Abstract
Previous studies suggest that the thiadiazole compound SCH-202676 (N-(2,3-diphenyl-1,2,4-thiadiazol-5-(2H)-ylidene)methanamine) acts as an allosteric modulator of a variety of structurally distinct G protein-coupled receptors (GPCRs). It was postulated that SCH-202676 would directly bind a structural motif in the receptor molecule common to divergent members of the GPCR family. The molecular mechanisms of such a promiscuous action, however, remain obscure.
To clarify the mechanism of SCH-202676 action, we used the functional approach of [35S]GTPγS autoradiography with rat brain cryostat sections together with classical membrane [35S]GTPγS binding assays to evaluate how the thiadiazole affects G protein activity mediated by various receptors linked to the Gi-family of G proteins.
We found that in the absence of dithiotreitol (DTT), SCH-202676 (10−7–10−5 M) elicits nonspecific effects in the [35S]GTPγS-based G protein activation assays, thereby severely compromising interpretations on the compounds ability to allosterically inhibit receptor-mediated G protein activity. Such a nonspecific behaviour was fully reversed upon addition of DTT (1 mM), revealing thiol-based mechanism of action.
In routine incubations containing DTT, SCH-202676 had no effect on receptor-driven G protein activity, as assessed for adenosine A1, α2-adrenergic, cannabinoid CB1, lysophosphatidic acid LPA1, muscarinic M2/M4, purinergic P2Y12 or sphingosine 1-phosphate receptors, suggesting that the thiadiazole does not act as an allosteric modulator of GPCR function.
1H NMR analysis indicated that SCH-202676 underwent structural changes after incubation with the reducing agent DTT or with brain tissue.
We conclude that SCH-202676 modulates GPCRs via thiol modification rather than via true allosteric mechanisms.
Keywords: Allosteric modulator, cysteine, dithiotreitol, heterotrimeric G protein, G protein-coupled receptor, [35S]GTPγS autoradiography, SCH-202676, sulphydryl, thiadiazole, thiol
Introduction
Previous studies have suggested that the thiadiazole compound, SCH-202676 (N-(2,3-diphenyl-1,2,4-thiadiazol-5-(2H)-ylidene)methanamine), acts as an allosteric modulator of various G protein-coupled receptors (GPCRs). By definition, an allosteric modulator is a ligand that although silent per se, is capable of modulating (increase or decrease) the action of the ‘orthosteric' ligand (agonist or antagonist) by binding to a distinct, that is, allosteric site on the receptor molecule (Kenakin, 2004). In the pioneering work by Fawzi and co-workers, it was demonstrated that low micromolar concentrations of SCH-202676 inhibited both agonist and antagonist radioligand binding to a number of structurally distinct GPCRs including human μ-, δ-, and κ-opioid, α- and β-adrenergic, muscarinic M1 and M2 acetylcholine and dopaminergic D1 and D2 receptors (Fawzi et al., 2001). It was further shown that SCH-202676 had no direct effect on heterotrimeric G protein activity but at similar concentrations, the compound totally abolished α2A-adrenoceptor-dependent [35S]GTPγS binding responses (Fawzi et al., 2001). These data were interpreted to suggest that SCH-202676 might directly bind a structural motif in the receptor molecule common to divergent members of the GPCR family.
Further studies on the muscarinic M1 acetylcholine receptor led the authors to conclude that SCH-202676 may use a dual mode of ligand–receptor interaction involving both extra- and intracellular attachment points on the M1 receptor that are distinct from the allosteric binding site recognized by prototypical modulators (Lanzafame & Christopoulos, 2004). Two additional studies that have been conducted using this compound concentrated on the interaction of SCH-202676 with adenosine and P2Y receptors and showed its divergent effects on the purinergic receptors. SCH-202676 inhibited radioligand binding to adenosine A1, A2A and A3 receptors and affected their dissociation kinetics, but interestingly, it had no effect on the P2Y1 receptor (Gao et al., 2004; van den Nieuwendijk et al., 2004).
From the viewpoint of drug discovery, allosteric modulators could present an alternative approach with potential benefits, as compared to the more traditional scheme aiming at development of drugs targeting the orthosteric site. Allosteric modulators might have a natural limitation of their effect, as the endogenous agonist needs to be present and would also show tissue selectivity, as the endogenous ligand concentrations may vary over different tissues. Although receptor-subtype specific allosteric modulators have been identified for several GPCRs (Kenakin, 2004), the promiscuous activity of SCH-202676 among diverse GPCRs raises an obvious question as to its molecular mechanism of action. In an effort to address this important question, we have used the functional approach of [35S]GTPγS autoradiography with brain cryostat sections to systematically study how SCH-202676 affects GPCR function, as this technique allows selective detection of receptor-dependent G protein activity simultaneously for several GPCRs in multiple brain regions (Laitinen, 2004). To complement the autoradiography approach, we used classical membrane [35S]GTPγS binding assays to provide a quantitative measure for the effects of the thiadiazole on the signalling of a panel of receptors coupling to the Gi-family of G proteins. This study demonstrates that SCH-202676 is a thiol-reactive compound rather than a true allosteric modulator, and disrupts GPCR signalling in a dithiotreitol (DTT)-sensitive manner in [35S]GTPγS-based functional assays. While this manuscript was in preparation, another study reached a similar conclusion by demonstrating that SCH-202676 and related thiadiazole compounds elicit their modulatory actions on adenosine A1 receptor via sulphydryl modification (Göblyös et al., 2005).
