Abstract
Cryptochrome blue-light photoreceptors are found in both plants and animals and have been implicated in numerous developmental and circadian signaling pathways. Nevertheless, no action spectrum for a physiological response shown to be entirely under the control of cryptochrome has been reported. In this work, an action spectrum was determined in vivo for a cryptochrome-mediated high-irradiance response, the blue-light-dependent inhibition of hypocotyl elongation in Arabidopsis. Comparison of growth of wild-type, cry1cry2 cryptochrome-deficient double mutants, and cryptochrome-overexpressing seedlings demonstrated that responsivity to monochromatic light sources within the range of 390 to 530 nm results from the activity of cryptochrome with no other photoreceptor having a significant primary role at the fluence range tested. In both green- and norflurazon-treated (chlorophyll-deficient) seedlings, cryptochrome activity is fairly uniform throughout its range of maximal response (390–480 nm), with no sharply defined peak at 450 nm; however, activity at longer wavelengths was disproportionately enhanced in CRY1-overexpressing seedlings as compared with wild type. The action spectrum does not correlate well with the absorption spectra either of purified recombinant cryptochrome photoreceptor or to that of a second class of blue-light photoreceptor, phototropin (PHOT1 and PHOT2). Photoreceptor concentration as determined by western-blot analysis showed a greater stability of CRY2 protein under the monochromatic light conditions used in this study as compared with broad band blue light, suggesting a complex mechanism of photoreceptor activation. The possible role of additional photoreceptors (in particular phytochrome A) in cryptochrome responses is discussed.
Blue-light responses are found in organisms throughout the biological kingdom; in many instances, these responses are mediated by photoreceptors that specifically absorb blue light and are relatively ineffective at other wavelengths. In Arabidopsis, flavoprotein blue-light photoreceptors known as cryptochromes have been implicated in light responses including inhibition of hypocotyl elongation, anthocyanin accumulation, internode and petiole elongation, seed germination, blue-light-regulated gene expression, initiation of flowering time, phototropism, and the entrainment of circadian rhythms (Ahmad and Cashmore, 1996; Ahmad, 1999). Cryptochrome-type photoreceptors have recently been found in animal systems as well wherein they seem to play essential roles in the entrainment and maintenance of circadian rhythms (Sancar, 2000). Cryptochromes are characterized by their striking homology to certain classes of DNA photolyases, or blue-light-dependent DNA repair enzymes, which catalyze a blue-light-dependent electron transfer reaction (Deisenhofer, 2000). In Arabidopsis there are two similar genes encoding cryptochrome photoreceptors, CRY1 (or HY4) and CRY2, whose encoded proteins differ primarily in their respective C-terminal domains. In addition, the CRY1 photoreceptor is stable in plants grown in high intensities of blue light, whereas CRY2 photoreceptor fails to accumulate and appears to be rapidly degraded under conditions wherein the photoreceptor is active (Ahmad et al., 1998a; Lin et al., 1998). Cryptochromes contain the chromophore-binding domain of photolyases, and have been shown to bind both a pterin and flavin chromophore; however, they lack the apparent pyrimidine dimer binding or repair activity of photolyases (Malhotra et al., 1995; Lin et al., 1995). In addition, some cryptochromes contain C-terminal extensions not found in photolyases, which are shown to be necessary for photoreceptor functions (Ahmad et al., 1995; Yang et al., 2000). Given the striking homology of cryptochromes to photolyases, a primary mechanism of action via blue-light-dependent electron transfer is likely (see Ahmad and Cashmore, 1996; Ahmad, 1999; Lin, 2000, and refs. therein). Recent studies with fruitfly (Drosophila melanogaster) and animal cryptochromes also suggest electron transfer mechanisms (Lin et al., 2001).
Although the cryptochrome photoreceptors are well characterized and have been studied in a variety of plant and animal systems, very little is known about their mechanism of action and photochemistry. Photolyases, to which the cryptochrome photoreceptors are most closely related, are unusual among flavoproteins in that they are activated with flavin present in the reduced form. Reduced flavin, in contrast to oxidized flavin, has a peak of absorption at around 360 nm and absorbs very little visible light. As a result, photolyases rely on an antennae pigment, a folate, or deazaflavin derivative for the bulk of their activity in blue light (400–500 nm). The peak of absorption of the antennae pigment can be between 380 to 440 nm depending on the precise composition of the chromophore (Sancar et al., 1987; Ahmad and Cashmore, 1996) and seems to occur at near 420 nm for purified recombinant cryptochrome (Malhotra et al., 1995). By contrast, most flavoenzymes occur in the oxidized form, showing a peak of absorption at 450 nm, as is found in the phototropin-like photoreceptors (Briggs et al., 2001). Therefore, an important clue regarding the mechanism of action of the cryptochrome photoreceptors would be to ascertain the absorption characteristics of the photoreceptor in its active state within the plant. Such data is provided by the production of an action spectrum, a dose-response curve at multiple wavelengths, for a certain plant activity or physiological response that is under the control of this photoreceptor.
Although many action spectra for plant blue-light responses exist in the literature, their interpretation is complicated by the fact that multiple plant photoreceptors in addition to cryptochromes are active in blue light. Unrelated blue-light photoreceptors, distinct from the cryptochromes, have recently been identified and shown to be flavoproteins, absorbing maximally at 450 nm (Briggs and Huala, 1999; Briggs et al., 2001). In addition, plant phytochrome photoreceptors, which respond principally to red/far-red light, show some absorption of blue light, rendering the interpretation of classical blue-light action spectra more difficult (Shinomura et al., 2000; Shinomura et al., 1996). In the case of higher plants, the situation is even further complicated by the observation that a signal from phytochrome is necessary for full activity of the cryptochrome blue-light photoreceptors in hypocotyl growth inhibition and anthocyanin accumulation (Ahmad and Cashmore, 1997). Therefore, it is not possible to infer that any given published blue-light action spectra represents the activity of cryptochrome.
