Abstract
The gravitational field controls plant growth, morphology, and development. However, the underlying transduction mechanisms are not well understood. Much indirect evidence has implicated the cytoplasmic free calcium concentration ([Ca2+]c) as an important factor, but direct evidence for changes in [Ca2+]c is currently lacking. We now have made measurements of [Ca2+]c in groups of young seedlings of Arabidopsis expressing aequorin in the cytoplasm and reconstituted in vivo with cp-coelenterazine, a synthetic high-affinity luminophore. Distinct [Ca2+]c signaling occurs in response to gravistimulation with kinetics very different from [Ca2+]c transients evoked by other mechanical stimuli (e.g. movement and wind). [Ca2+]c changes produced in response to gravistimulation are transient but with a duration of many minutes and dependent on stimulus strength (i.e. the angle of displacement). The auxin transport blockers 2,3,5-tri-iodo benzoic acid and N-(1-naphthyl) phthalamic acid interfere with gravi-induced [Ca2+]c responses and addition of methyl indole-3-acetic acid to whole seedlings induces long-lived [Ca2+]c transients, suggesting that changes in auxin transport may interact with [Ca2+]c. Permanent nonaxial rotation of seedlings on a two-dimensional clinostat, however, produced a sustained elevation of the [Ca2+]c level. This probably reflects permanent displacement of gravity-sensing cellular components and/or disturbance of cytoskeletal tension. It is concluded that [Ca2+]c is part of the gravity transduction mechanism in young Arabidopsis seedlings.
Many studies have demonstrated that the cytoplasmic free Ca2+ concentration ([Ca2+]c) is affected by environmental stimuli such as touch, wind, cold, drought, light, and oxidative stress. These stimuli induce transient elevations of [Ca2+]c that modify the activity of many Ca2+-dependent proteins. When integrated together with other molecular processes of signal transduction, [Ca2+]c transients may help mediate biochemical and physiological responses of the plant (for review, see Plieth, 2001). The variety of stimuli that modify [Ca2+]c is extensive and discussions of possible mechanisms to explain how [Ca2+]c might specifically mediate each stimulus are frequent (e.g. Trewavas and Malhó, 1997; Malhó, 1999; Sanders et al., 1999).
[Ca2+]c has also been suggested many times to be an important factor for producing an appropriate graviresponse (Sinclair and Trewavas, 1997; Weisenseel and Meyer, 1997; Chen et al., 1999; Chatterjee et al., 2000). Interactions between the effects of auxin on growth and external Ca2+ have also been reported a number of times (Lee et al., 1983a, 1983b) and other data suggest a correlated transport of Ca2+ across the wall during gravitropic bending (Roux and Serlin, 1987). Evidence relating to the involvement of [Ca2+]c in gravitropism is extensive but mainly indirect, often relying on inhibitors (Belavskaya, 1996), calcium-binding proteins (Stinemetz et al., 1987), or other cellular messengers known to be related to [Ca2+]c signaling such as IP3 (Perera et al., 1999). [Ca2+]c changes in response to gravistimulation in maize (Zea mays) coleoptiles have been reported (Gehring et al., 1990), but the data have neither yet been kinetically analyzed nor reproduced. One extremely thorough imaging investigation failed to observe any change of [Ca2+]c in root tissue (Legue et al., 1997).
The simplest method to measure [Ca2+]c in whole seedlings is to use aequorin transformation (Knight et al., 1991). We have tried several times to detect [Ca2+]c transients after gravitational stimulation using this method but failed to observe substantive [Ca2+]c changes in single transformed seedlings (M.R. Knight and A.J. Trewavas, unpublished data). To resolve this important issue, we have now redesigned equipment to accommodate larger numbers of transformed seedlings and reconstituted in vivo with cp-coelenterazine (CTZ), which gives a much more sensitive Ca2+ indicator. With these improvements, we can now routinely detect changes in [Ca2+]c involved in graviresponse.
