al., 1996; Selley et al., 1998; Hosohata et al., 1998) and neuroblastoma cells (Harrison et al., 1998). On the other hand, nalorphine was less efficacious than NalBzoH, indicating that the CHO/MOR cell system used allowed the detection of a range of agonist efficacies.
A number of studies have previously shown that NalBzoH blocked MOR-mediated responses (Gistrak et al., 1989; Paul et al., 1990; France & Woods, 1992; Berzetei-Gurske et al., 1995; Dunnill et al., 1996) and the compound is considered as a potent MOR antagonist (Gutstein & Akil, 2001). In CHO expressing the cloned MOR, Brown & Pasternak (1998) reported that NalBzoH inhibited [3H]diprenorphine binding with potencies (Ki=0.9–2.8 nM) close to that observed in the present study, but failed to cause a significant inhibition of FSK-stimulated cyclic AMP accumulation, indicating that the compound was a pure antagonist devoid of any partial agonist activity. The reason for the discrepant results between the study by Brown & Pasternak (1998) and the present study is not clear. A difference in receptor densities is unlikely to account for the different functional activity, as the Bmax value of [3H]diprenorphine binding in CHO/MOR cell membranes used in the present study (350 fmol mg−1 protein) was even lower than that of [3H]NalBzoH binding (450 fmol mg−1 protein) in the recombinant cell system used by Brown & Pasternak (1998). It is noteworthy that Brown & Pasternak (1998) observed that the binding of [3H]NalBzoH to MOR showed a biphasic dissociation kinetic with a slow and a rapid component and that the addition of guanine nucleotides or cell treatment with pertussis toxin abolished the slow dissociation component. These observations are in line with the present finding that NalBzoH acts as a partial agonist, rather than a pure antagonist, at the MOR.
In CHO/KOR cells, NalBzoH acted as a potent and full agonist similarly to (−)-U-50,488, but exhibited a lower Ki/EC50 ratio and, consequently, a lower efficacy value. This finding suggests the possibility that, under conditions of stimulus–response efficiency lower than that provided by the transfected cells used in the present study, NalBzoH may behave as a KOR partial agonist. Indeed, there are reports that the compound may act as a mixed agonist–antagonist at KOR endogenously expressed in peripheral tissues. In the longitudinal muscle/myenteric plexus of guinea-pig ileum, NalBzoH elicited agonist effects that were blocked by either nor-BNI or the irreversible KOR antagonist UPHIT (Berzetei-Gurske et al., 1995; Dunnill et al., 1996). On the other hand, Berzetei-Gurske et al. (1995) reported that in the same tissue preparation and in mouse vas deferens NalBzoH was also able to antagonize the muscle relaxant effects of the KOR agonist U-69,593, although with potencies that were several fold lower than the reported affinity of NalBzoH at the KOR.
Despite NalBzoH has been largely used as an opioid receptor ligand, relatively little information has been provided on the functional activity of this compound at DOR. Paul et al. (1990) reported that in mice NalBzoH antagonized the analgesia induced by intrathecal injection of DPDPE, whereas Standifer et al. (1994) showed that in BE(2)-C neuroblastoma cells, which expressed a functionally active DOR, NalBzoH potently displaced [3H]DPDPE binding, but inhibited cyclic AMP accumulation independently of DOR. The present study, on the contrary, shows that in CHO/DOR cells NalBzoH behaved as a potent agonist with maximal responses almost equal to those of DPDPE. However, as observed at KOR, the estimated efficacy of NalBzoH was much lower than that of DPDPE, indicating that the compound may behave as a full or partial agonist depending on the experimental setting. Indeed, we observed that in NG 108-15 cells naturally expressing DOR NalBzoH stimulated [35S]GTPγS binding and inhibited FSK-stimulated cyclic AMP accumulation with Emax values corresponding to 81 and 70% of those displayed by DPDPE, respectively (Olianas & Onali, unpublished results). Moreover, NalBzoH behaved as a partial agonist in eliciting DOR-mediated responses in rat olfactory bulb (Onali & Olianas, 2004).