Methods
Animals and preparation of cryostat sections
All animal experiments were approved by the local ethics committee and were conducted in vitro using brain sections from 4-week-old male Wistar rats. The animals lived in a 12-h light, 12-h dark cycle (lights on 0700) with water and food ad libitum. The rats were killed by decapitation, 8 h after lights on, whole brains were dissected out, dipped in isopentane on dry ice and stored at −80°C. Coronal or sagittal sections (20 μm thick) were cut at −14°C using a Leica cryostat. In some experiments (illustrated in Figure 2), spleen sections (20 μm thick) from four-week-old animals were also used. Tissue was thaw-mounted onto SuperFrost* Plus slides (Menzel-Gläser, Germany) at 20°C. The sections were stored at −80°C for up to 8 months without any apparent loss of binding responses.
Figure 2.
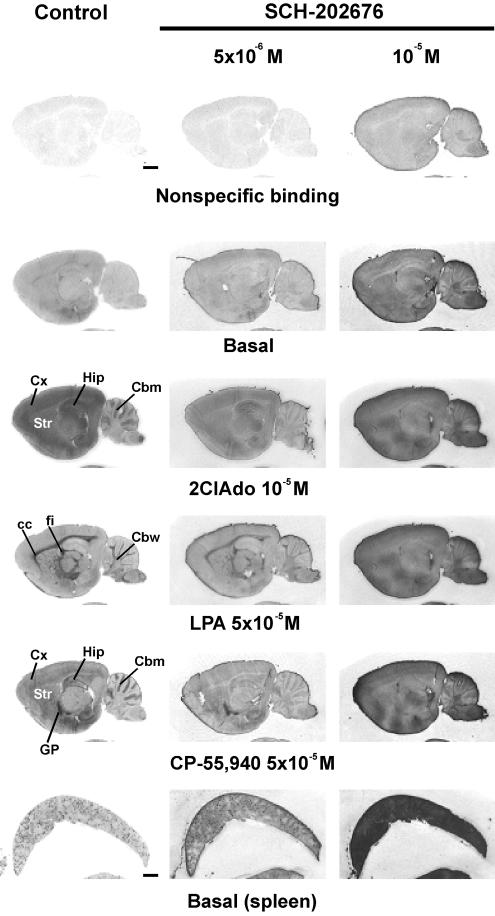
In the absence of DTT, SCH-202676 elicits nonspecific binding responses in [35S]GTPγS-based G protein activation assays. [35S]GTPγS autoradiography of rat brain (sagittal plane) and spleen sections was conducted using a three-step protocol with ADA (1 U ml−1) present throughout steps 2 and 3, as detailed in the Methods section. SCH-202676 was present at the indicated concentrations during the [35S]GTPγS labeling (step 3). The GPCR agonists 2-chloroadenosine (2ClAdo), lysophosphatidic acid (LPA), and CP-55,940 were present during step 3 to stimulate adenosine A1, lysophosphatidic acid LPA1 and cannabinoid CB1 receptors, respectively. In the control panel (left), the anatomical brain loci where the receptor agonists typically activate G proteins at this sagittal plane are indicated. Note SCH-202676-evoked nonspecific and dose-dependent increase in [35S]GTPγS binding that is not restricted to the tissue sections but is apparent to glass slides (background) as well. Note also severe blunting of agonist-stimulated responses in the presence of 5 × 10−6 M SCH-202676 and their total absence with 10−5 M thiadiazole concentration. Abbreviations: cc, corpus callosum; Cbm, cerebellar molecular layer; Cbw, cerebellar white matter; Cx, cerebral cortex; fi, fimbria of the hippocampus; Hip, hippocampus; GP, globus pallidus; Str, striatum. Scale bars=2 mm.
Chemicals
Adenosine deaminase (ADA) was obtained from Roche Molecular Biochemicals (Ingelheim, Germany) whereas BSA (fatty acid free), DTT, DPCPX, GDP, GTPγS, lysophosphatidic acid (LPA), and 2-methylthio ADP (2MeSADP) were from Sigma-RBI (St Louis, MO, U.S.A.). CP-55,940 and SCH-202676 were purchased from Tocris Cookson Ltd. (Bristol, U.K.). [35S]GTPγS (initial specific activity 1250 Ci mmol−1) was from NEN (Boston, MA, U.S.A.). The stock solution was diluted 1 : 50 in 10 mM Tris-HCl, pH 7.4 containing 10 mM DTT and stored as single-use aliquots at −80°C. All other chemicals were of highest purity available.