RESULTS
Gravitational Signals Induce Changes in [Ca2+]c That Are Different to Other Mechanical Stimuli
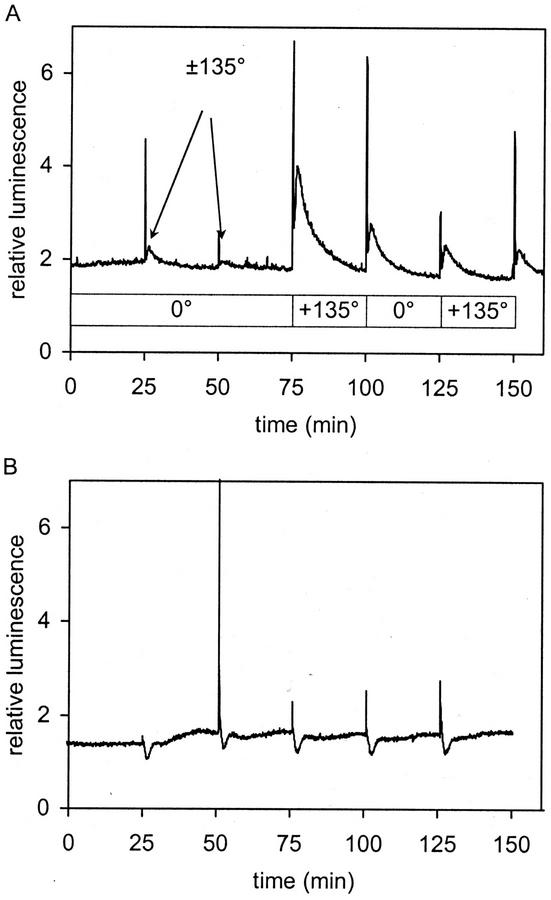
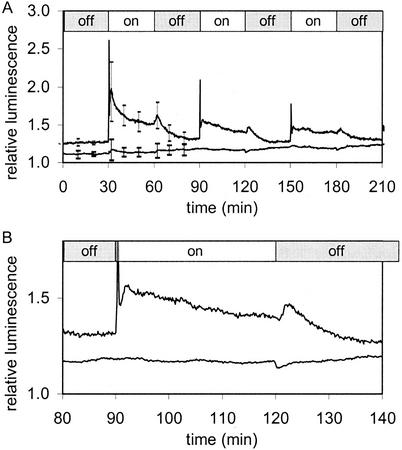
Transgenic Arabidopsis seedlings expressing aequorin in the cytoplasm were grown on vertical agar in a petri dish and reconstituted with cp-CTZ. Seedlings were fixed to a wheel (Fig. 1) in front of a PMT, inside a chemiluminometer, and rotated to various angles relative to the gravity vector. Initially, a plate of seedlings was turned through 135° in the chemiluminometer and returned within 5 s to the vertical position. A short luminescence transient was observed (Figs. 2A and 3B; ±135°), followed by a slight shoulder. However, when the seedlings were left at +135°, the initial spike (lasting about 25 s) and the second shoulder transient (peaking at 90 s and lasting about 15 min) were much more evident (Figs. 2A and 3A). Extrapolation of the shoulder back along the time axis suggests it may be initiated at time zero (i.e. time of movement). The initial spike and the shoulder, therefore, may have different origins. Further rotation to restimulate the seedlings produced a reduced response (Figs. 2A and 4).
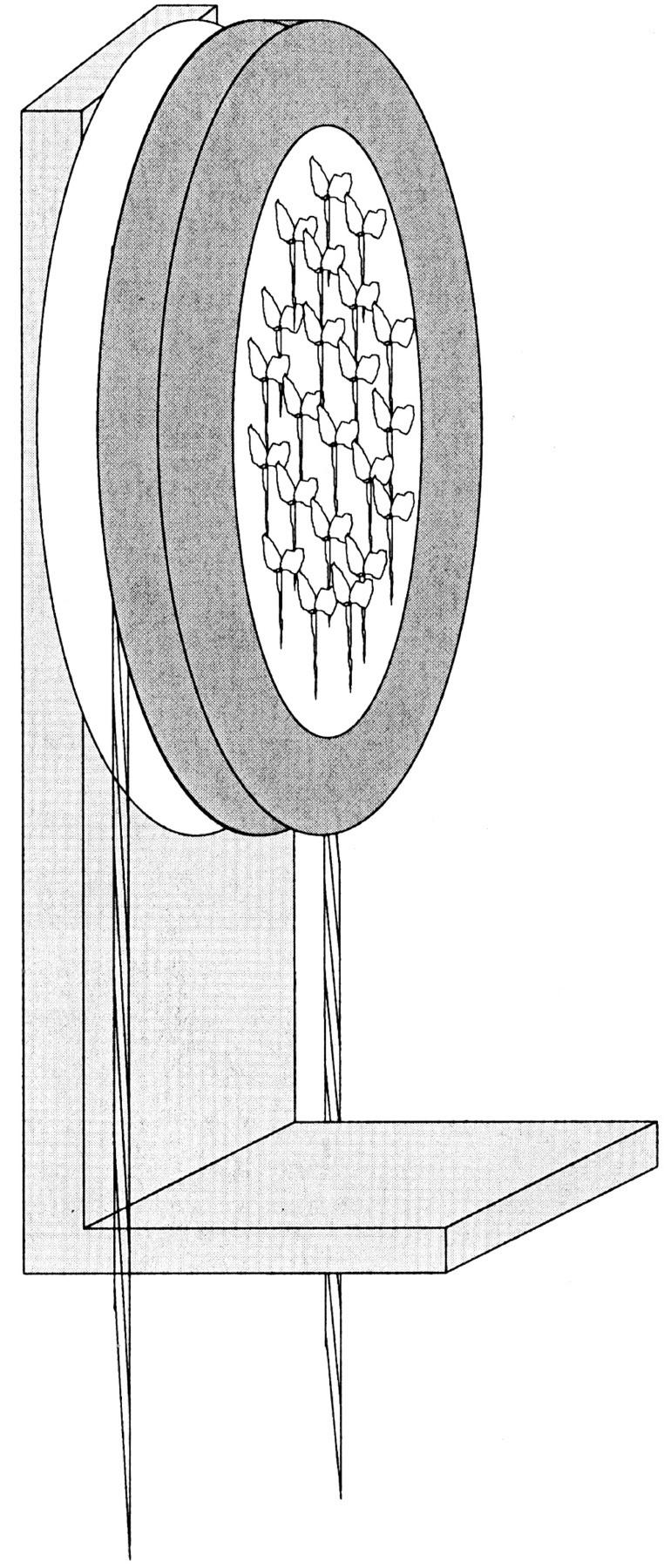
Figure 1.
Scheme of the rotatable wheel (two-dimensional clinostat) for mounting Arabidopsis seedling vertically in front of a photo multiplier tube (PMT).
Figure 2.