In this study, an action spectrum is generated for a response demonstrated to be under the control of cryptochrome, namely blue-light-dependent inhibition of hypocotyl elongation in Arabidopsis. In previous studies in Arabidopsis (Young et al., 1992; Goto et al., 1993), the extent of growth inhibition resulting from cryptochrome activity (both CRY1 and CRY2) had not been determined, nor had there been correction for potential artifacts resulting from shading of the blue-light receptors by chlorophylls and carotenoids. In the present study, we show that cry1cry2 double-mutant seedlings show no measurable growth inhibition at wavelengths from 390 to 530 nm under the given light intensities, indicating cryptochrome as the primary photoreceptor species involved. Growth inhibition biosynthesis studies in the presence of norflurazon, a carotenoid inhibitor resulting in photobleaching of Arabidopsis seedlings, were performed to correct for possible shifts in the action spectra due to shading by chlorophyll. Further clues into possible photoreceptor mechanism of action and photochemistry is provided by examination of action spectra of a cryptochrome-1-overexpressing transgenic line and an analysis of photoreceptor concentration and stability, in particular that of light-labile CRY2. These action spectra are compared with the absorption spectra of purified Arabidopsis blue-light photoreceptors, and the results are discussed in relation to the known photoreceptors that might contribute to this response in Arabidopsis and other plants.
RESULTS
Inhibition of Hypocotyl Elongation in Arabidopsis Activity Spectrum for Cryptochrome in a High-Irradiance Response
In Arabidopsis, inhibition of hypocotyl elongation is a sensitive and quantitative light response in which multiple photoreceptors participate, including phytochromes, CRY1, and CRY2. There are currently two published action spectra for blue-light-dependent inhibition of hypocotyl elongation in Arabidopsis (Young et al., 1992; Goto et al., 1993), but in neither case did the authors identify the component of the response due solely to cryptochrome (CRY1 and CRY2). In the present study, the growth response of wild-type Arabidopsis seedlings was compared with that of double cryptochrome cry1cry2 mutants, allowing unequivocal determination of an activity spectrum for cryptochrome. In addition, the responsivity of seedlings overexpressing CRY1 protein was evaluated for possible wavelength-specific effects, because such seedlings show a hypersensitive response to blue light under broad band conditions (Lin et al., 1996; Ahmad et al., 1998a). In darkness, the lengths of the hypocotyls of wild type, mutant seedlings and CRY1 overexpressors were essentially the same (Fig. 1A). Hypocotyl growth inhibition in light-treated seedlings was measured at multiple light intensities at wavelengths of 10- to 20-nm intervals spanning the range of 380- to 450-nm bandwidth, with additional points in the red/far-red and near-UV region (not shown). The degree of inhibition of hypocotyl growth was plotted as a percentage of the growth of seedlings retained in complete darkness (no growth inhibition) at wavelengths in which a robust cryptochrome response was obtained (Fig. 1B). These experiments were repeated in a total of five separate trials; in all cases, the results were qualitatively similar and internally consistent. The differential growth inhibition of seedlings lacking all cryptochrome (cry1cry2), and containing a superabundance of cryptochrome photoreceptor (Oe) allows the determination of a high-irradiance action spectrum for a cryptochrome response.
Figure 1.
Inhibition of hypocotyl elongation in Arabidopsis seedlings. A, Hypocotyl lengths of seedlings germinated as described in “Materials and Methods” and maintained for the indicated lengths of time in darkness. Error bars represent the se. Wt, Wild type; Oe, CRY1 overexpressor; C1C2, cry1cry2 double-mutant seedlings. B, Seedlings were plated and placed under monochromatic light sources as indicated in “Materials and Methods.” Measurements of seedling hypocotyl growth are presented as percentages of dark-grown control seedlings (where there is no growth inhibition). Error bars represent the se. OE, Transgenic seedlings overexpressing CRY1 protein; Wt, wild-type seedlings; C1C2, cry1cry2 double-mutant seedlings. C, Plot of cryptochrome action spectrum for 20% and 30% hypocotyl growth inhibition, respectively, as calculated from the data used in B. Oe, Overexpressor of CRY1; Wt, wild type. Peaks represent peak activity (maximal sensitivity) of the photoreceptor.
Significantly, cry1cry2 double-mutant seedlings did not show growth inhibition at any wavelength of monochromatic light tested between 311 and 550 nm at the photon fluence rates used in this study. Both wild-type and CRY1-overexpressing seedlings showed maximal growth inhibition between 380 and 500 nm (Fig. 1B). Very little response was seen below 365 nm, using UV sources of light fluence 0.5 to 1.0 μmol m−2 s−1. From 500 to 550 nm, little growth inhibition of wild type was observed at the output (30–50 μmol m−2 s−1) our lamps could generate, although increased growth inhibition of CRY1-overexpressing seedlings as compared with wild type or cry1cry2 double mutant was observed until 550 nm (not shown). At wavelengths longer than 570 nm, there were no detectable differences in light-dependent hypocotyl growth inhibition between cryptochrome-deficient and cryptochrome-overexpressing seedlings, even at quite high light intensities (80–100 μmol m−2 s−1; not shown). This result is consistent with cryptochromes being active principally in the range from 365 to 550 nm.
To more precisely ascertain the wavelength at which cryptochrome is maximally effective in these seedlings, an action spectrum was generated in which the photon fluence rate resulting in 20% or 30% growth inhibition was plotted as a function of wavelength (Fig. 1C). The action spectrum is plotted such that peaks occurring in the plot correspond to peak sensitivity of the photoreceptor. In the case of wild type, the action spectrum is almost flat with no more than a 2-fold variation in effectiveness of light between 380 and 480 nm. Similar to wild type, the CRY1-overexpressing seedlings showed a fairly flat action spectrum, although they were considerably more light-sensitive than wild type at all wavelengths tested. However, in contrast to wild type, maximal activity in the CRY1 overexpressor occurred near 480 nm, with a greater relative responsivity at 500 nm (only 4-fold less than that of wild type, which showed a 10-fold drop in activity at 500 nm compared with 450 nm; Fig. 1C). Thus, increasing the dosage of cryptochrome photoreceptor appears to result in shift of photoreceptor sensitivity toward higher bandwidths.
