Abstract
One of the challenges of oncolytic virotherapy is an inability to easily track or monitor virus activity during treatment. Here we describe the construction and functional characterization of Ad/hTC-GFP-E1, an oncolytic virus whose transgenes GFP and E1A are both under the control of a synthetic promoter (hTC). This promoter consists of sequences from the human telomorase reverse transcriptase promoter and a minimal cytomegalovirus early promoter. The tumor-specific expression of E1A and GFP was demonstrated by Western blot and fluorescent microscope analyses, and the tumor-specific cytotoxcity by crystal violet staining and cell viability assays. Viral replication and tumor cell lysis occured at multiplicities of infection (MOI) as low as 100 viral particles per cell in sensitive cell lines. No overt cytotoxic effect was observed in normal human fibroblasts, even at MOIs over 2000 vp. The presence of oncolytic vector was easily visualized and quantitated in vitro and in vivo, in correlation with viral replication. Intralesional administration of the virus into subcutaneous H1299 (NSCLC) tumor xenografts significantly suppressed tumor growth and provided a survival benefit. Together, these results demonstrate that an hTERT-specific oncolytic adenovirus expressing an hTERT-specific transgene is applicable for cancer therapy.
Keywords: adenovirus, virotherapy, telomerase, E1A, non-small cell lung cancer
Oncolytic virotherapy is designed to selectively replicate and kill tumor cells by using tumor-dependent viral mutants or by using tumor-specific promoters to control viral replication. Tumor-specific promoters, such those for alpha fetoprotein 1,2, prostate-specific antigen 3, and MUC-1 4 have been used to promote virus replication-essential genes in oncolytic virus. Although there is evidence that controlling viral gene expression by tumor-specific promoters is feasible, this approach can be hindered by the typically weak activity of many tumor- and tissue-specific promoters. We recently found that a human telomerase reverse transcriptase (hTERT) promoter coupled with a small fragment of the cytomegalovirus (CMV) promoter has much higher activity than wild-type hTERT promoter but retains hTERT specificity.5 To test whether this promoter can be used to control viral replication, we constructed an oncolytic adenovector whose essential early gene, E1A, is driven by this hTERT-CMV (hTC) fusion promoter. Due to the small size of the promoter, it is also possible to include a separate transgene expression cassette. The addition of an hTERT specific reporter, such as GFP, allows us to easily and effectively monitor the vectors ability to infect, replicate, express, and persist in cancer cells. Here we report our in vitro and in vivo characterization of this tumor-specific, GFP-expressing oncolytic vector, Ad/hTC-GFP-E1.
Vector construction and in vitro characterization
The hTC fusion promoter consists of a fragment of the hTERT promoter (bases −456 to −2 upstream of the start codon; GenBank: AH007699) and a minimized fragment of the CMV promoter (bases −1017 to −901 upstream of the human Cytomegalovirus major immediate early protein start codon, GenBank: M21295). The shuttle plasmid, pAd/hTC-GFP-E1, was constructed by placing hTC upstream of both an eGFP expression cassette and the E1A gene. The oncolytic vector was then constructed in 293 cells with the shuttle plasmid and a Cla I digested 35 kb fragment of adenovirus type 5. The recombinant oncolytic vector, Ad/hTC-GFP-E1 (Figure 1A), was identified by cytopathic effects and accompanying GFP fluorescence. The vector was plaque-purified, expanded in 293K cells and purified by two cycles of CsCl banding. The sequences in the hTC-GFP cassette and hTC-E1 region were verified by PCR and DNA sequencing. Vector amplification, purification, titration, and quality tests were performed as we have previously reported.6,7 Viral particles:plaque forming unit (vp:pfu) ratios were usually between 30:1 and 100:1.
Figure 1.