[35S]GTPγS autoradiography
The assay was conducted under optimized conditions, where noise due to tonic adenosine A1 receptor activity has been eliminated (Laitinen, 1999; Laitinen et al., 2001). Experiments were conducted in light-protected chambers. Briefly, the assay consisted of preincubation for 20 min at 20°C in buffer A (50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 100 mM NaCl, 5 mM MgCl2), followed by GDP loading for 1 h at 20°C in buffer A, containing additionally 2 mM GDP and DPCPX (10−6 M) or ADA (1 U ml−1) to eliminate tonic adenosine A1 receptor activity. For agonist-stimulated [35S]GTPγS binding, sections were incubated for 90 min at 20°C in buffer A, containing additionally 80 pM [35S]GTPγS, 2 mM GDP, DPCPX (10−6 M) or ADA (1 U ml−1), plus the receptor agonists in alone or in combination with SCH-202676 (10−7–10−5 M) and DTT (1 mM), as detailed in the Results section. Nonspecific binding (Nsb) was determined in the presence of 10 μM GTPγS. The sections were washed twice at 0°C for 5 min in washing buffer (50 mM Tris-HCl, 5 mM MgCl2, pH 7.4), rinsed in ice-cold deionised water for 30 s, air dried and apposed to Biomax™ MR film (Kodak) for 6–11 days. Autoradiography films were scanned at 150 dpi resolution to generate digital images.
[35S]GTPγS membrane binding assays
The incubations were carried out for 90 min at 25°C under constant shaking essentially as previously described (Kurkinen et al., 1997; Savinainen et al., 2003). The final concentrations of the components in the binding reaction were 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 100 mM NaCl, 5 mM MgCl2, 0.5% BSA, 10 μM GDP, 0.5 U ml−1 ADA, 150 pM [35S]GTPγS and varying concentrations of SCH-202676 (10−6, 5 × 10−6 or 10−5 M) and DTT (1 mM), as detailed in the Results sections. Nsb was defined using 10 μM GTPγS. Reaction was quenched by the addition of 4 ml ice-cold wash buffer (50 mM Tris-HCl, pH 7.4 and 5 mM MgCl2) followed by rapid filtration through Whatman GF/B glass fibre filters (Whatman, Maidstone, U.K.) and two additional 4 ml washes with the buffer. Radioactivity in filters was counted with Wallac Rackbeta liquid scintillation counter (Wallac, Turku, Finland).
Nuclear magnetic resonance
1H-NMR spectra were recorded at 300 K on a Bruker Avance DRX 500 spectrometer equipped with a normal 2.5 mm BBI probe. The samples were evaporated to dryness and dissolved in 130 μl of D2O and 1H-NMR spectra were measured in a 2.5 mm microtube.
Data analysis
[35S]GTPγS-membrane binding data were analysed with GraphPad Prism software (GraphPad, San Diego, CA, U.S.A.). Assays were run with duplicate samples and repeated independently at least three times. Statistical analyses were made with one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test with P<0.05 considered as statistically significant.
Results
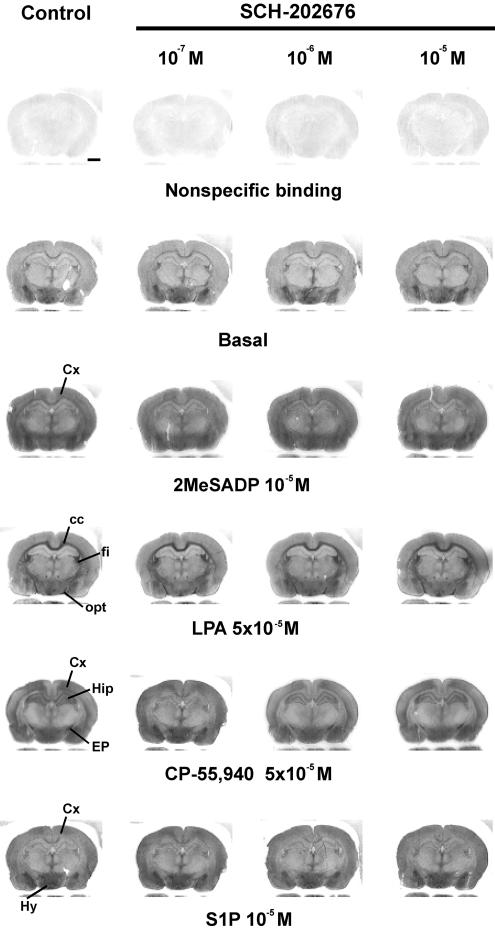
Previous studied indicate that SCH-202676 exhibits an extraordinary steep and narrow dose–response range (typical effective concentration range 10−7–10−5 M) in modulating radioligand binding to GPCRs (Fawzi et al., 2001; Gao et al., 2004; Lanzafame & Christopoulos, 2004; van den Nieuwendijk et al., 2004). Therefore, our initial studies were set up to clarify whether SCH-202676 at this concentration range affects receptor-dependent G protein activation, as assessed in rat brain sections using the optimised standard protocol for [35S]GTPγS autoradiography. We focused the initial experiments on four Gi-linked receptors, namely the purinergic P2Y12, the lysophophatidic acid LPA1, the cannabinoid CB1 and the sphingosine 1-phosphate (S1P) receptors, as G protein activity upon stimulation of these receptors has been previously characterized using the autoradiography approach and each receptor shows a unique anatomical distribution pattern in the developing rat brain (Laitinen, 2004). As depicted in Figure 1, the P2Y receptor agonist 2MeSADP activated G proteins both in gray and white matter regions, producing a heterogeneous activity pattern as described earlier (Laitinen et al., 2001; Vasiljev et al., 2003). Our previous work has established that the 2MeSADP-stimulated [35S]GTPγS binding responses in rat brain sections are mediated by a P2Y receptor subtype that pharmacologically corresponds to P2Y12 (Laitinen et al., 2001; Vasiljev et al., 2003). As further evident from Figure 1, LPA-stimulated Gi protein activity was largely restricted to the myelinating white matter tracts, such as the corpus callosum (cc), the fimbria of the hippocampus (fi) and the optic tract (opt), closely reflecting the anatomical distribution of the LPA1 receptor subtype (Waeber & Chiu, 1999; Laitinen, 2004). In contrast, CB1 and S1P receptor responses show widespread distribution mainly in the grey matter regions (Waeber & Chiu, 1999; Laitinen, 2004), such regions visible in Figure 1 include, for example, the cerebral cortex (Cx) and the hippocampus (Hip). In contrast to our expectations, SCH-202676 at concentrations previously shown to inhibit radioligand binding to various GPCRs (10−7–10−5 M) and to totally abolish α2A-adrenoceptor-mediated [35S]GTPγS binding responses in CHO cell membranes (Fawzi et al., 2001) had no apparent effect on the G protein activity mediated by the tested GPCRs (Figure 1). These experiments also indicated that nonspecific and basal [35S]GTPγS binding was unaffected by the thiadiazole (Figure 1). Additional studies (not shown) further indicated that SCH-202676 (10−5 M) had no detectable effect on G protein activity mediated by an additional set of Gi-coupled receptors, including the α2-adrenergic, adenosine A1, and muscarinic M2/M4 receptors. This negative outcome was rather unexpected in light of previous findings demonstrating the promiscuous ability of SCH-202676 to modulate various GPCRs.