A concern with the interpretation of action spectra in de-etiolated plant material is shading by non-photoreceptor pigments, in particular chlorophylls and carotenoids, both of which absorb in blue light. It had been previously shown for both Sinapis alba (Beggs et al., 1980) and Chenopodium rubrum (Holmes and Wagner, 1982) that chlorophyll significantly altered the action spectrum of hypocotyl growth inhibition in these plant species. To correct for possible shading artifacts, action spectra for seedling growth inhibition were repeated on petri plates containing the herbicide norflurazon, an inhibitor of carotenoid biosynthesis that also prevents chlorophyll accumulation in light-grown plants (Beggs et al., 1980; Holmes and Wagner, 1982).
Wild type, cry1cry2 mutant seedlings, and CRY1 overexpressors grown on norflurazon showed identical rates of growth in darkness, red light (660 nm), and far-red light (713 nm; Fig. 2A). This indicates that there was no activity of cryptochrome under these conditions, and also that the various mutant lines were comparable in growth capacity. At wavelengths from 392 to 530 nm, there was no observable growth inhibition in cry1cry2 double-mutant seedlings (Fig. 2B), indicating that no additional blue-light photoreceptor was contributing measurably to the primary response at these wavelengths. As an additional control, the degree of growth inhibition obtained for seedlings at 450 nm was measured after 30 h of continuous growth instead of 60 h. These data were compared with the data at 450 nm obtained for growth inhibition at 60 h in Figure 2B. No difference in fluence response characteristics was observed, indicating that no major distortion in the action spectra is likely to occur at least during the latter one-half of the growth period (not shown). The action spectrum for the norflurazon-grown seedlings was calculated from the data used for Figure 2B and plotted for 20%, 30%, and 50% growth inhibition for wild-type and CRY1-overexpressing seedlings (Fig. 2C).
Figure 2.
Inhibition of hypocotyl elongation in Arabidopsis seedlings grown on norflurazon. A, Hypocotyl lengths of seedlings germinated as described in “Materials and Methods” and maintained for the indicated lengths of time in darkness (upper panel) or for 60 h continuous growth in 660-nm red light (41 μmol m−2 s−1) or 713 nm far red light (29 μmol m−2 s−1). Error bars represent the se. Wt, Wild type; Oe, CRY1 overexpressor; C1C2, cry1cry2 double-mutant seedlings. B, Seedlings were plated and placed under monochromatic light sources as indicated in “Materials and Methods.” Measurements of seedling hypocotyl growth are presented as percentages of dark-grown control seedlings (where there is no growth inhibition). Error bars represent the se. Oe, Transgenic seedlings overexpressing CRY1 protein; Wt, wild-type seedlings; C1C2, cry1cry2 double-mutant seedlings. C, Plot of cryptochrome action spectrum for 20%, 30%, and 50% hypocotyl growth inhibition, respectively, as calculated from the data presented in B. Oe, Overexpressor of CRY1; Wt, wild type. Peaks represent peak activity (maximal sensitivity) of the photoreceptor.
The action spectra obtained on norflurazon for wild-type seedlings is also fairly flat in shape with no more than 2.5-fold variation in peak photon effectiveness in the wavelength range from 380 to 480 nm (Fig. 2C). In contrast to untreated seedlings, some growth inhibition was observed for cry1cry2 double-mutant seedlings at 380 nm (Fig. 2B, see 380-nm curve), indicating the activity of additional photoreceptors absorbing in the UV that were shaded in untreated seedlings and that were, thereby, not detected. In addition, wild-type seedlings were more sensitive to blue light on norflurazon and responded to lower light intensities than was the case for untreated seedlings, again suggesting that chlorophyll significantly shades the relevant photoreceptors in green tissues. Action spectra obtained on norflurazon for the cryptochrome-overexpressing lines did not show a peak near 480 nm as for untreated seedlings but, instead, showed a more pronounced shoulder than seen for the wild type.
Differential Stability of CRY2 Protein in Broad Band and Monochromatic Light
It has been previously shown that both CRY1 and CRY2 contribute to the growth inhibition response in Arabidopsis (Ahmad et al., 1998a; Lin et al., 1998). However, unlike CRY1, the CRY2 photoreceptor of Arabidopsis appears to be light labile, and levels of photoreceptor protein in seedlings are significantly lower in broad band blue, UV-A, and green light (in which the photoreceptor is active) than in red light or dark (in which the photoreceptor shows no activity). To assess the possible contribution of CRY2 protein to the cryptochrome action spectrum, the stability of CRY2 protein was evaluated at several wavelengths of monochromatic light throughout the range of maximum cryptochrome effectiveness (Fig. 3). Western-blot analysis of seedlings of Arabidopsis were performed with anti-CRY2 antibody, and levels of CRY2 protein were compared with levels of accumulation in dark. Surprisingly, at 400, 450, and 500 nm, the amount of CRY2 protein did not decrease dramatically from that found in dark-grown control seedlings. The light photon fluences used were relatively high; at 450 nm, the intensity of 8 μmol m−2 s−1 was sufficient to induce more than 50% hypocotyl growth inhibition in wild-type seedlings, for example, indicating considerable cryptochrome photoreceptor activity. These results are in contrast to the marked instability of CRY2 protein in broad band blue light (Fig. 3).
Figure 3.
Stability of CRY2 protein under monochromatic light conditions. Equal amounts of protein from 3-d-old seedlings grown under the indicated light conditions were loaded onto each lane of an SDS-polyacrylamide gel, transferred to nitrocellulose, and probed with anti-CRY2 antibody. Light intensities used are 10 μmol m−2 s−1 for red and blue light under broad band conditions and 10, 8, and 25 μmol m−2 s−1 light fluence for 400-, 450-, and 500-nm monochromatic light, respectively.