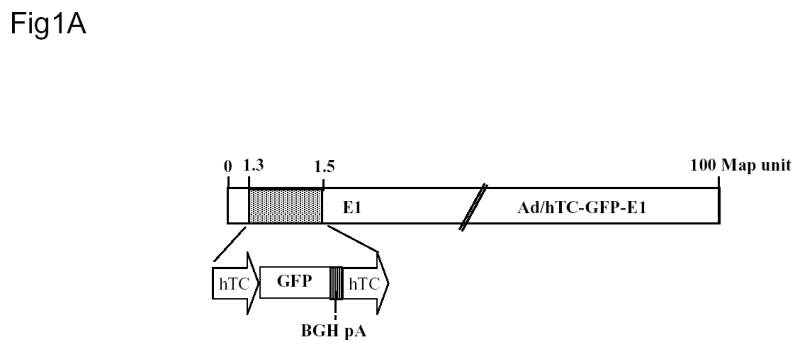
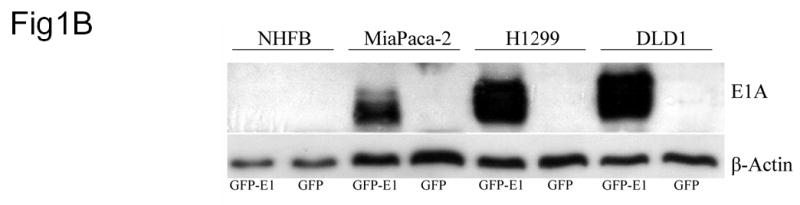
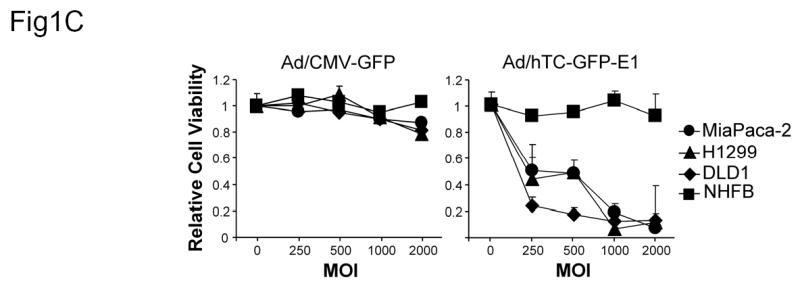
Construction and Characterization of Ad/hTC-GFP-E1. (A) Diagram of Ad/hTC-GFP-E1. (B) Western blot analysis of E1A expression in cells infected with Ad/CMV-GFP or Ad/hTC-GFP-E1 at an MOI of 100 and incubated for 5 days. Similar results were observed on days 3, 7, and 10. β-Actin was used as a loading control. (C) Dose-response analysis of cell viability after infection with Ad/CMV-GFP or Ad/hTC-GFP-E1 at various MOIs for 5 days. Data points represent means from triplicate experiments, normalized to control (PBS) viability. Error bars represent standard deviations.
To quickly assess the replication competency of the virus, DLD-1 human colon cancer cells were infected with Ad/hTC-GFP-E1 and the non-replicating control vector, Ad/CMV-GFP, at an MOI of 500 or 2000. The lysates of the infected cells were transferred separately into fresh DLD-1 cells, which were allowed to incubate for 4 days. GFP expression and cytotoxicity were evident in cells given lysates from Ad/hTC-GFP-E1-infected cells, whereas no visible GFP expression or cytotoxicity was observed in cells given lysates from Ad/CMV-GFP-infected cells, demonstrating that effective viral replication occurred in cells infected with Ad/hTC-GFP-E1 but not in cells infected with Ad/CMV-GFP.
We also tested hTC-driven E1A expression in lung cancer cell line H1299, pancreatic cancer cell line MiaPaca-2, colon cancer cell line DLD-1, and normal human fibroblasts, NHFB. Cells were infected with Ad/hTC-GFP-E1 or Ad/CMV-GFP at an MOI of 100 vp. Five days later, the cells were harvested and analyzed by western blotting for viral E1A expression. Only cancer cells infected with Ad/hTC-GFP-E1 expressed E1A (Figure 1B). No E1A expression was detected in any Ad/CMV-GFP-infected cells or in Ad/hTC-GFP-E1-infected NHFBs, indicating that hTC can be used to restrict viral E1 expression to rapidly dividing cancer cells.