Figure 1.
Under the routine conditions of [35S]GTPγS autoradiography, SCH-202676 has no effect on receptor-dependent G protein activity in rat brain cryostat sections. [35S]GTPγS autoradiography of coronal brain sections was conducted using a three-step protocol with 10−6 M DPCPX present throughout steps 2 and 3, as detailed in the Methods section. SCH-202676 was present at the indicated concentrations together with 1 mM DTT during the [35S]GTPγS labeling (step 3). The GPCR agonists 2-methylthio ADP (2MeSADP), lysophosphatidic acid (LPA), CP-55,940 and sphingosine 1-phosphate (S1P) were present during step 3 to stimulate purinergic P2Y12, lysophosphatidic acid LPA1, cannabinoid CB1 and sphingosine 1-phosphate S1P1 receptors, respectively. In the control panel (left), the anatomical loci where the receptor agonists typically activate G proteins at this coronal plane are indicated. Note the apparent lack of effect of SCH-202676 on nonspecific, basal or agonist-stimulated responses. Abbreviations: cc, corpus callosum; Cx, cerebral cortex; EP, entopeduncular nucleus fi; fimbria of the hippocampus; Hip, hippocampus, Hy, hypothalamus; opt, optic tract. Scale bar=2 mm.
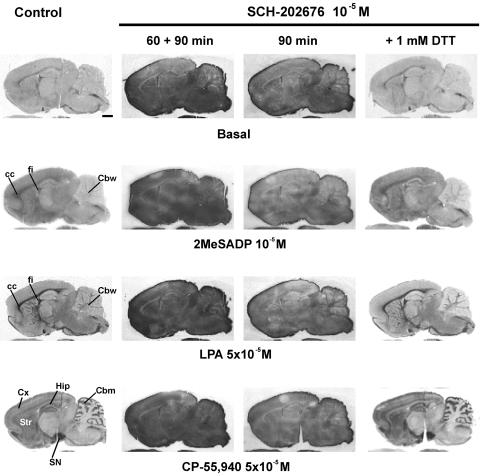
To resolve the reason behind this discrepant outcome, we modified the standard incubation protocol by omitting DTT, a reducing agent that is frequently included in [35S]GTPγS binding assays to reduce the levels of nonspecific and basal binding (Happe et al., 2001). As anticipated, this modification resulted in a dramatically different outcome with respect to the behaviour of SCH-202676. In the absence of DTT, the thiadiazole (5 × 10−6 and 10−5 M) caused an overall, nonspecific, and dose-dependent increase in [35S]GTPγS labelling, not only to brain sections but to glass slides as well (Figure 2). Such a behaviour was not restricted to rat brain sections, as the thiadiazole similarly compromised specific [35S]GTPγS labelling to sections of mouse brain (data not shown) and rat spleen (Figure 2, bottom row), as well as to rat brain membrane preparations (see below). It was notable that under the DTT-free conditions, all agonist-stimulated [35S]GTPγS binding responses were blunted with 5 × 10−6 M SCH-202676 and practically absent with the higher (10−5 M) thiadiazole concentration (Figure 2). Additional studies indicated that SCH-202676 at only slightly smaller concentrations (10−6 and 2 × 10−6 M) failed to affect basal or receptor-dependent responses in brain sections (data not shown). These data indicate, in agreement with previous radioligand binding studies (Fawzi et al., 2001; Gao et al., 2004; Lanzafame & Christopoulos, 2004; van den Nieuwendijk et al., 2004), that SCH-202676 exhibits an extraordinary narrow effective dose-range also in [35S]GTPγS autoradiography assessing GPCR function. Prolonging the incubation time of SCH-202676 (10−5 M) with tissue further increased the nonspecific [35S]GTPγS labelling of brain sections (Figure 3), indicating that the compound likely reacted with tissue components as present during the binding assay. Importantly, the thiadiazole action was fully reversed upon addition of 1 mM DTT (Figure 3), revealing that sulphydryl-sensitive mechanisms were responsible for the [35S]GTPγS binding-interfering action of SCH-202676.