[Ca2+]c changes in Arabidopsis seedlings expressing the calcium indicator aequorin in the cytoplasm in response to different mechanical stimuli. A, Gravistimulation by turning seedlings growing on a vertical agar plate upside down in front of a luminometer. At t = 25 min and t = 50 min, seedlings were turned 135° upside down and immediately back to vertical position (±135°). At t = 75 min, seedlings were turned through 135° and left for 25 min in this position. At 100 min, seedlings were turned back to normal position. At t = 125 min, seedlings were turned to 135° position and at 150 min, turned back. B, Mechanical stimulation by blowing ambient air over seedlings growing on a vertical agar plate. Pulses of air (50 mL s−1 for 1 s) were applied at t = 25, 50, 75, 100, and 125 min.
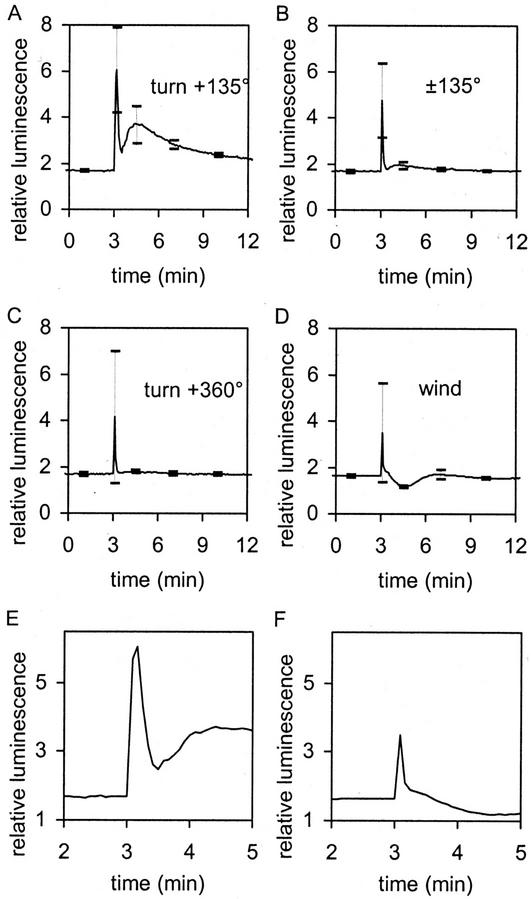
Figure 3.
Effect of different stimuli on the [Ca2+]c response. A, Gravistimulation: at t = 3 min, the plate with seedlings was turned through 135°. B, Control: At t = 3 min, the plate was turned 135° and immediately (within the same sample interval) back to vertical position. C, Control: at t = 3 min, the plate was turned 360°. D, Control: stimulation by wind; at t = 3 min, 50 mL of ambient air was blown in 1 s through the plate. sds are given at t = 1.0, 3.1, 4.5, 7.0, and 10 min with n = 5 for all curves. E and F, Enlargements of A and D, respectively, to show main initial [Ca2+]c spike after stimulation and reveal the difference in time scale.
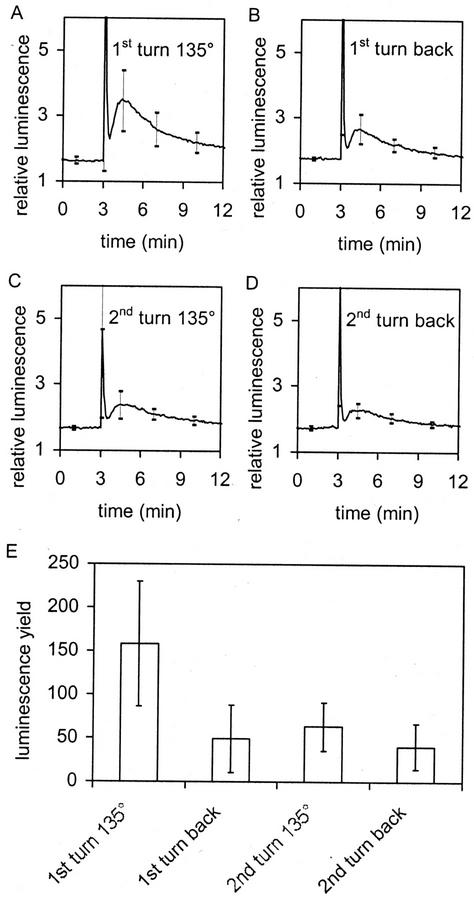
Figure 4.
Attenuation of the gravity-induced slow [Ca2+]c phase, i.e. desensitization, as measured by decreasing amplitudes with repeated gravistimulation. Curves in A through D are averages of five. sds are given at t = 1.0, 3.1, 4.5, 7.0, and 10 min. E, Light yields produced by stimulation (i.e. integrated areas under the curves shown in A through D minus background).
Gravistimuli have sometimes been regarded as kind of inside-out mechanical stimuli (Trewavas and Knight, 1994). Figure 2B shows the effect of brief mechanical stimulations on seedling luminescence by blowing air over seedlings. Whereas the initial spike might be similar to that shown in Figure 2A, the dip after the spike indicates a marked difference in the [Ca2+]c characteristics (Fig. 3, A versus D and E versus F).