In contrast to the appreciable agonist activity exhibited at classical opioid receptors, NalBzoH was unable to activate the cloned NOP receptor. In the [35S]GTPγS binding assay, the compound failed to affect basal activity and antagonized the stimulation elicited by N/OFQ. These results are similar to those reported in other functional studies using transfected cell lines (Noda et al., 1998; Seki et al., 1999; Hawkinson et al., 2000) and different tissue preparations (Calo' et al., 2000). Bigoni et al. (2002) and McDonald et al. (2003) observed that in membranes of CHO/NOP cells, NalBzoH caused a small stimulatory effect on basal [35S]GTPγS binding (13% of the N/OFQ effect) at 5 μM GDP (the same concentration used in the present study), but had no agonist effect and behaved as an antagonist at higher GDP concentrations (100 μM). Bigoni et al. (2002) also reported that in intact CHO/NOP cells, NalBzoH inhibited cyclic AMP accumulation by 44% with a potency (pEC50 value=6.00) almost 10-fold lower than that displayed in blocking N/OFQ (pA2=6.93) and concluded that the compound may display a partial agonist activity under strict experimental conditions. In CHO cells expressing the KOR-3 receptor, the mouse homologue of the human NOP (Pan et al., 1995), NalBzoH was found to maximally inhibit cyclic AMP by approximately 20% at the concentration of 100 nM (Pan et al., 1996). In the present study, we failed to observe any effect of NalBzoH on cyclic AMP accumulation. Moreover, as occurred in the [35S]GTPγS binding assay, NalBzoH antagonized the N/OFQ-induced cyclic AMP inhibition. A possible explanation for our failure to detect a partial agonist activity of NalBzoH in the [35S]GTPγS binding assay is that the transfected CHO/NOP cells had a receptor density (400 fmol mg−1 protein) much lower than that of CHO/NOP cells used by McDonald et al. (2003) (1900 fmol mg−1protein). However, with regard to the NalBzoH effects on cyclic AMP levels, it should be noted that a lack of inhibition has also been reported by other investigators using CHO/NOP cell systems with receptor densities ranging from 61 fmol mg−1protein (Seki et al., 1999) to 2100 fmol mg−1 protein (Noda et al. 1998).
To investigate whether the agonist effects of NalBzoH could be detected also at opioid receptors expressed under native conditions, we examined the effect of NalBzoH in a rat brain area, the dorsal striatum, which is known to express all the four types of opioid receptors (Mansour et al., 1995; Darland et al., 1998). We found that NalBzoH stimulated [35S]GTPγS binding and this response was antagonized by CTAP, NTI and nor-BNI in a saturable manner and with high potencies, indicating the participation of MOR, DOR and KOR. In addition, like other opioid receptor agonists, NalBzoH inhibited the FSK-stimulated adenylyl cyclase activity of rat striatal membranes and also this effect appeared to involve all the three classical opioid receptors.
In conclusion, the present study demonstrates for the first time that NalBzoH exhibits agonist activity at both recombinant and native MOR, KOR and DOR. The data do not support the current classification of NalBzoH as a selective κ3-opioid receptor agonist and pure MOR antagonist and suggest caution when the compound is used as NOP receptor antagonist to identify N/OFQ-mediated effects. Nonetheless, they also reveal that, among the various opioid receptor ligands, NalBzoH possesses a pharmacologically unique profile, as it has the ability to stimulate the classical opioid receptors mediating analgesia and to block NOP receptors inducing supraspinal nociception.
Acknowledgments
We thank the University of Missouri-Rolla cDNA Resource Center (www.cdna.org) for providing the cDNAs encoding the human opioid receptors.
Abbreviations
- BSA
bovine serum albumin
- CHO
Chinese hamster ovary
- CHO/DOR, CHO/KOR, CHO/MOR and CHO/NOP cells
CHO cells expressing the recombinant human δ-, κ-, μ- and NOP receptor, respectively