Figure 3.
Time-dependent and fully DTT-reversible effects of SCH-202676 on [35S]GTPγS-based G protein activation assays with brain cryostat sections. [35S]GTPγS autoradiography of rat brain sagittal sections was conducted using a three-step protocol with 10−6 M DPCPX present throughout steps 2 and 3, as detailed in the Methods section. SCH-202676 was present at the indicated concentrations either throughout steps 2 and 3 (60+90 min) or only during the [35S]GTPγS labeling (step3, 90 min). Where indicated, DTT (1 mM) was included during step3. The GPCR agonists 2-methylthio ADP (2MeSADP), lysophosphatidic acid (LPA), and CP-55,940 were present during step 3 to stimulate purinergic P2Y12, lysophosphatidic acid LPA1, and cannabinoid CB1 receptors. In the control panel (left), the anatomical loci where the receptor agonists typically activate G proteins at this sagittal plane are indicated. Note the time-dependent increase in the SCH-202676-evoked nonspecific [35S]GTPγS labeling. Note also complete reversal of the thiadiazole action in the presence of DTT. Abbreviations: cc, corpus callosum; Cbm, cerebellar molecular layer; Cbw, cerebellar white matter; Cx, cerebral cortex; fi, fimbria of the hippocampus; Hip, hippocampus; Str, striatum; SN, substantia nigra. Scale bar=2 mm.
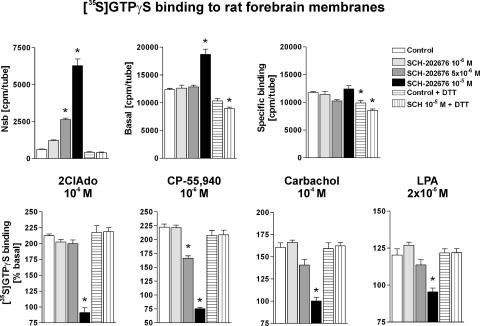
To rule out the possibility that the above findings were an artefact inherent to the autoradiography approach, classical membrane [35S]GTPγS binding assays were conducted in parallel. In accordance with the autoradiography results, SCH-202676 when tested under DTT-free conditions and at concentrations of 10−6, 5 × 10−6 and 10−5 M, caused a nonspecific and dose-dependent increase in [35S]GTPγS labelling to rat forebrain membranes (Figure 4). Notably, Nsb, which under the control conditions represents approximately 0.3% of total added radioactivity, was increased up to 10-fold in the presence of 10−5 M SCH-202676 (Figure 4, upper left). Similarly, total membrane-associated [35S]GTPγS binding under the basal condition was significantly increased with the highest thiadiazole concentration (Figure 4, upper middle), whereas specific binding, obtained after subtracting nonspecific from total binding, was not significantly affected (Figure 4, upper right). When DTT (1 mM) was included in the binding cocktail, 10−5 M SCH-202676 had no effect on the above-mentioned parameters. Furthermore and in full accordance with the autoradiography results, agonist-stimulated [35S]GTPγS binding responses under the DTT-free assay conditions were variably blunted in the presence of 5 × 10−5 M thiadiazole, and totally abolished at 10−5 M concentration of the compound (Figure 4, bottom panel). In the presence of DTT (1 mM), SCH-202676 (10−5 M) had no effect on the agonist potency or efficacy at the four studied receptors (Figure 4 and Table 1).
Figure 4.
SCH-202676 elicits nonspecific and fully DTT-sensitive [35S]GTPγS binding responses in filtration-based G protein activation assays. Rat forebrain membranes (5 μg per tube) were incubated for 90 min at 25°C in a final volume of 0.4 ml with the indicated combinations of compounds in the presence of 0.5 U ml−1 ADA, as detailed in the Methods section. Where indicated, incubations contained 1 mM DTT. The GPCR agonists 2-chloroadenosine (2ClAdo), CP-55,940, carbachol and lysophosphatidic acid (LPA) were used to stimulate adenosine A1, cannabinoid CB1, muscarinic M2/M4 and lysophosphatidic acid LPA1 receptors, respectively. The data represent the mean±s.e. from two to three independent experiments performed in duplicate. An asterix denotes a statistically significant difference (P<0.05; ANOVA followed by Tukey's multiple comparison) as compared to the respective control condition.
Table 1.
In the presence of DTT SCH-202676 has no effect on agonist potency of efficacy in rat forebrain [35S]GTPγS binding assays of various Gi-coupled receptors
| Control | SCH-202676 10−5 M | |||
|---|---|---|---|---|
| Receptor (agonist) | log(EC50) | Emax (%) | log(EC50) | Emax (%) |
| M2/M4 mAChRs (CCh) | −5.1±0.1 | 169±4 | −5.0±0.1 | 168±4 |
| Adenosine A1 (2ClAdo) | −6.5±0.1 | 229±6 | −6.5±0.3 | 238±16 |
| Cannabinoid CB1 (CP55940) | −7.0±0.1 | 197±4 | −6.9±0.1 | 199±5 |
| LPA1 (LPA) | −5.5±0.5 | 125±7 | −5.9±0.5 | 120±5 |
Membranes were incubated in control conditions or in the presence of 10−5 M SCH-202676 for 90 min with 1 mM DTT included in the assay buffer, as detailed in the Methods section. Values are mean±s.e. from three independent experiments performed in duplicate. Emax is expressed in percentage over basal with nonspecific binding subtracted.