Is the initial spike increased by leaving the seedlings at 135° or is it simply induced by rotation itself? Figure 3B shows that turning the seedlings to 135° and back again within 5 s leads to a reduced luminescence compared with seedlings turned to 135° and left in that position (Fig. 3A). A single rotation around 360° (Fig. 3C) also leads to a smaller spike, thereby lacking any subsequent shoulder. Figure 3, E and F, which expand the time scale, indicate that the kinetics of this initial gravi-induced spike are different to those of wind. The gravispike lasts some 25 s, whereas wind or touch is limited largely to a 5-s response as reported before (Knight et al., 1991). Figure 3, A and D, confirm the pronounced difference between the gravity-induced changes in [Ca2+]c and those of wind in the second part of the response.
The [Ca2+]c transients in response to gravistimulation of plants grown on Suc-free medium did not differ from those in plants grown on full medium including 1% (w/v) Suc (data not shown). Also, there was no significant difference in gravity-induced [Ca2+]c kinetics between dark-grown and control seedlings (data not shown).
Signal Adaptation
A number of signals lead to adaptation in [Ca2+]c responsiveness such that successive stimulation either fails to elicit a second [Ca2+]c peak within a defined time period of several minutes or even hours or that successive stimuli elicit much smaller peaks (e.g. Knight et al., 1992; Plieth et al., 1999b). Figures 2A and 4 indicate that adaptation to gravistimuli may also be present. Figure 4A shows the response of seedlings rotated to 135° and left for 15 min, and Figure 4B shows that subsequent return to the vertical induces an additional luminescence signal. Surprisingly, the turn back to the vertical induces a luminescence response similar in character to that of the initial response, although the amplitude of the shoulder is lower. This suggests that the turn-back luminescence is also a gravitropic response. Upon a second turn to 135° (Fig. 4C) and a subsequent second turn back to the vertical (Fig. 4D), similar kinetics of luminescence change are observed again except that the shoulder is again smaller. The initial spike remains almost unchanged, indicating a lack of adaptation of this response with the experimental design used here. Figure 4E reports total luminescence produced by these experiments, suggesting that the major reduction is in the second turn to 135°.
Size of [Ca2+]c Response Is Related to the Angle of Displacement
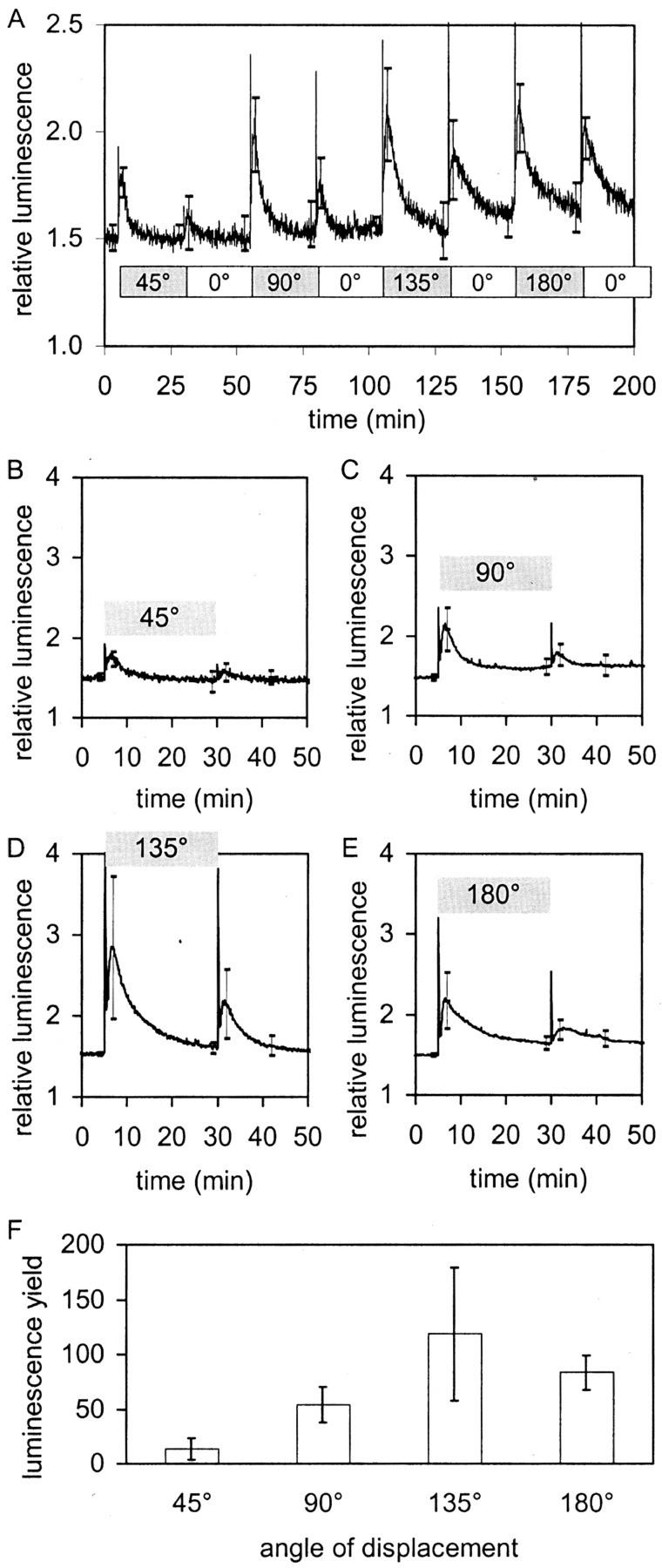
The optimum angle for gravitropic stimulation has been shown to be between 120° and 135°, and slower responses are obtained at 45°, 90°, and 180° (Larsen, 1969). It is thought that 135° provides the optimal situation for statocyte amyloplasts to slide down the cell wall, providing maximal gravitropic stimulation. Batches of seedlings were subjected to successively increasing angles of stimulation to detect any relationship between the apparent strength of the stimulus and the luminescence response (Fig. 5A). The bar underneath the trace in Figure 5A indicates the sequence of changes in the angle of stimulation. Independent experiments (Fig. 5, B–E) show that the maximum of luminescence was obtained at 135°. The total transient length is clearly shorter at 45° and 90° as well, being only about 6 min at 45°.