- DAMGO
(D-Ala2-N-methyl-Phe-Gly-ol5)-enkephalin
- DMSO
dimethyl sulfoxide
- DOR
δ-opioid receptor
- DPDPE
(2-D-penicillamine,5-D-penicillamine)-enkephalin)
- FSK
forskolin
- KOR
κ-opioid receptor
- MOR
μ-opioid receptor
- NalBzoH
naloxone benzoylhydrazone
- N/OFQ
nociceptin/orphanin FQ
- NTI
naltrindole
- nor-BNI
nor-binaltorphimine
References
- ABDULLA F.A., SMITH P.A. Nociceptin inhibits T-type Ca2+ channel current in rat sensory neurons by a G-protein-independent mechanism. J. Neurosci. 1997;17:8721–8728. doi: 10.1523/JNEUROSCI.17-22-08721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERZETEI-GURSKE I.P., WHITE A., POLGAR W., DeCOSTA B.R., PASTERNAK G.W., TOLL L. The in vitro pharmacological characterization of naloxone benzoylhydrazone. Eur. J. Pharmacol. 1995;277:257–263. doi: 10.1016/0014-2999(95)00088-3. [DOI] [PubMed] [Google Scholar]
- BIGONI R., CAO G., RIZZI A., OKAWA H., REGOLI D., SMART D., LAMBERT D.G. Effects of naloxone benzoylhydrazone on native and recombinant nociceptin/orphanin FQ receptors. Can. J. Physiol. Pharmacol. 2002;80:407–412. doi: 10.1139/y02-040. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- BROWN G.P., PASTERNAK G.W. 3H-Naloxone benzoylhydrazone binding in MOR-1-transfected chinese hamster ovary cells: evidence for G-protein-dependent antagonist binding. J. Pharmacol. Exp. Ther. 1998;286:376–381. [PubMed] [Google Scholar]
- CALO' G., BIGONI R., RIZZI A., GUERRINI R., SALVADORI S., REGOLI D. Nociceptin/orphaninFQ receptor ligands. Peptides. 2000;21:935–947. doi: 10.1016/s0196-9781(00)00230-8. [DOI] [PubMed] [Google Scholar]
- CHENG J., ROQUES B.P., GACEL G.A., HUANG E., PASTERNAK G.W. κ3-Opiate receptor binding in the mouse and rat. Eur. J. Pharmacol. Mol. Pharmacol. Sect. 1992;226:15–20. doi: 10.1016/0922-4106(92)90077-9. [DOI] [PubMed] [Google Scholar]
- CHENG J., STANDIFER K.M., TUBLIN P.R., SU W., PASTERNAK G.W. Demonstration of κ3-opioid receptors in the SH-SY5Y human neuroblastoma cell line. J. Neurochem. 1995;65:170–175. doi: 10.1046/j.1471-4159.1995.65010170.x. [DOI] [PubMed] [Google Scholar]
- CHENG Y.C., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3102. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CHIOU L.-C. Differential antagonism by naloxone benzoylhydrazone of the activation of inward rectifying K+ channels by nociceptin and a μ-opioid in rat periaqueductal grey slices. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:583–589. doi: 10.1007/s002100100402. [DOI] [PubMed] [Google Scholar]
- CISZEWSKA G.R., GINOS J.A., CHARTON M., STANDIFER K.M., BROOKS A.I., BROWN G.P., RYAN-MORO J.P., BERZETEI-GURSKE I., TOLL L., PASTERNAK G.W. Synthesis and characterization of substituted benzoylhydrazones of naloxone. Synapse. 1996;24:193–201. doi: 10.1002/(SICI)1098-2396(199610)24:2<193::AID-SYN11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- CLARK J.A., LIU L., PRICE M., HERSH B., EDELSON M., PASTERNAK G.W. Kappa opiate receptor multiplicity: evidence for two U50,488-sensitive κ1 subtypes and a novel κ3 subtype. J. Pharmacol. Exp. Ther. 1989;251:461–468. [PubMed] [Google Scholar]
- COX V., CLARKE S., CZYZYK T., ANSONOFF M., NITSCHE J., HSU M.-S., BORSODI A., TOMBOLY C., TOTH G., HILL R., PINTAR J., KITCHEN I. Autoradiography in opioid triple knock-out mice reveals opioid and opioid receptor like binding of naloxone benzoylhydrazone. Neuropharmacology. 2005;48:228–235. doi: 10.1016/j.neuropharm.2004.09.016. [DOI] [PubMed] [Google Scholar]
- DARLAND T., HEINRICHER M.M., GRANDY D.K. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Pharmacol. Sci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- DUNNILL R.J., KAKIZAWA K., McKNIGHT A.T., HENDERSON G. Characterization of the actions of naloxone benzoylhydrazone at μ-opioid, κ-opioid and ORL1 receptors in isolated tissues from rat and guinea pig. Br. J. Pharmacol. 1996;119:275P. [Google Scholar]