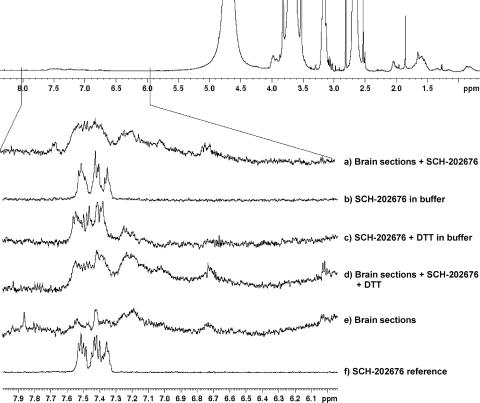
To shed further light on the DTT-sensitive behaviour of SCH-202676, we evaluated the compound stability using 1H-NMR (Figure 5). The reference spectrum (spectrum f, SCH-202676 in DMSO) showed the expected chemical shifts at 7.6–7.3 p.p.m. due to aromatic hydrogens, indicating chemical integrity of the compound. Identical spectrum was recorded after storage for several days at room temperature (data not shown), or after incubations of the compound for 90 min in the buffer system of our study (spectrum b). This analysis indicated that the thiadiazole was chemically stable in the buffer system used in [35S]GTPγS binding assays. In contrast, addition of DTT to the buffer resulted in dramatic changes in the aromatic hydrogen signals at 7.6–7.3 p.p.m. (compare spectrum c against that of b and f), indicating that the presence of thiol groups per se induced chemical decomposition of the thiadiazole molecule. Similarly, incubation of SCH-202676 together with brain sections (Figure 5a) resulted in notable changes in the chemical shifts at 7.6–7.3 p.p.m. Interestingly, no further modification was evident when incubations with brain sections contained additionally DTT (compare spectrum d against a). This structural analysis indicates that SCH-202676 undergoes chemical decomposition after reacting with the reducing agent DTT or with brain tissue.
Figure 5.
SCH-202676 undergoes structural changes after incubation with the reducing agent DTT with brain tissue. The thiadiazole (6 × 10−5 M) was incubated at 20°C for 90 min in Tris-based assay buffer (50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 100 mM NaCl, 5 mM MgCl2) together with DTT (1 mM) or with brain sections, as indicated in traces a–e. The postincubation supernatant was collected and 1H-NMR spectra determined as detailed in the Methods section. Trace f represents the 1H-NMR spectrum of SCH-202676 in DMSO. Note chemical stability of SCH-202676 in the aqueous buffer (b) and structural changes (altered 1H NMR spectra) after incubation with DTT (c) or brain sections (a).
Discussion
The present study demonstrated that in incubations containing no DTT, the ‘allosteric modulator' SCH-202676 is a thiol-reactive substance and elicits nonspecific effects in [35S]GTPγS binding-based G protein activation assays, thereby challenging previous conclusions regarding the compound's ability to allosterically modulate GPCR function. The nonspecific behaviour was fully reversed upon addition of DTT, indicating the involvement of a thiol-based mechanism of action. Indeed, in routine incubations containing DTT, SCH-202676 had no detectable effect on agonist-stimulated G protein activity, as determined for various GPCRs, arguing against true allosteric modulatory action. Structural analysis using 1H NMR revealed that SCH-202676 was prone to decomposition after reacting with the reducing agent DTT or with brain tissue. While our study was in progress, another group independently demonstrated that SCH-202676 and related thiadiazoles elicit their modulatory action on adenosine A1 receptor via sulphydryl modification rather than via true allosteric mechanism (Göblyös et al., 2005). Using several bioanalytical approaches, this study elegantly demonstrated that the thiadiazoles were reduced into their thiourea precursors by thiol groups of cysteine residues, thus explaining the promiscuous activity towards divergent members of the GPCR family. The present findings are in good agreement with the observations of Göblyös and co-workers.
Collectively, the seminal observations made in these two studies can likely explain the promiscuous action of SCH-202676 towards various GPCRs reported in previous studies (Fawzi et al., 2001; Gao et al., 2004; Lanzafame & Christopoulos, 2004; van den Nieuwendijk et al., 2004). The pioneering work demonstrated that SCH-202676 had no direct effect on heterotrimeric G protein activity as assessed by [35S]GTPγS binding to purified recombinant Gαo subunit or Gβγ-stimulated ADP-ribosylation of Gαo by pertussis toxin (Fawzi et al., 2001). Quite opposite, SCH-202676 at similar concentrations, totally inhibited α2A-adrenoceptor-dependent [35S]GTPγS binding responses in the same study. Curiously, the experiments assessing direct action on G proteins were conducted in the presence of millimolar concentrations of DTT whereas the [35S]GTPγS binding assay measuring α2A-adrenoceptor function contained no DTT. In retrospect, this methodological deviation was actually the first to catch our attention, leading to modify the standard assay protocol so that DDT was omitted. Indeed, such a modification was already successfully applied in our recent study characterizing the highly receptor-specific modulation of GPCR signalling by S-nitrosothiols (Kokkola et al., 2005). As presently shown, attempts to address the allosteric action of SCH-202676 with [35S]GTPγS-based G protein activation assays not containing DTT are severely compromised due to the nonspecific, sulphydryl-involving action of the compound. On the other hand, the reported lack of direct activity of SCH-202676 on G proteins (Fawzi et al., 2001) can be fully explained by the inclusion of DTT in those assays.