Cryptochrome Activity Is Modified by Receptor Dosage in a Wavelength-Specific Manner
An unexpected feature of the cryptochrome action spectrum is that CRY1-overexpressing seedlings show relatively greater activity than wild type at wavelengths longer than about 430 nm (Figs. 1C and 2C). There is an approximately 4-fold greater sensitivity to 400 nm in CRY1-overexpressing seedlings as compared with wild type, whereas the difference in sensitivity is almost 20-fold at 480 nm light. The observed shift in peak activity to 480 nm of cryptochrome-overexpressing seedlings (Fig. 1C) is significantly less pronounced in norflurazon-treated seedlings (Fig. 2C), suggesting it may have been partially resulting from the higher chlorophyll content of such seedlings in continuous light conditions. Nevertheless, a greater sensitivity to higher wavelength light in CRY1-overexpressing seedlings holds even in the presence of norflurazon. For instance, CRY1-overexpressing seedlings on norflurazon are about 4-fold more sensitive than wild-type seedlings between 392 and 413 nm monochromatic light (Fig. 2, B and C). By contrast, the difference in sensitivity is close to 10-fold at several longer wavelengths, particularly between 472 and 491 nm.
This effect is illustrated in Figure 4A, showing a typical experiment where seedlings of wild-type and overexpressing lines were subjected to decreasing photon fluences of monochromatic light at 491 and 400 nm light, respectively. Wild-type seedlings show similar sensitivity to the initial light intensity chosen. However, CRY1-overexpressing seedlings are considerably more sensitive to decreasing intensities of 491 nm than of 400 nm light.
Figure 4.
CRY1 dosage dependence of hypocotyl growth inhibition. A, Relative growth inhibition of norflurazon-treated wild-type (wt) and cryptochrome-overexpressing seedlings at decreasing photon fluence rates of 491- and 400-nm monochromatic light, respectively. Initial photon fluence rates (designated 100%) were chosen that resulted in identical growth inhibition of wild-type seedlings (7 μmol m−2 s−1 at 491 nm; 2.4 μmol m−2 s−1 at 400 nm). Growth of seedlings was compared at decreasing intensities of light (60%, 33%, and 13%), and growth inhibition of CRY1-overexpressing seedlings was compared under the two wavelengths. Overexpressing seedlings (Oe) showed greater relative sensitivity to 491-nm than to 400-nm light. B, CRY1 cryptochrome photoreceptor concentration in the seedlings did not change at any light treatment. Western blots were prepared from Wt and Oe (overexpressing) seedlings from A and compared with seedlings grown in continuous darkness. C, Comparison of photoreceptor concentration between cryptochrome-overproducing and wild-type seedlings. Lane 1, Wt and Oe indicates equivalent concentrations of total proteins of wild-type and overexpressing seedlings, respectively. Lanes 0.5 and 0.2 represent a dilution of 2- and 5-fold, respectively, of plant extract from the cryptochrome-overexpressing seedlings.
A simple explanation for the increased sensitivity to blue light in CRY1-overexpressing Arabidopsis lines would be a proportionate increase in the concentration of the cryptochrome photoreceptors. To test this possibility, the levels of CRY1 and CRY2 protein were compared in wild-type and overexpressing Arabidopsis lines by western-blot analysis under the different growth conditions used in Figure 4A (Fig. 4B). At none of the light intensities examined was there any variation relative to dark levels of cryptochrome photoreceptor, either in overexpressing (Oe) or wild-type lines. Finally, a dilution series of protein extracted from cryptochrome-overexpressing lines indicated the increase in cryptochrome photoreceptor concentration in the CRY1-overexpressing Arabidopsis lines was not greater than a 5-fold increase as compared with wild type (Fig. 4C).
DISCUSSION
In this study we have measured hypocotyl growth inhibition in a number of cryptochrome mutants over the wavelength range 311 to 730 nm. To correct for possible shading artifacts by chlorophyll or carotenoids (Beggs et al., 1980; Holmes and Wagner, 1982), the action spectra were also performed with seedlings grown on the herbicide norflurazon, which eliminates chlorophyll in light-grown seedlings. Based on comparisons of wild-type, cry1cry2 double-mutant, and CRY1-overexpressing Arabidopsis seedlings, it was determined that maximal cryptochrome activity occurs between 380- to 500-nm bandwidth, and that, moreover, there is no significant independent contribution by other blue-light receptors to stem growth inhibition between 392 to 530 nm under the conditions used in this study. There was some residual cryptochrome activity at shorter wavelengths (up to 365 nm) and also at longer wavelengths up to 550 nm, but no cryptochrome-specific activity in either red (660 nm) or far-red (713 nm) light, consistent with several prior studies (Goto et al., 1993; Lin et al., 1996) and somewhat in contrast to recent suggestions for a role for cryptochrome in red light (Devlin and Kay, 2000).
In overall shape and fluence threshold, the data we present for wild-type seedlings are in agreement with the results from prior studies investigating hypocotyl growth in Arabidopsis as a function of wavelength, involving a comparison of wild-type with phytochrome-deficient hy2 mutant seedlings (Goto et al., 1993) and to blu1, an allele of hy4 (deficient in CRY1; Young et al., 1992). However, the action spectra obtained from norflurazon-treated seedlings, though similar in shape to those of untreated seedlings for wild type, showed significantly greater sensitivity at all light intensities, in agreement with prior observations for other plant species (Beggs et al., 1980; Holmes and Wagner, 1982).