Oncolytic effect of Ad/hTC-GFP-E1
To test whether Ad/hTC-GFP-E1 causes oncolysis, we evaluated cell viability of DLD1, H1299, and NHFB after 4, 8, 13, and 17 days after infection with Ad/CMV-GFP, Ad/hTC-GFP-E1. As a positive control for adenoviral infection and normal cell toxicity, we also included wild-type adenovirus type 5 (WT). Cells treated with PBS were used as a mock control, and all data was normalized according to the mock group. For the replicating viruses, Ad/hTC-GFP-E1 and WT, 100 MOI was sufficient to cause complete lysis in DLD1. H1299, though visibly fluorescing GFP shortly after 100 MOI infection, succombed slower and developed cytopathic effects only after 13 days. Complete lysis was achieved in these cells after 20–25 days (data not shown). 1000 MOI, however, was sufficient to cause complete lysis in both DLD1 and H1299 fairly quickly, with overt cytopathic effects observed after only a few days of infection. Time course and dose dependent analysis of Ad/hTC-GFP-E1 compared to wild-type adenovirus revealed similar patterns of oncolysis, indicating that the addition of the hTC promoter and GFP transgene to the oncolytic virus did not diminish its ability to infect and replicate. In NHFB cells, treatment with wild-type virus at 1000 MOI resulted in more than 70% cell killing at day 8 and more than 99% cell death at day 17. Detectable, increasing levels of toxicity were also seen at 100 MOI, similar to the effects observed in H1299 at the same MOI. In comparison, Ad/hTC-GFP-E1 did not cause any measurable toxicity at 100 MOI, and very low levels of toxicity at 1000 MOI in NHFB cells.
We also performed dose-dependent toxicity assays using DLD1, H1299, MiaPaca-2, and NHFBs. Cells were infected with Ad/CMV-GFP and Ad/hTC-GFP-E1 at an MOI of 250, 500, 1000, or 2000. PBS was used as a mock control (MOI = 0), and groups were normalized according to the mock group’s viability data. The cells were incubated for 5 days and then analyzed for viability using XTT assay. No toxicity was observed in any of the cell lines infected with Ad/CMV-GFP, but high levels of toxicity were observed in Ad/hTC-GFP-E1-infected cancer cells at all doses, especially at MOIs of 1000 and 2000 (Figure 1C). No notable toxicity was evident in NHFBs infected with Ad/hTC-GFP-E1, even at an MOI of 2000.
Suppression of tumor growth by Ad/hTC-GFP-E1 in vivo
To evaluate the antitumor activity of Ad/hTC-GFP-E1 in established human xenograft tumors, H1299 human non-small-cell lung cancer cells were inoculated subcutaneously into the dorsal rear flanks of nude mice. Treatment began when the tumors reached 3–5 mm in diameter. Virus injections were administered 9, 13, 19, and 26 days post inoculation. Tumor growth and survival was then monitored. Compared to treatments with PBS (mock control) and Ad/CMV-LacZ (adenovirus control), treatment with Ad/hTC-GFP-E1 caused significant retardation of tumor growth (P < 0.005; Figure 2A). Treatment with Ad/hTC-GFP-E1 also significantly prolonged the survival of the tumor-bearing animals (P < 0.007; Figure 2B). The median duration of survival for animals treated with PBS, Ad/CMV-GFP, and Ad/hTC-GFP-E1 was 57, 61, and 98 days, respectively. Five of the 13 mice treated with Ad/hTC-GFP-E1 showed no visible tumor growth and remained tumor free throughout the experiment. These results indicate that Ad/hTC-GFP-E1 is highly active against established tumors.
Figure 2.