Previous studies indicate that thiol-reactive agents can have profound effects on Gi-mediated receptor signalling, as assesses using [35S]GTPγS autoradiography. For example, treatment of brain sections with N-ethylmaleimide (NEM), an irreversible thiol-alkylating reagent targeting the C-terminal cysteine residue in the α subunits of Gi family members, results in total loss of receptor-stimulated G protein activity (Laitinen, 2004). On the other hand, S-nitrosothiols were shown to modulate GPCR signalling via reversible mechanisms probably involving S-nitrosylation (Kokkola et al., 2005). In contrast to the inhibitory action of NEM, S-nitrosothiols were capable of very targeted regulation of GPCR signalling, as they potentiated muscarinic M2/M4 receptor responses, inhibited P2Y12, LPA1, and CB1 receptors, and only marginally affected several other receptors (Kokkola et al., 2005). The behaviour of SCH-202676 is clearly different from those of the above-mentioned thiol agents, as the thiadiazole elicited a generalized nonspecific inhibitory action in [35S]GTPγS binding assays (evident in autoradiography experiments throughout the glass slides and tissue sections, see Figures 2 and 3). Importantly, DTT could fully reverse this effect. NMR analysis indicated that the presence of sulphydryl groups per se (as was the case with DTT) was sufficient to induce chemical decomposition of SCH-202676. We made no further attempts to identify the chemical structure of the thiol-modified product but Göblyös and co-workers (2005) demonstrated that SCH-202676 and related thiadiazoles were reduced into their thiourea precursors by thiol groups of cysteine residues. This study further showed that the thiadiazoles underwent structural changes only after reaction with cysteine, but not other amino acids.
Although not always evident from the original methods description, there is a good reason to assume that the previous radioligand binding assays characterizing the allosteric action of SCH-202676 on various GPCRs were actually conducted in the absence of DTT (Fawzi et al., 2001; Lanzafame & Christopoulos, 2004; Gao et al., 2004, van den Nieuwendijk et al., 2004). As radioligand binding to most GPCRs appears to be sensitive to sulphydryl modification, the allosteric effect of SCH-202676 in these assays is thus also likely best explained by mechanisms involving thiol groups. It is noteworthy that among the P2Y receptors, sensitivity to sulphydryl modification is characteristic for the P2Y12 receptors but not the P2Y1 receptors (Macfarlane et al., 1983, Mills, 1996; Savi et al., 2001; Ding et al., 2003). Interestingly, P2Y1 is so far also the only receptor that in radioligand binding studies was fully resistant to the actions of SCH-202676 (Gao et al., 2004). Thus, SCH-202676 is best described as a sulphydryl-reactive compound rather than a true allosteric modulator.
Acknowledgments
Anna M. Lewandowicz is a medical student from Lodz, Poland and participated in this study as an IFMSA (International Federation of Medical Students' Association) exchange student. We are thankful to Mrs Taina Vihavainen and Mrs Taija Vaarala for skillful technical assistance and to Mrs Sandra Perez for conducting some of the membrane [35S]GTPγS binding assays. Mr Pasi Soininen is acknowledged for his help in generating the NMR figure.
Abbreviations
- 2ClAdo
2-chloroadenosine
- 2MeSADP
2-methylthio ADP
- [35S]GTPγS
guanosine-5′-O-(3-[35S]-thio)-triphosphate
- ADA
adenosine deaminase
- CCh
carbachol
- CP-55,940
(−)-3-[2-hydroxy-4-(1,1-dimethylheptyl)-phenyl]-4-[3-hydroxypropyl]cyclohexan-1-ol
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- DTT
dithiotreitol
- GPCR(s)
G protein-coupled receptor(s)
- LPA
lysophosphatidic acid
- Nsb
nonspecific binding
- SCH-202676
N-(2,3-diphenyl-1,2,4-thiadiazol-5-(2H)-ylidene)methanamine
References
- DING Z., KIM S., DORSAM R.T., JIN J., KUNAPULI S.P. Inactivation of the human P2Y12 receptor by thiol reagents requires interaction with both extracellular cysteine residues, Cys17 and Cys270. Blood. 2003;101:3908–3914. doi: 10.1182/blood-2002-10-3027. [DOI] [PubMed] [Google Scholar]
- FAWZI A.B., MACDONALD D., BENBOW L.L., SMITH-TORHAN A., ZHANG H., WEIG B.C., HO G., TULSHIAN D., LINDER M.E., GRAZIANO M.P. SCH-202676: an allosteric modulator of both agonist and antagonist binding to G protein-coupled receptors. Mol. Pharmacol. 2001;59:30–37. doi: 10.1124/mol.59.1.30. [DOI] [PubMed] [Google Scholar]