- EHLERT F.J. The relationship between muscarinic receptor occupancy and adenylate cyclase inhibition in the rabbit myocardium. Mol. Pharmacol. 1985;28:410–421. [PubMed] [Google Scholar]
- EMMERSON P.J., CLARK M.J., MANSOUR A., AKIL H., WOODS J.H., MEDZIHRADSKY F. Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the μ opioid receptor. J. Pharmacol. Exp. Ther. 1996;278:1121–1127. [PubMed] [Google Scholar]
- FLAU K., REDMER A., LIEDTKE S., KATHMANN M., SCHLICKER E. Inhibition of striatal and retinal dopamine release via nociceptin/orphanin FQ receptors. Br. J. Pharmacol. 2002;137:1355–1361. doi: 10.1038/sj.bjp.0704998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEMING W.W., WESTFALL D.P., DE LA LANDE S., JELLET L.B. Log-normal distribution of equieffective doses of norepinephrine and acetylcholine in several tissues. J. Pharmacol. Exp. Ther. 1972;181:339–345. [PubMed] [Google Scholar]
- FRANCE C.P., WOODS J.H. Naloxone benzylhydrazone is a μ-selective opioid antagonist without kappa agonist effects in rhesus monkeys. Behav. Pharmacol. 1992;3:133–141. [PubMed] [Google Scholar]
- GISTRAK M.A., PAUL D., HAHN E.F., PASTERNAK G.W. Pharmacological actions of a novel mixed opiate agonist/antagonist: naloxone benzoylhydrazone. J. Pharmacol. Exp. Ther. 1989;251:469–476. [PubMed] [Google Scholar]
- GUTSTEIN H.B., AKIL H.Opioid analgesics Goodman & Gilman's The Pharmacological Basis of Therapeutics 2001New York: McGraw-Hill Book Company; 569–619.ed. Hardman, J.G., Limbird, L.E. & Goodman Gilman, A. pp [Google Scholar]
- HARRISON L.M., KASTIN A.J., ZADINA J.E. Differential effects of endomorphin-1, endomorphin-2, and Tyr-W-MIF-1 on activation of G proteins in SH-SY5Y human neuroblastoma membranes. Peptides. 1998;19:749–753. doi: 10.1016/s0196-9781(98)00022-9. [DOI] [PubMed] [Google Scholar]
- HAWKINSON J.E., ACOSTA-BURRUEL M., ESPITIA S.A. Opioid activity profiles indicate similarities between the nociceptin/orphanin FQ and opioid receptors. Eur. J. Pharmacol. 2000;389:107–114. doi: 10.1016/s0014-2999(99)00904-8. [DOI] [PubMed] [Google Scholar]
- HOSOHATA K., BURKEY T.H., ALFARO-LOPEZ J., VARGA E., HRUBY V.J., ROESKE W.R., YAMAMURA H.Y. Endomorphin-1 and endomorphin-2 are partial agonists at the human μ-opioid receptor. Eur. J. Pharmacol. 1998;346:111–114. doi: 10.1016/s0014-2999(98)00117-4. [DOI] [PubMed] [Google Scholar]
- MANSOUR A., FOX C.A., AKIL H., WATSON S.J. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- MATHIS J.P., MANDYAM C.D., ALTEMEMI G.F., PASTERNAK G.W., STANDIFER K.M. Orphanin FQ/nociceptin and naloxone benzoylhydrazone activate distinct receptors in BE(2)-C human neuroblastoma cells. Neurosci. Lett. 2001;299:173–176. doi: 10.1016/s0304-3940(01)01524-5. [DOI] [PubMed] [Google Scholar]
- McDONALD J., BARNES T.A., OKAWA H., WILLIAMS J., CALO' G., ROWBOTHAM D.J., LAMBERT D.G. Partial agonist behaviour depends upon the level of nociceptin/orphanin FQ receptor expression: studies using the ectysone-inducible mammalian expression system. Br. J. Pharmacol. 2003;140:61–70. doi: 10.1038/sj.bjp.0705401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEUNIER J.C. The potential therapeutic value of nociceptin receptor agonists and antagonists. Exp. Opin. Ther. Patents. 2000;10:371–388. [Google Scholar]
- MEUNIER J.C., MOLLERAU C., TOLL L., SUAUDEAU C., MOISAND C., ALVINERIE P., BUTOUR J.L., GUILLEMOT J.C., FERRARA P., MONSERRAT B., MAZARGUIL H., VASSART G., PARMENTIER M., COSTENTIN J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MINOWA S., ISHIHARA S., TSUCHIYA S., HORIE S., WATANABE K., MURAYAMA T. Involvement of glutamate and gamma-amino-butyric acid receptor systems on gastric acid secretion induced by activation of kappa-opioid receptors in the central nervous system in rats. Br. J. Pharmacol. 2003;138:1049–1058. doi: 10.1038/sj.bjp.0705082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOGIL J.S., PASTERNAK G.W. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol. Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- MUNSON P.J., RODBARD D. Ligand: a versatile computerized approach for the characterization of ligand binding systems. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- NABESHIMA T., NODA Y., MAMIYA T. The role of nociceptin in cognition. Brain. Res. 1999;848:167–173. doi: 10.1016/s0006-8993(99)01906-x. [DOI] [PubMed] [Google Scholar]