The absence of response in cry1cry2 double mutants in blue light is important because it suggests that no other photoreceptor has a significant direct role in blue-light-dependent hypocotyl growth inhibition. Therefore, other photoreceptors that have been proposed to play a direct role, in particular phytochrome A (phyA; Casal and Mazella, 1998; Neff and Chory, 1998; Poppe et al., 1998) most likely act indirectly through modification/interaction with the cryptochrome-dependent signaling pathway. An enhancement of CRY2 function by phytochrome is demonstrated by pulse experiments, in which red-light treatment increases CRY2-dependent growth inhibition in Arabidopsis seedlings (Fig. 5). In addition, possible interaction between CRY2 and phytochrome has been documented both in vitro (Ahmad et al., 1998b) and in vivo (Mas et al., 2000). Recent studies on the role of phyA in early events of blue-light-mediated stem growth inhibition support an indirect role for phyA in some cryptochrome responses (Folta and Spalding, 2001b).
Figure 5.
Enhancement of CRY1 and CRY2 action by phytochrome. Seedlings of the indicated genotypes (wt, wild type; cry1cry2, double mutant of cryptochrome; OECRY1, overexpressor of CRY1; OECRY2, overexpressor of CRY2; Ahmad et al., 1998a) were germinated as described in “Materials and Methods” and then placed for 72 h under the following light conditions: BL, 0.05 μmol m−2 s−1 blue-light intensity; BL + RL, seedlings were kept at 0.05 μmol m−2 s−1 blue-light intensity and subjected to 10-min red light pulses once every 3 h at a fluence of 3 μmol m−2 s−1; RL, seedlings were kept in continuous darkness and subjected to 10-min red light pulses once every 3 h at a fluence of 3 μmol m−2 s−1. Hypocotyl lengths of 20 seedlings per light treatment were averaged; error bars represent the se.
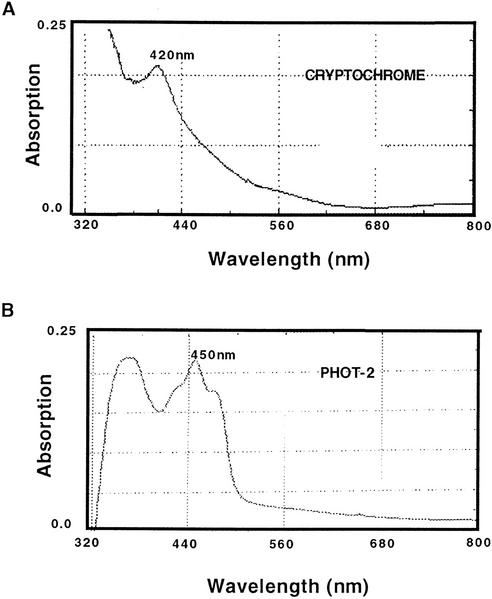
Action spectra have traditionally been constructed as a tool for proposing the molecular identity of the relevant photoreceptor. The absorption spectra of putative photoreceptors is compared with the action spectrum of the response, and the best “match” is presumed to be the photoreceptor. There exist a number of published absorption spectra for recombinant cryptochrome expressed in heterologous systems. Plant cryptochrome expressed heterologously in Escherichia coli shows a pronounced peak of absorption near 420 nm as a result of the pterin chromophore (Malhotra et al., 1995), with the absorption spectrum trailing off rapidly to near zero by 500 nm. CRY1 photoreceptor has, in addition, been expressed in insect cell systems, from which it has been isolated as an oxidized flavoprotein lacking a secondary pterin chromophore (Lin et al., 1995). However, a difference spectrum between insect cell extracts expressing high levels of CRY1 photoreceptor protein and control extracts (not expressing CRY1) shows an absorption spectrum identical to the E. coli expression product, with a major peak near 420 nm (Fig. 6A). Therefore, the lack of a secondary chromophore in these earlier insect cell experiments may have occurred as a result of the purification process, in which the pterin chromophore was lost.
Figure 6.
Absorption spectra of purified blue-light photoreceptors. A, Absorption spectrum of CRY1 protein expressed in baculovirus-infected Sf9 insect cells. Characteristics of absorption spectra are as in published plant cryptochrome spectra (Malhotra et al., 1995). B, Absorption spectrum of amino terminal NPL1 or PHOT-2 protein fragment expressed in E. coli. Characteristics of absorption spectra are as in published NPH1 spectra (Christie et al., 1998; Salomon et al., 2000).
Surprisingly, comparison of the action spectra (Figs. 1C and 2C) with the cryptochrome absorption spectrum (Fig. 6A) shows little correspondence. There is no pronounced peak at 420 nm in the action spectrum, and activity at 500 nm is relatively high, particularly in overexpressing seedlings, in comparison with the absorption spectrum. This lack of correspondence between action spectrum and absorption spectrum is all the more surprising because, in the case of type I photolyases, to which plant cryptochromes show the greatest degree of homology, action spectra for DNA photorepair are essentially superimposable upon the absorption spectra of the purified proteins (Jorns et al., 1986; Sancar et al., 1987). Cryptochrome may in fact be active in a different form (perhaps using oxidized flavin, thereby explaining the peak at 450 nm) than is photolyase, thus, raising the intriguing possibility of a differing primary mechanism of action of cryptochrome from photolyase.
An alternative explanation for the lack of correspondence between absorption spectra and action spectra may be complex events downstream of the point of photoreception, which distort the action spectra. This is particularly a possibility with action spectra involving long periods of irradiation (HIR spectra). To determine whether distortion might occur over the period of total irradiation, we measured degree of growth inhibition after a shorter (30 h) growth period for seedlings in 450 nm light, but observed no significant distortion at least after the 1st d of growth (not shown). Nevertheless, only a much more rapid assay for cryptochrome function can definitively exclude the possibility of artifact in these action spectra. It would also be preferable to devise an assay for cryptochrome function in dark-grown seedlings, because in this way, indirect light effects on concentrations of other photoreceptors and/or signaling intermediates may be avoided.