Figure 5.
Effect of different angles of displacement on the [Ca2+]c response in Arabidopsis. A, The plate with seedlings was tilted as indicated by the bar. Trace is average of four. sds are given at 3, 7, 28, 32, 53, 57, 78, 82, 103, 107, 128, 132, 153, 157, 178, and 182 min. B through E, Independent averaged experiments: The plate was tilted at t = 5 min by the angle indicated in the figures and turned back to vertical at t = 30 min. All traces are averages of five. sds are given at t = 4, 7, 29, 32, and 42 min. F, Light yields produced by stimulation with different angles of displacement (i.e. integrated areas from t = 5–30 min under the curves shown in B–E minus background).
Possible Relationship of Graviresponse in [Ca2+]c with Auxin
N-(1-Naphthyl) phthalamic Acid (NPA) and 2,3,5-tri-iodo benzoic acid (TIBA) are inhibitors that have been used for many years to influence the polar transport of auxin and inhibit gravitropic responses (Katekar and Geissler, 1980). Reorientation of growth induced by asymmetric gravity stimulation is in part the result of modified auxin movement and normally commences with a lag period of about 15 to 20 min. On this basis, we reasoned that NPA and TIBA should have no effects on the gravistimulated changes in luminescence that have finished by this time.
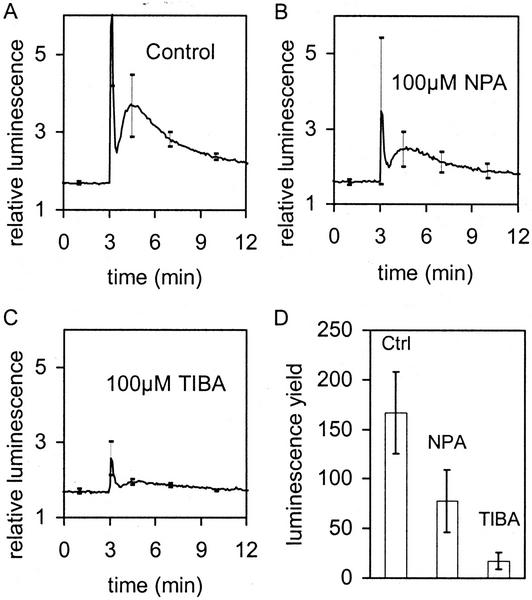
Seedlings were pretreated with NPA and TIBA and the luminescence response to a 135° displacement measured (Fig. 6). Both inhibitors had a substantive effect on both components of the luminescence response, the spike and the shoulder.
Figure 6.
Effect of auxin transport inhibitors on the [Ca2+]c response after gravistimulation (A–C: At t = 3 min, the plate with seedlings was turned through 135°). A, Control: as Figure 3A. B, Pretreatment of seedlings with 100 μm NPA. C, Pretreatment of seedlings with 100 μm TIBA. Curve in A is average of five. Curves in B and C are averages of four. sds are given at t = 1.0, 3.1, 4.5, 7.0, and 10 min. D, Light yields produced by stimulation (i.e. integrated areas under the curves shown in A–C minus background).
If there is a direct requirement for continued polar transport for the gravi-[Ca2+]c response, can auxin actually increase [Ca2+]c in these seedlings? We have tried for some years to obtain [Ca2+]c changes in response to auxin application and found the responses small and very variable (M.K. Watahiki and A.J. Trewavas, unpublished data). However, the sporadic positive results suggested that the rate of penetration of auxin into the tissue may limit the rate at which the ultimate [Ca2+]c responses occur. As a consequence, a perfusion system has now been used (Plieth et al., 1999a) to avoid unstirred layers and methyl-indole-3-acetic acid (IAA) to increase penetration of the effector through cuticle and cell membranes. With these improvements, a [Ca2+]c response could easily be detected (Fig. 7). There is a lag of several minutes before luminescence starts to increase and the single [Ca2+]c transient induced is unlike that shown for the gravitropic response. However, an external treatment with auxin is unlike interruption of a continually moving stream of auxin by inhibitors, so this may not be surprising. We also performed control experiments with the auxin analogs sodium-butyrate and butyrate-methyl-ester, both classified as class 3 auxins (http://www-z.embl-heidelberg.de:8080/ExternalInfo/wade/pub/data/auxin_data/classes.html) with no physiological hormone activity. Butyrate, as a weak acid, is able to acidify the cytoplasm (Plieth et al., 1997) with the result that [Ca2+]c increases in parallel (Plieth, 2001). The data (Fig. 7B) show that the low-pH-induced [Ca2+]c changes are small and demonstrate that there is a significant effect of active IAA on [Ca2+]c.
Figure 7.