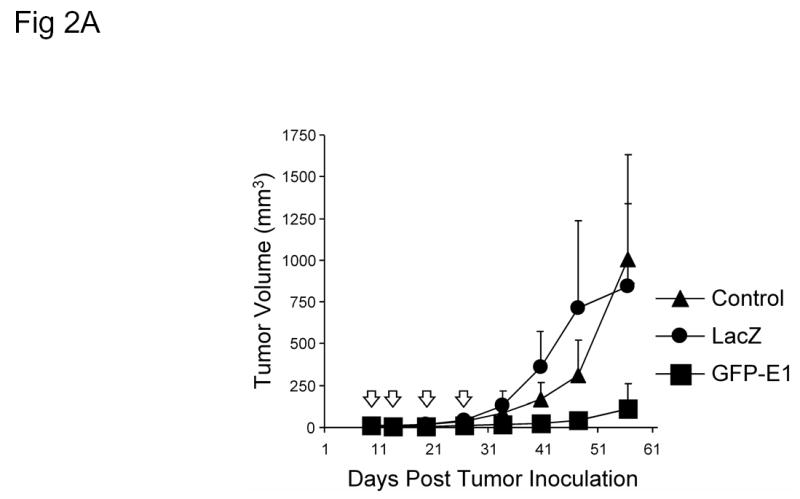
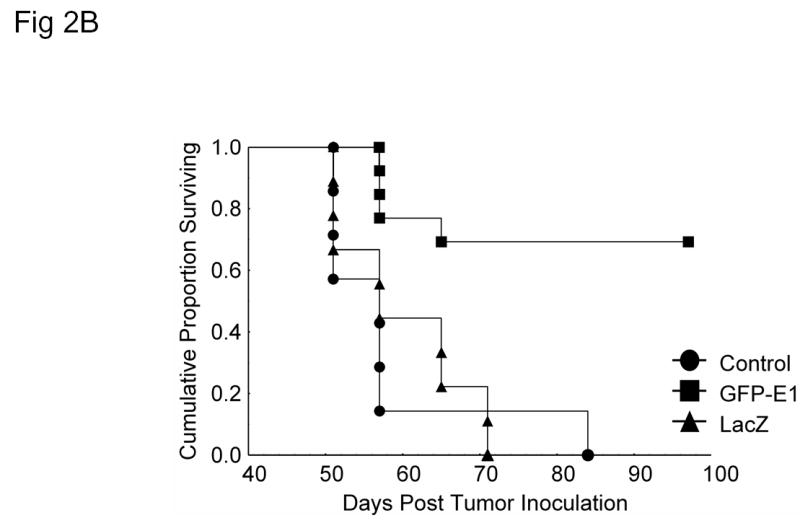
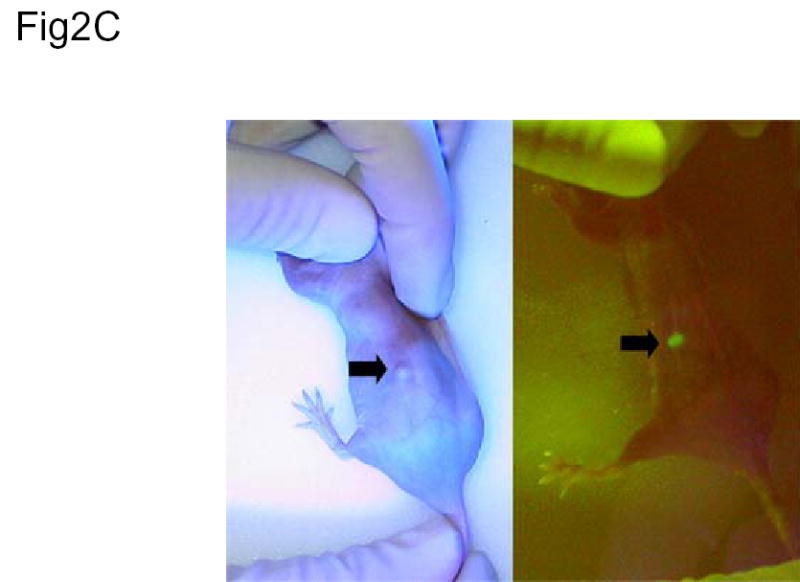
In vivo application. (A) Tumor growth analysis of nude mice with H1299 non-small-cell lung cancer xenografts infected with Ad/CMV-LacZ or Ad/hTC-GFP-E1. Ad/hTC-GFP-E1 significantly (P < 0.005) suppressed tumor growth compared to the other treatments, as shown by repeated-measures one-way ANOVA comparing Ad/hTC-GFP-E1 to both control and Ad/LacZ. (B) Kaplan-Meier survival analysis of mice in (B). Mice infected with Ad/hTC-GFP-E1 lived significantly longer (P < 0.007) than those in the other treatments groups. (C) In vivo imaging. Dorsal flank tumor of nude mouse treated with Ad/hTC-GFP-E1 displaying GFP expression 5 days post final treatment. Similar levels of expression could be seen in all mice treated with Ad/hTC-GFP-E1, however only one is shown here. The same mouse is pictured in both panels. Left panel is under normal light conditions. Right panel is under fluorescent light. Arrow indicates tumor position.