A second class of plant blue-light photoreceptor for which the absorption spectra have been determined are the plant phototropins, PHOT1 and PHOT2. This class of photoreceptor bind oxidized flavin and have been implicated in a variety of blue-light responses including phototropism and blue-light-dependent chloroplast movements (Briggs et al., 2001). The absorption spectrum of purified recombinant PHOT1 has been identified from both insect cell (Christie et al., 1998) and E. coli (Salomon et al., 2000) expression systems and shows a marked peak at 450 nm resulting from oxidized flavin. For the purposes of this discussion, we have expressed a fragment of PHOT2 comprising the flavin-binding domains of this photoreceptor in E. coli and present the absorption spectrum below (Fig. 6B). Interestingly, the cryptochrome action spectrum is somewhat more similar to the phototropin absorption spectra than it is to that of purified cryptochrome. The position of the peak (450 nm) and shoulders (Figs. 1C and 2C) in wild-type seedlings corresponds more closely with those of the phototropin absorption spectrum, although the cryptochrome curve is much flatter. We have determined that there is a small effect of PHOT1 on hypocotyl elongation at high-intensity broad band blue light (M. Ahmad, unpublished data). A role for phototropin in inhibition of hypocotyl elongation is also suggested in recent publications investigating blue-light-dependent activation of ion channels (Folta and Spalding, 2001a). However, given that cry1cry2 double mutants show no growth inhibition at the conditions used in the present work, any contribution by such PAS-domain-containing photoreceptors to the action spectrum is likely to be indirect and/or minor.
Evidence of wavelength-specific effects on the cryptochrome photoreceptors is found in the differential stability of CRY2 protein. Under broad band blue-light conditions, CRY2 protein is rapidly degraded and accumulates to only very low concentrations in seedlings. This may be due to targeting by degradative enzymes upon activation of the photoreceptor, by analogy to the situation with phyA. However, under the narrow band light conditions used in these action spectra, CRY2 protein appears to be more stable and accumulates even at relatively high blue-light intensities. It is possible that cryptochromes may cycle between multiple conformations upon activation by light and that only one of these forms is recognized by protein degradative enzymes. It will be intriguing to examine whether combinations of monochromatic light of different wavelengths reduce the stability of CRY2 protein and whether the conformation of CRY2 photoreceptor under monochromatic light conditions differs from that under broad band blue light.
The action spectra of CRY1-overexpressing seedlings show an unexpected feature in that the responsivity varies between a 4-fold to a more than 10-fold increase compared with wild type, even though the increase in photoreceptor concentration is not more than 5-fold at any wavelength. In particular, there is greater responsivity of cryptochrome-overexpressing seedlings above 430 nm in comparison with wild-type seedlings of both untreated and norflurazon-treated plant material. A similar shift to longer wavelengths in the action spectrum has been observed for transgenic Arabidopsis seedlings overexpressing phytochrome (McCormac et al., 1993; Shinomura et al., 1998) and was explained in terms of wavelength dependent differences in the absolute amounts of Pfr generated in overexpressing lines (Shinomura et al., 1998). It is intriguing to speculate that dosage dependence in cryptochrome photoreceptor mutants may also reflect differential accumulation and/or lifetime of multiple interchangeable receptor conformations.
In summary, we present here an action spectrum for a response demonstrated to be under the control of cryptochrome under the fluence range investigated. The action spectrum shows several unexpected features, in particular a lack of correspondence with the absorption spectrum of the purified cryptochrome photoreceptor from several heterologous systems. This may indicate some difference in the biochemical mode of action between cryptochromes and photolyases, where absorption spectra and action spectra coincide closely. Furthermore, examination of cryptochrome responses in CRY1-overexpressing seedlings shows a complex dosage dependence at different wavelengths, suggesting a complex mechanism of activation. Finally, the stability of CRY2 photoreceptor is affected differentially by broad band and monochromatic light, further suggesting complex events within the photoreceptor at the point of absorption of the light signal. It will be of great interest to sort out the molecular basis of this intriguing physiology, both by biophysical investigation of the purified photoreceptor and by detailed examination of cryptochrome interactions with potential substrates as a function of wavelength.
MATERIALS AND METHODS
Arabidopsis Seedling Germination and Growth
For all light irradiation experiments Arabidopsis seeds were surface sterilized by a brief wash in 100% (v/v) ethanol, followed by air drying under a laminar flow hood. Seeds were sown on one-half-strength Murashige and Skoog salts medium (Sigma, St. Louis) on petri plates containing 2% (w/v) Suc and 0.8% (w/v) agar. Norflurazon was added at a final concentration of 5 × 10−6 M and was a kind gift of Dr. Klaus Kreuz (Novartis, Basel). Plates were stored for 2 d at 4°C to break dormancy, and were subsequently transferred to white light (30 μmol m−2 s−1) for 24 h to induce germination. Hy4-2.23N and fha alleles were used for CRY1 and CRY2 mutations, respectively; CRY1- and CRY2-overexpressing seedlings were as described (Ahmad et al., 1998a).
Interference Filters and Light Sources
Monochromatic light was produced by slide projectors (Prado Universal 31047, Ernst Leitz GmBH, Wetzler, Germany) in combination with 5-mm KGI heat-absorbing filters (Schott Glaswerke, Mainz, Germany) placed before interference filters of 8- to 10-nm half-bandwidth (Schott Glaswerke, Mainz, Germany). Broad band light was produced by using cool-white fluorescent bulbs (Philips, Eindhoven, The Netherlands) in association with broad band plexiglass filters of 100-nm bandwidth as used in prior studies (Ahmad et al., 1998a).