[Ca2+]c in response to auxin and auxin-analogs in 5-d-old Arabidopsis seedlings expressing aequorin in the cytoplasm and reconstituted in vivo with cp-CTZ. Seedlings were placed in a perfusion cuvette. The effector was given at t = 30 min and washed out at t = 90 min as indicated by the black bar. A, Upper trace, 1.0 mm IAA-methylester; lower trace, 0.1 mm IAA-methylester. B, Upper trace, 1.0 mm Na-butyrate; lower trace, 1.0 mm butyrate-methylester. All experiments were performed in KCl, MgCl2, and CaCl2 0.1 mm each with 1.0 mm MES/NaOH at pH = 5.7. All traces are averages of five. sds are given at t = 20, 40, 60, 80, 94, and 120 min.
Effect of Clinostat Rotation on [Ca2+]c
The clinostat has been used for over 100 years to try to simulate weightlessness on the earth's surface. However, the use of the clinostat has generally fallen out of favor because of undesired side effects and an appreciation that plant growth may be different in true weightlessness compared with a clinostat treatment (Sievers and Hejnowicz, 1992; Hoson et al., 1997).
We reasoned that if a single rotation initiates a single initial spike of [Ca2+]c, then continuous rotation of seedlings grown on a vertical plate might initiate a series of spikes, perhaps with a maximum each time seedlings reached 135° and the shoulder to the [Ca2+]c signal shown in Figures 2 through 6 is eliminated. Also, if a clinostat abolishes gravisensing altogether with seedlings grown on a horizontal plate (i.e. “true clinostating” = “axial rotation”), then the typical response should appear when the motor is switched off, giving the gravitational force opportunity to take effect on the plants. Thus, this might provide a useful tool to investigate the cellular origin of both spike and shoulder further.
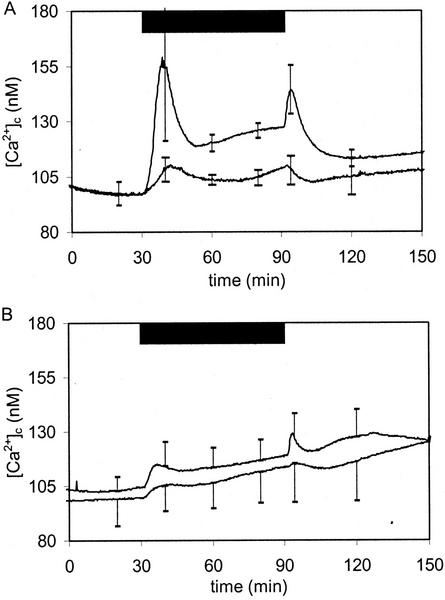
The wheel (Fig. 1) was connected to a motor and used as two-dimensional clinostat rotating at low speed. We applied a rotation speed of 3 rpm and switched the motor on and off every 30 min. Figure 8 shows the effect of permanent rotation on luminescence. Surprisingly, there was no significant luminescence yield when seedlings had been grown on a horizontal plate leading to axial rotation (“screwdriver rotation”) when mounted on the wheel (Fig. 8, A and B, lower traces). However, luminescence signal with permanent rotation of seedlings grown on vertical plate (“hands-of-a-clock rotation”) was a sustained increase rather than a transient (Fig. 8, A and B, upper traces). This information suggests that the clinostat results in permanent stimulation of the seedlings when rotating nonaxially. The large initial spikes seen in Figures 2 through 6 are still present when the motor is switched on, but are lacking when switched off. The dip in the luminescence when the motor is switched off (lower traces in Fig. 8, A and B) indicates that axial rotation (“true clinostating”) is sensed and that some Ca2+ pumping is going on.
Figure 8.
Clinostat experiments with 4-d-old Arabidopsis seedlings expressing aequorin in the cytoplasm. The plate was mounted on a rotatable sample holder with horizontal axis (i.e. two-dimensional clinostat). A motor placed outside the measuring chamber was connected with the sample holder by a timing belt (see Fig. 1). Rotation speed of the agar plate with seedlings was adjusted to 3 rpm. Motor was switched on and off every 30 min as indicated by the bar. Upper traces, [Ca2+]c responses to rotation of seedlings grown on vertical agarplate (i.e. “hands-of-a-clock” rotation). Lower traces, [Ca2+]c responses to rotation of seedlings grown on horizontal agarplate (i.e. axial “screwdriver” rotation). Curves are averages of six. sds are given at t = 10, 20, 32, 40, 50, 62, 70, and 80 min. B is magnification of A.