We also tested possible treatment related toxicity by analyzing serum liver enzyme activity. Analysis of blood collected 5 days after the final treatment showed that aminotransferase and alanine aminotransferase levels were in normal ranges in all animals tested, regardless of the treatment they received (data not shown), suggesting that Ad/hTC-GFP-E1 was well tolerated.
To test whether infection of tumor by Ad/hTC-GFP-E1 can be monitored by detecting GFP expression, we examined GFP expression in mice bearing tumors 5 days post the final injection. GFP expression was easily detectable in Ad/hTC-GFP-E1-infected tumors (Figure 2C). This finding indicates that viral transgene expression and replication were active in the tumors, providing a useful tool for assessing vector function and persistence. Taken together, our data demonstrate that the hTC-GFP expression cassette in an oncolytic vector can be used to monitor tumor-specific infection and expression of the vector in tumors and surrounding tissue.
In summary, our results showed that the hTC promoter is useful for construction of hTERT specific oncolytic adenovectors and that incorporation of a GFP transgene in the oncolytic vector can be useful for monitoring vector replication. Incorporating GFP in an oncolytic vector facilitates in vitro and in vivo qualitative and quantitative monitoring of virus infection, replication, cell susceptibility, permissiveness, and promoter activity. Studies of the diagnostic capabilities of hTERT-specific GFP expression have been done in oncolytic settings 8, but not as a single vector. Umeoka, and others, previously demonstrated that intrathoracic co-administration of an hTERT-restricted oncolytic adenovector with a CMV-promoted, GFP-expressing adenovector demonstrated tumor-specific amplification of GFP expression and replication of both vectors. Since GFP expression, even though not tumor-specifically restricted, would be enhanced in areas of virus replication, it was thus was readily detected by a three-chip color cooled charged-coupled device (CCD) camera. This allowed visualization of subcutaneous and pleurally disseminated tumors. In our case, a single oncolytic virus, combining high level, tumor-specific GFP fluorescence with hTERT-restricted replication will not only identify and lyse over-active areas of hTERT expression, but can also provide further real-time indications for presence, persistence, and therapeutic activity of the virus in a quick and practical method.