Inhibition of Hypocotyl Elongation Assays
Approximately 50 seeds of wild-type, cryptochrome-overexpressing, and cryptochrome-deficient Arabidopsis lines were plated within a single petri dish for each light treatment. In this way, hypocotyl growth of seedlings is directly comparable and did not suffer from possible inconsistencies in light treatments or experimental manipulation between plates. Seedlings were plated an average of 1 to 2 mm apart to minimize self-shading through the course of the light irradiations. Plates were stacked in columns under light sources, and irradiated from above, such that the light intensity at subsequent positions in the stack were reduced by a defined amount. Beams of light from slide projectors were shone through interference filters by objectives that penetrated the wall at each position. Irradiations were performed for a continuous 60 h after the 24-h white-light treatment to induce germination of seedlings (see above). For each experiment, a number of plates were wrapped in foil and maintained for 60 h as dark controls. Light intensities at the surface of the petri plates were measured for each plate in a stack after the completion of the experiment. In all instances, dark controls showed no evidence of differential growth between the different genotypes, indicating that this growth regime does not activate the cryptochrome photoreceptors in the absence of light. Subsequent to termination of the light treatments, samples for determination of protein concentrations were taken immediately. Additional plates of seedlings were stored at 4°C for a period not greater than 8 h, during which time from 10 to 20 seedlings were measured per plate per Arabidopsis line. Error bars represent the se; in general, growth was very uniform, and variation within a population was not more than 10% of total seedling length. Light treatments under broad band filters were carried out under light conditions (filters and fluorescent bulbs) as described previously (Ahmad et al., 1998a).
Western-Blot Analysis
Western-blot analysis of seedlings was performed using anti-CRY1 and anti-CRY2 antibodies prepared to the C terminus of the respective proteins expressed in recombinant form, essentially as described previously (Ahmad et al., 1998a). All seedlings used for western-blot analysis were quick-frozen in liquid nitrogen subsequent to light treatments. Samples were then ground in SDS sample buffer (Laemmli) and equivalent amounts of protein run on each lane of an SDS polyacrylamide gel, before western transfer and detection by antisera.
Absorption Spectra of Recombinant Photoreceptors
Cryptochrome-1 protein was expressed in insect cell (Sf9) system as described previously (Lin et al., 1995). Insect cell cultures expressing recombinant cryptochrome were precipitated and lysed as described (Lin et al., 1995) in parallel with an equal amount of uninfected Sf9 cells. The cell suspensions were clarified by ultracentrifugation at 40,000 rpm for 1 h, and the supernatants were adjusted to an equivalent total protein concentration (1 mg mL−1). The absorption spectrum of the extract from both cryptochrome-expressing and control cells was taken in a DU7400 spectrophotometer (Beckman Coulter, Inc., Fullerton, CA). Insect cell control cultures not expressing recombinant cryptochrome showed no measurable absorption at the concentration used in visible (380–800 nm) light. The absorption spectrum of cryptochrome was assessed as the difference spectra between extracts from cryptochrome-expressing and uninduced cell cultures, similarly to the determination of recombinant PHOT1 absorption spectra from insect cell culture (Christie et al., 1998).
An N-terminal fragment of PHOT2 photoreceptor comprising a flavin-binding or LOV domain (amino acids 1–900) was expressed in Escherichia coli using the PET21 vector expression system (Novagen, Madison, WI). Recombinant truncated NPL1 protein was purified via an HIS affinity tag on a nickel affinity column by methods recommended by the manufacturer. The purified recombinant protein was eluted, and the absorption spectrum taken in a Beckman Coulter DU7400 spectrophotometer. The spectrum is identical to published spectra (Salomon et al., 2000).
ACKNOWLEDGMENTS
We thank Alfred Batschauer for use of action spectroscopy facilities and critical reading of the manuscript; Jean-Pierre Bouly for critical reading of the manuscript; and Emile Miginiac, Jean-Claude Kader, and the members of the plant science laboratory at the University of Paris for their assistance and support.
Footnotes
This work was supported by a Contrat Atipe Blanche from the Centre National de la Recherche Scientifique (to M.A.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010969.
LITERATURE CITED
- Ahmad M. Seeing the world in red and blue: insight into plant vision and photoreceptors. Curr Opin Plant Biol. 1999;2:230–235. doi: 10.1016/S1369-5266(99)80040-5. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Cashmore AR. Seeing blue: the discovery of cryptochrome. Plant Mol Biol. 1996;30:851–861. doi: 10.1007/BF00020798. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Cashmore AR. The blue-light receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana. Plant J. 1997;11:421–427. doi: 10.1046/j.1365-313x.1997.11030421.x. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Jarillo JA, Cashmore AR. Chimeric proteins between cry1 and cry2 Arabidopsis blue light photoreceptors indicate overlapping functions and varying protein stability. Plant Cell. 1998a;10:197–208. doi: 10.1105/tpc.10.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M, Jarillo J, Smirnova O, Cashmore AR. The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Mol Cells. 1998b;1:939–948. doi: 10.1016/s1097-2765(00)80094-5. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Lin C, Cashmore AR. Mutations throughout an Arabidopsis blue-light photoreceptor impair blue-light-responsive anthocyanin accumulation and inhibition of hypocotyl elongation. Plant J. 1995;8:653–658. doi: 10.1046/j.1365-313x.1995.08050653.x. [DOI] [PubMed] [Google Scholar]