DISCUSSION
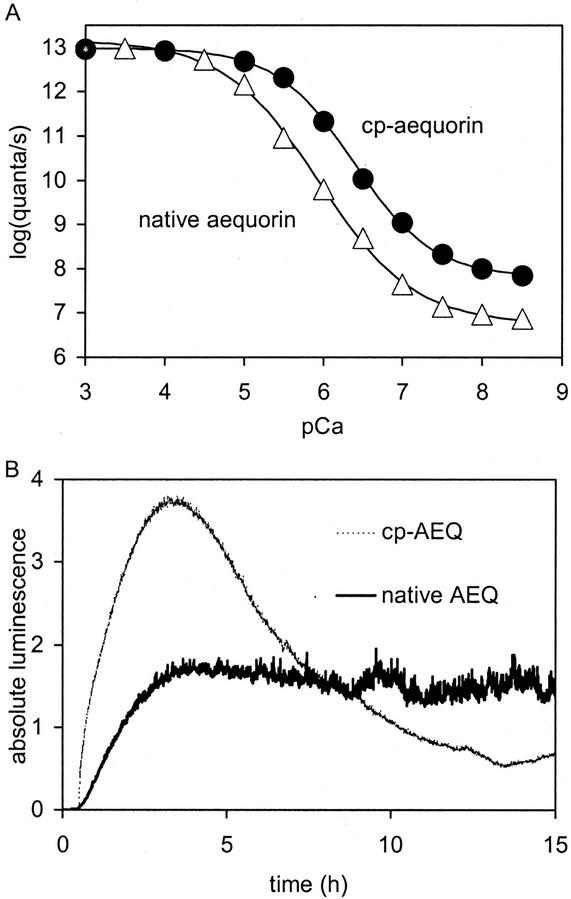
The information reported here indicates that a gravitropic stimulus does induce changes in [Ca2+]c. There are several possible reasons why [Ca2+]c changes induced by reorientation of plants have not been detected so far: Legue et al. (1997) used the fluorescent calcium indicator dye indo-1, which very likely dampens small calcium transients (Bormann et al., 2001) because of slow dissociation (or “off”) rates. Indo-1 has a high Ca2+ affinity (half saturation at 250 nm; Grynkiewicz et al., 1985) compared with cp-aequorin (half saturation at 400 nm; Fig. 9A), which might influence cellular [Ca2+] transients substantially. Here, we used aequorin, which emits light only when Ca2+ is bound. It is not the balance of Ca2+-bound and unbound indicator that reports [Ca2+] as is the case with fluorescent dyes. As a consequence, aequorin is consumed while reporting [Ca2+] (no dissociation reaction). Therefore, aequorin luminescence has an extremely high signal to background ratio that allows detection of even tiny [Ca2+]c changes and/or [Ca2+]c transients in a few responding cells embedded in non-responding tissue. Finally, with this method, the integrated signal of several hundreds of seedlings is obtained at once, whereas Legue et al. (1997) focused on small parts of a root from a single plant with each experiment. Thus, we obtained a global [Ca2+]c signal at the price of less spatial information (i.e. no information about origin and/or location of the [Ca2+]c transient in the seedling). So, either the aequorin technique is sensitive to a pool of calcium that was obscured or not measured in the previous study, or it is measuring changes in calcium in a different part of the seedling than was measured by Legue et al. (1997). To find the origin and/or location of [Ca2+]c transients produced by gravistimulation, one can express aequorin in certain tissues or cells (e.g. columella cells, pericycle cells, and bundle sheath cells; Kiegle et al., 2000; Plieth, 2001; J. Love, personal communication), to make the indicator report just from these tissues.
Figure 9.
Differences between native aequorin and cp-aequorin. A, Relationship between [Ca2+] and luminescence (data taken from Shimomura et al., 1993, and fitted with sigmoidal Bolzmann fit). B, In vivo reconstitution of aequorin in the cytoplasm of Arabidopsis roots with native CTZ (dark line) and cp-CTZ (light line). Addition of CTZ at t = 0.5 h. Absolute luminescence (×10,000) is given in B, which is luminescence of each integration interval divided by total luminescence produced by the specimen. Relative luminescence (×10,000) is given in all other figures, which is luminescence of each integration interval divided by luminescence still remaining in the specimen. The characteristics of the fitted curves in A are: ▵, native aequorin, 50% midpoint of 5.9 pCa, background of 6.8 log quanta s−1; and ●, cp-aequorin, 50% midpoint of 6.4 pCa, background of 7.8 log quanta s−1.
Two characteristics noted in the data here are an initial [Ca2+]c spike followed by a much longer shoulder of luminescence that peaks about 90 s after gravitropic stimulation has been induced. However, rapid single spikes are characteristic of mechanical stimuli such as touch or wind but as a luminescence spike they rarely last more than 5 s (Knight et al., 1991, 1992). However, the luminescence spikes observed here are 20 to 25 s (Figs. 2 and 3). Nevertheless, gravitropic stimulation can be interpreted as a kind of inside-out mechanical stimulation (Trewavas and Knight, 1994; Sinclair et al., 1996). So, the gravity-induced [Ca2+]c spikes might be part of a group typical for mechanical stimuli-like touch (Knight et al., 1991), wind (Knight et al., 1992), and raindrops (M. Viry-Moussaïd and A.J. Trewavas, unpublished data). As Figure 8 shows, even permanent rotation gives rise to only one initial spike. The size of the spike is also influenced by NPA and TIBA (Fig. 6), but does not appear to experience significant adaptation (Fig. 4). If the specificity of these two inhibitors NPA and TIBA can be believed (Katekar and Geissler, 1980), then the [Ca2+]c signal underpinning the spike may be related to auxin movement. Does this indicate that changes in auxin flux are more rapid than previously thought?
There are two parameters used in gravitropic experiments that might relate to those gravi-induced [Ca2+]c. The presentation time, that is the shortest time to elicit a graviresponse, can be as small as 12 s (Volkmann and Sievers, 1979; Hejnowicz et al., 1998); 20 s has been measured for Arabidopsis (Kiss et al., 1989). The perception time, in contrast, is the minimum period necessary for perception of a stimulus even though a response cannot be detected. It can be deduced using intermittent stimulation on a clinostat; periods as short as 0.5 s have been reported (Volkmann and Sievers, 1979). The initial gravi-induced [Ca2+]c spike might then be related to these early steps in gravitropic sensing. Figure 3 indicates that the size of the spike is smaller with a complete rotation of seedlings within a 5-s period, compared with the maximum amplitude of spike obtained when seedlings are left at 135°. A longer gravistimulation period than 5 s is necessary for a full [Ca2+]c spike and could be related to the presentation time. Because starchless mutants exhibit a much longer presentation and perception time (Kiss et al., 1989), incorporation of aequorin into several of these mutants should help clarify the nature of this relationship.