- NODA Y., MAMIYA T., NABESHIMA T., NISHI M., HIGASHIOKA M., TAKESHIMA H. Loss of antinociception induced by naloxone benzoylhydrazone in nociceptin receptor-knockout mice. J. Biol. Chem. 1998;273:18047–18051. doi: 10.1074/jbc.273.29.18047. [DOI] [PubMed] [Google Scholar]
- ONALI P., OLIANAS M.C. G protein activation and cyclic AMP modulation by naloxone benzoylhydrazone in distinct layers of rat olfactory bulb. Br. J. Pharmacol. 2004;143:638–648. doi: 10.1038/sj.bjp.0705951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAN Y.-X., CHENG J., XU J., ROSSI G., JACOBSON E., RYAN-MORO J., BROOKS A.I., DEAN G.E., STANDIFER K.M., PASTERNAK G.W. Cloning and functional characterization through antisense mapping of a κ3-related opioid receptor. Mol. Pharmacol. 1995;47:1180–1188. [PubMed] [Google Scholar]
- PAN Y.-X., XU J., RYAN-MORO J., MATHIS J., HOM J.S.H., MEI J., PASTERNAK G.W. Dissociation of affinity and efficacy in KOR-3 chimeras. FEBS Lett. 1996;395:207–210. doi: 10.1016/0014-5793(96)01023-x. [DOI] [PubMed] [Google Scholar]
- PAUL D., LEVISON J.A., HOWARD D.H., PICK C.G., HAHN E.F., PASTERNAK G.W. Naloxone benzoylhydrazone (NalBzoH) analgesia. J. Pharmacol. Exp. Ther. 1990;255:769–774. [PubMed] [Google Scholar]
- PELTON J.T., KAZMIERSKI W., GULYA K., YAMAMURA H.I., HRUBY V.J. Design and synthesis of conformationally constrained somatostatin analogues with high potency and specificity for μ opioid receptors. J. Med. Chem. 1986;29:2370–2375. doi: 10.1021/jm00161a037. [DOI] [PubMed] [Google Scholar]
- PICK C.G., PETER Y SCHREIBER S., WEIZMAN R. Pharmacological characterization of buprenorphine, a mixed agonist-antagonist with kappa 3 analgesia. Brain Res. 1997;744:41–46. doi: 10.1016/s0006-8993(96)01069-4. [DOI] [PubMed] [Google Scholar]
- PRICE M., GISTRAK M.A., ITZHAK Y., HAHN E.F., PASTERNAK G.W. Receptor binding of [3H]naloxone benzoylhydrazone: a reversible κ and slowly dissociable μ opiate. Mol. Pharmacol. 1989;35:67–74. [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MOSMA F.J., CIVELLI O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- REISINE T., PASTERNAK G.W.Opioid analgesics and antagonists Goodman & Gilman's The Pharmacological Basis of Therapeutics 1996New York: McGraw-Hill Book Company; 521–555.ed. Hardman, J.G., Limbird, L.E. & Goodman Gilman, A. pp [Google Scholar]
- SALOMON Y., LONDOS D., RODBELL M. A highly sensitive adenylate cyclase assay. Anal. Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- SEKI T., AWAMURA S., KIMURA C., IDE S., SAKANO K., MINAMI M., NAGASE H., SATOH M. Pharmacological properties of TRK-820 on cloned μ-, δ-, and κ-opioid receptors and nociceptin receptor. Eur. J. Pharmacol. 1999;376:159–167. doi: 10.1016/s0014-2999(99)00369-6. [DOI] [PubMed] [Google Scholar]
- SELLEY D.E., LIU Q., CHILDERS S.R. Signal transduction correlates of mu opioid agonist intrinsic efficacy: receptor-stimulated [35S]GTPγS binding in mMOR-CHO cells and rat thalamus. J. Pharmacol. Exp. Ther. 1998;285:496–505. [PubMed] [Google Scholar]
- STANDIFER K.M., CHENG J., BROOKS A.I., HONRADO C.P., SU W., VISCONTI L.M., BIEDLER J.L., PASTERNAK G.W. Biochemical and pharmacological characterization of mu, delta and kappa3 opioid receptors expressed in BE(2)-C neuroblastoma cells. J. Pharmacol. Exp. Ther. 1994;270:1246–1255. [PubMed] [Google Scholar]
- STANDIFER K.M., MURTHY L.R., KINOUCHI K., STEELE L., PASTERNAK G.W. Affinity labeling of μ and κ receptors with naloxone benzoylhydrazone. Mol. Pharmacol. 1991;39:290–298. [PubMed] [Google Scholar]