Other optimizations of the hTC oncolytic system may provide even more evidence of its applicability in cancer therapy. Not all cell types can be infected by and release adenoviruses efficiently, but modifications to the vector and its therapeutic transgene can help overcome this problem. For example, adenovirus vectors with modified RGD motifs have been shown to be much more infectious than wild-type adenoviruses in cell types that are resistant to adenoviral infection 9. In addition, several proteins have been shown to enhance and assist viral replication and release from infected cells, and apoptotic genes such as p53 or pro-drug activating genes may provide additional tumor-specific toxicity 10. Though not typical of lytic viral infections, enhanced levels of apoptosis may allow faster, earlier release of the virus, thus reducing the time required for infected cells to release complete viral particles. In addition, immune-stimulating genes such as granulocyte/macrophage colony-stimulating factor, have been used to increase immune surveillance of infected tumors in the hope that the immune system will recognize previously undetected tumor antigens. Conversely, should the immune system not have a significant role in oncolytic therapy, it may be in the interest of the virus to express immune-suppressing proteins that remove immune interference with virus replication; this may allow increased levels of infection and high circulating titers of virus. By providing the ability to tumor-specifically replicate and express a beneficial transgene, our development and use of hTC promoters to control both transgene and viral E1A expression might be useful for constructing tumor-specific oncolytic vectors expressing additional therapeutic genes.
Acknowledgments
We thank Pierrette Lo for editorial review. This article represents partial fulfillment of the requirements for a Ph.D. degree by J.J.D.
Footnotes
Grant support: National Cancer Institute grants RO1 CA 092487-01A1 and RO1 CA 098582-01A1 (both to B. Fang), National Cancer Institute Lung Specialized Program of Research Excellence, and National Institutes of Health Core Grant CA-16672, and Lockton Grant-Matching Funds.
References
- 1.Li Y, et al. A hepatocellular carcinoma-specific adenovirus variant, CV890, eliminates distant human liver tumors in combination with doxorubicin. Cancer Res. 2001;61:6428–6436. [PubMed] [Google Scholar]
- 2.Takahashi M, et al. E1B-55K-deleted adenovirus expressing E1A-13S by AFP-enhancer/promoter is capable of highly specific replication in AFP-producing hepatocellular carcinoma and eradication of established tumor. Mol Ther. 2002;5:627–634. doi: 10.1006/mthe.2002.0589. [DOI] [PubMed] [Google Scholar]
- 3.Yu DC, et al. Antitumor synergy of CV787, a prostate cancer-specific adenovirus, and paclitaxel and docetaxel. Cancer Res. 2001;61:517–525. [PubMed] [Google Scholar]
- 4.Kurihara T, Brough DE, Kovesdi I, Kufe DW. Selectivity of a replication-competent adenovirus for human breast carcinoma cells expressing the MUC1 antigen. J Clin Invest. 2000;106:763–771. doi: 10.1172/JCI9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu G, et al. A novel synthetic hTERT-Mini-CMV chimera promoter-driven tumor-selective and high-efficiency expression of transgene for systemic cancer gene therapy. Proc Am Assoc Cancer Res. 2005;46 [Google Scholar]
- 6.Gu J, et al. Tumor-specific transgene expression from the human telomerase reverse transcriptase promoter enables targeting of the therapeutic effects of the Bax gene to cancers. Cancer Res. 2000;60:5359–5364. [PubMed] [Google Scholar]
- 7.Lin T, et al. Targeted expression of green fluorescent protein/tumor necrosis factor-related apoptosis-inducing ligand fusion protein from human telomerase reverse transcriptase promoter elicits antitumor activity without toxic effects on primary human hepatocytes. Cancer Res. 2002;62:3620–3625. [PubMed] [Google Scholar]
- 8.Umeoka T, et al. Visualization of intrathoracically disseminated solid tumors in mice with optical imaging by telomerase-specific amplification of a transferred green fluorescent protein gene. Cancer Res. 2004;64:6259–6265. doi: 10.1158/0008-5472.CAN-04-1335. [DOI] [PubMed] [Google Scholar]
- 9.Vigne E, et al. RGD inclusion in the hexon monomer provides adenovirus type 5-based vectors with a fiber knob-independent pathway for infection. J Virol. 1999;73:5156–5161. doi: 10.1128/jvi.73.6.5156-5161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauthoff H, et al. Late expression of p53 from a replicating adenovirus improves tumor cell killing and is more tumor cell specific than expression of the adenoviral death protein. Hum Gene Ther. 2002;13:1859–1871. doi: 10.1089/104303402760372954. [DOI] [PubMed] [Google Scholar]