- Beggs CJ, Holmes MG, Jabben M, Schafer E. Action spectra for the inhibition of hypocotyl growth by continuous irradiation in light and dark-grown Sinapis alba L. seedlings. Plant Physiol. 1980;66:615–618. doi: 10.1104/pp.66.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs W, Huala E. Blue-light photoreceptors in higher plants. Annu Rev Cell Dev Biol. 1999;15:33–62. doi: 10.1146/annurev.cellbio.15.1.33. [DOI] [PubMed] [Google Scholar]
- Briggs WR, Beck CF, Cashmore AR, Christie JM, Hughes J, Jarillo JA, Kagawa T, Kanegae H, Liscum E, Nagatani A et al. The phototropin family of photoreceptors. Plant Cell. 2001;13:993–997. doi: 10.1105/tpc.13.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ, Mazella MA. Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phyA, phyB, and hy4 simple, double, and triple mutants in Arabidopsis. Plant Physiol. 1998;118:19–25. doi: 10.1104/pp.118.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs W. Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science. 1998;282:1698–1701. doi: 10.1126/science.282.5394.1698. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J. DNA photolyases and cryptochromes. Mutat Res. 2000;460:143–149. doi: 10.1016/s0921-8777(00)00023-9. [DOI] [PubMed] [Google Scholar]
- Devlin PF, Kay SA. Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell. 2000;12:2499–2510. doi: 10.1105/tpc.12.12.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta KM, Spalding EP. Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J. 2001a;26:471–478. doi: 10.1046/j.1365-313x.2001.01038.x. [DOI] [PubMed] [Google Scholar]
- Folta KM, Spalding EP. Opposing roles of phytochrome A and phytochrome B in early cryptochrome-mediated growth inhibition. Plant J. 2001b;28:333–340. doi: 10.1046/j.1365-313x.2001.01157.x. [DOI] [PubMed] [Google Scholar]
- Goto N, Yamamoto K, Watanabe M. Action spectra for inhibition of hypocotyl growth of wild-type plants and of the hy2 long-hypocotyl mutant of Arabidopsis thaliana. Photochem Photobiol. 1993;57:867–871. [Google Scholar]
- Holmes MG, Wagner E. The influence of chlorophyll on the spectral control of elongation growth in Chenopodium rubrum L. hypocotyls. Plant Cell Physiol. 1982;23:745–750. [Google Scholar]
- Jorns MS, Baldwin ET, Sancar GB, Sancar A. Action mechanism of Escherischia coli DNA photolyase. J Biol Chem. 1986;262:486–491. [PubMed] [Google Scholar]
- Lin C. Plant blue-light receptors. Trends Plant Sci. 2000;5:337–342. doi: 10.1016/s1360-1385(00)01687-3. [DOI] [PubMed] [Google Scholar]
- Lin C, Ahmad M, Cashmore AR. Arabidopsis cryptochrome 1 is a soluble protein mediating blue light-dependent regulation of plant growth and development. Plant J. 1996;10:893–902. doi: 10.1046/j.1365-313x.1996.10050893.x. [DOI] [PubMed] [Google Scholar]
- Lin C, Robertson DE, Ahmad M, Raibekas AA, Jorns MS, Dutton PL, Cashmore AR. Association of flavin adenine dinucleotide with the Arabidopsis blue light receptor CRY1. Science. 1995;269:968–970. doi: 10.1126/science.7638620. [DOI] [PubMed] [Google Scholar]
- Lin C, Yang H, Guo H, Mockler T, Chen J, Cashmore AR. Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue-light receptor cryptochrome 2. Proc Natl Acad Sci USA. 1998;95:7686–7690. doi: 10.1073/pnas.95.5.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FJ, Song W, Meyer-Bernstein E, Naidoo N, Sehgal A. Photic signaling by cryptochrome in the Drosophila circadian system. Mol Cell Biol. 2001;21:7287–7294. doi: 10.1128/MCB.21.21.7287-7294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra K, Sang-Tae K, Batschauer A, Dawut L, Sancar A. Putative blue-light photoreceptors from Arabidopsis thaliana and Sinapis alba with a high degree of sequence homology to DNA photolyase contain the two photolyase cofactors but lack DNA repair activity. Biochemistry. 1995;34:6892–6899. doi: 10.1021/bi00020a037. [DOI] [PubMed] [Google Scholar]
- Mas P, Devlin PF, Panda S, Kay SA. Functional interaction of phytochrome B and cryptochrome 2. Nature. 2000;408:207–211. doi: 10.1038/35041583. [DOI] [PubMed] [Google Scholar]
- McCormac AC, Wagner D, Boylan MT, Quail PH, Smith H, Whitelam GC. Photoresponses of transgenic Arabidopsis seedlings expressing introduced phytochrome B-encoding cDNA: evidence that phytochrome A and phytochrome B have distinct photoregulatory functions. Plant J. 1993;4:19–27. [Google Scholar]
- Neff MM, Chory J. Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998;118:27–36. doi: 10.1104/pp.118.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe C, Sweere U, Drumm-Herrel H, Schafer E. The blue-light receptor cryptochrome 1 can act independently of phytochrome A and B in Arabidopsis thaliana. Plant J. 1998;16:465–471. doi: 10.1046/j.1365-313x.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- Salomon M, Christie JM, Knieb E, Lempert U, Briggs WR. Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry. 2000;39:9401–9410. doi: 10.1021/bi000585+. [DOI] [PubMed] [Google Scholar]
- Sancar A. CRYPTOCHROME: the second photoactive pigment in the eye and its role in circadian photoreception. Annu Rev Biochem. 2000;69:31–67. doi: 10.1146/annurev.biochem.69.1.31. [DOI] [PubMed] [Google Scholar]
- Sancar GB, Jorns MS, Payne G, Fluke DJ, Rupert CS, Sancar A. Action mechanism of Escherischia coli DNA photolyase. J Biol Chem. 1987;262:492–498. [PubMed] [Google Scholar]
- Shinomura T, Hanzawa H, Schafer E, Furuya M. Mode of phytochrome B action in the photoregulation of seed germination in Arabidopsis thaliana. Plant J. 1998;13:583–590. doi: 10.1046/j.1365-313x.1998.00049.x. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M, Furuya M. Action spectrum for phytochrome-A and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:8129–8133. doi: 10.1073/pnas.93.15.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomura T, Uchida K, Furuya M. Elementary processes of photoperception by phytochrome A for high-irradiance response of hypocotyl elongation in Arabidopsis. Plant Physiol. 2000;122:147–156. doi: 10.1104/pp.122.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HQ, Wu YJ, Tang RH, Liu D, Liu Y, Cashmore AR. The C termini of Arabidopsis cryptochromes mediate a constitutive light response. Cell. 2000;103:815–827. doi: 10.1016/s0092-8674(00)00184-7. [DOI] [PubMed] [Google Scholar]
- Young J, Liscum E, Hangarter RJ. Spectral-dependence of light-inhibited hypocotyl elongation in photomorphogenic mutants of Arabidopsis: evidence for a UV-A photosensor. Planta. 1992;188:106–114. doi: 10.1007/BF00198946. [DOI] [PubMed] [Google Scholar]