- GAO Z.G., GROSS A.S., JACOBSON K.A. Effects of the allosteric modulator SCH-202676 on adenosine and P2Y receptors. Life Sci. 2004;74:3173–3180. doi: 10.1016/j.lfs.2003.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOBLYOS A., DE VRIES H., BRUSSEE J., IJZERMAN A.P. Synthesis and biological evaluation of a new series of 2,3,5-substituted [1,2,4]-thiadiazoles as modulators of adenosine A1 receptors and their molecular mechanism of action. J. Med. Chem. 2005;48:1145–1151. doi: 10.1021/jm049337s. [DOI] [PubMed] [Google Scholar]
- HAPPE H.K., BYLUND D.B., MURRIN L.C. Agonist-stimulated [35S]GTPγS autoradiography: optimization for high sensitivity. Eur. J. Pharmacol. 2001;422:1–13. doi: 10.1016/s0014-2999(01)01043-3. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. Allosteric modulators: the new generation of receptor antagonist. Mol. Interv. 2004;4:222–229. doi: 10.1124/mi.4.4.6. [DOI] [PubMed] [Google Scholar]
- KOKKOLA T., SAVINAINEN J.R., MÖNKKÖNEN K.S., DURÁN RETAMAL M., LAITINEN J.T. S-Nitrosothiols modulate G protein-coupled receptor signaling in a reversible and highly receptor-specific manner. BMC Cell Biol. 2005;6:21. doi: 10.1186/1471-2121-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURKINEN K.M.A., KOISTINAHO J., LAITINEN J.T. [γ-35S]GTP autoradiography allows region-specific detection of muscarinic receptor-dependent G protein activation in the chick optic tectum. Brain Res. 1997;769:21–28. doi: 10.1016/s0006-8993(97)00663-x. [DOI] [PubMed] [Google Scholar]
- LAITINEN J.T. Selective detection of adenosine A1 receptor-dependent G-protein activity in basal and stimulated conditions of rat brain [35S]guanosine 5′-(γ-thio)triphosphate autoradiography. Neuroscience. 1999;90:1265–1279. doi: 10.1016/s0306-4522(98)00571-5. [DOI] [PubMed] [Google Scholar]
- LAITINEN J.T. [35S]GTPγS autoradiography: a powerful functional approach with expanding potential for neuropharmacological studies on receptors coupled to Gi family of G proteins. Curr. Neuropharmacol. 2004;2:191–206. [Google Scholar]
- LAITINEN J.T., URI A., RAIDARU G., MIETTINEN R. [35S]GTPγS autoradiography reveals a wide distribution of Gi/o-linked ADP receptors in the nervous system: close similarities with the platelet P2YADP receptor. J. Neurochem. 2001;77:505–518. doi: 10.1046/j.1471-4159.2001.00265.x. [DOI] [PubMed] [Google Scholar]
- LANZAFAME A., CHRISTOPOULOS A. Investigation of the interaction of a putative allosteric modulator, N-(2,3-diphenyl-1,2,4-thiadiazole-5-(2H)-ylidene) methanamine hydrobromide (SCH-202676), with M1 muscarinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2004;308:830–837. doi: 10.1124/jpet.103.060590. [DOI] [PubMed] [Google Scholar]
- MACFARLANE D.E., SRIVASTAVA P.C., MILLS D.C.B. 2-Methylthio-adenosine[β-32P]diphosphate. An agonist and radioligand for the receptor that inhibits the accumulation of cyclic AMP in intact blood platelets. J. Clin. Invest. 1983;71:420–428. doi: 10.1172/JCI110786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLS D.C.B. ADP receptors on platelets. Thromb. Haemost. 1996;76:835–856. [PubMed] [Google Scholar]
- SAVI P., LABOURET C., DELESQUE N., GUETTE F., LUPKER J., HERBERT J.M. P2Y12, a new platelet ADP receptor, target of clopidogrel. Biochem. Biophys. Res. Commun. 2001;283:379–383. doi: 10.1006/bbrc.2001.4816. [DOI] [PubMed] [Google Scholar]
- SAVINAINEN J.R., SAARIO S.M., NIEMI R., JÄRVINEN T., LAITINEN J.T. An optimised approach to study endocannabinoid signalling: evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors. Br. J. Pharmacol. 2003;140:1451–1459. doi: 10.1038/sj.bjp.0705577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DEN NIEUWENDIJK A.M., PIETRA D., HEITMAN L., GOBLYOS A., IJZERMAN A.P. Synthesis and biological evaluation of 2,3,5-substituted [1,2,4]thiadiazoles as allosteric modulators of adenosine receptors. J. Med. Chem. 2004;47:663–672. doi: 10.1021/jm030863d. [DOI] [PubMed] [Google Scholar]
- VASILJEV K.S., URI A., LAITINEN J.T. 2-Alkylthio-substituted platelet P2Y12 receptor antagonists reveal pharmacological identity between the rat brain Gi-linked ADP receptor and P2Y12. Neuropharmacology. 2003;45:145–154. doi: 10.1016/s0028-3908(03)00142-4. [DOI] [PubMed] [Google Scholar]
- WAEBER C., CHIU M.L. In vitro autoradiographic visualization of guanosine-5′-O-(3-[35S]thio)triphosphate binding stimulated by sphingosine 1-phosphate and lysophosphatidic acid. J. Neurochem. 1999;73:1212–1221. doi: 10.1046/j.1471-4159.1999.0731212.x. [DOI] [PubMed] [Google Scholar]