The second “shoulder” response noted here is a much more gradual and prolonged [Ca2+]c transient lasting about 15 min and peaking within 90 s (Figs. 2 and 3). Asymmetric growth generally starts within 15 min and again there might be significance in the time at which the shoulder transient finishes and asymmetric growth commences. However, a similar transient is induced when the seedlings are turned back to the vertical position, although the response is generally smaller. These data and those on subsequent restimulating again to 135° (Fig. 4) suggest some permanent modification in the cellular signaling system, reducing the size of this response. From this, we conclude that it is likely that these two facets of [Ca2+]c response, spike and shoulder, may have either different cellular or subcellular origins because the spike shows no apparent adaptation. The shoulder [Ca2+]c component does seem to be dependent on the angle of gravitropic stimulus, showing a maximum amplitude at 135° and a shorter total transient time at 45°. One attractive possibility that we are currently investigating is that this second [Ca2+]c shoulder could represent movement of amyloplasts displacing endoplasmic reticulum, from which [Ca2+]c is then released. The time required for amyloplast sedimentation of 6 to 12 min (Audus, 1969; Yoder et al., 2001) is in good agreement with the total transient time of the [Ca2+]c shoulder reported here.
It can be argued that reorientation of seedlings gives rise to changes in sustained mechanical stresses in the tissues because of their weight. This could also initiate changes in cytoplasmic calcium. Although we cannot dismiss this possibility, neither do we have evidence to support it. The fact that the angle at which the increase of [Ca2+]c is maximum is 135° (Fig. 5) suggests that the gravitropic response is the main cause for the [Ca2+]c increase. Mechanical stress is more likely to yield a maximum [Ca2+]c transient at 90° and sustained mechanical stress usually gives rise to a sustained [Ca2+]c increase (Fig. 8; C. Plieth and M.R. Knight, unpublished data). Therefore, the more attractive working hypothesis is that the shoulder [Ca2+]c component is more likely related to movement of amyloplasts.
The data in Figures 6 and 7 suggest that there might be a significant relationship between auxin and [Ca2+]c. Applied auxin can influence [Ca2+]c directly (Fig. 7) and NPA and TIBA both inhibited [Ca2+]c transients substantially (Fig. 6), but there is no selective inhibition of either the initial spike or the longer shoulder transient. Taken at their face value, might modification of polar auxin transport occur much earlier after gravistimulation than commonly supposed? Older models of modification of auxin transport suggest a fairly direct relationship between auxin movement and amyloplast sedimentation. However, whereas both inhibitors are known to impair auxin transport, other side effects could account for the inhibition observed here (Ross, 1998). Geldner et al. (2001) recently demonstrated that localization of the auxin efflux carrier candidate PIN1 is maintained by rapid trafficking between plasma membrane and endosomal compartments. This cycling (i.e. internalization and polar redistribution) is interrupted in the presence of TIBA, showing that auxin transport inhibitors generally interfere with membrane-trafficking processes. Hence, it is conceivable that treatment with either inhibitor eventually damages sensitive cells so they are no longer able to respond to gravity. Moreover, TIBA supposedly acts as a weak auxin (Katekar and Geissler, 1980), but treatment often reduces the total auxin present in the tissue (Goldsmith, 1969). Finally, TIBA reacts with sulfhydryl groups so side effects are likely, complicating interpretation. The recent isolation of mutants in which auxin transport is impaired (Lomax, 1997; Müller et al., 1998) offers a way forward if aequorin can be incorporated into such mutants.
However, the time when auxin redistribution is believed to commence has been estimated a number of times and it is thought that there is a lag of about 10 min in some tissues and even longer in others (Parker and Briggs, 1990). If these times are correct (and they are usually results obtained on shoots rather than roots), then the second long [Ca2+]c transient described here must be completed before auxin redistribution commences. In that case, our data can only conclude at the best that continued auxin movement may play a role for gravi-induced changes in [Ca2+]c. However, in some tissues, a change in sensitivity to auxin has also been detected (Rorabaugh and Salisbury, 1989) and with clear adaptation of the [Ca2+]c, suggesting some more permanent change has happened after the first gravitropic rotation (Fig. 4), a relationship between these two events may be more probable.
Redistribution of Ca2+ in the wall after gravitropic stimulation has been reported in which a higher wall Ca2+ is found in the slower growing half of the tissue (Björkman and Cleland, 1991; Lee et al., 1983a, 1983b; Slocum and Roux, 1983). Some relationship between wall Ca2+ redistribution and changes in [Ca2+]c is certainly possible and perhaps could be expected. Björkman and Cleland (1991) report that Ca2+ redistribution takes 10 min to start in maize roots but examination of their data suggests 5 min might be the real time of initiation. Furthermore, they show that the wall gradient may be essential for gravitropic curvature because it can be inhibited by EGTA derivatives or by fusicoccin. Because wall [Ca2+] is definitely regulated by the cell through [Ca2+]c (Trewavas and Malhó, 1997), a change in [Ca2+]c could alter wall [Ca2+] in turn.
However, there is a discrepancy in time. The shoulder [Ca2+]c changes described here peak between 1 and 2 min; compare this with the 5- to 10-min period for wall Ca2+ change in maize. However, Arabidopsis roots are much thinner than maize roots and wall [Ca2+] events may reach their target much quicker. A relationship between [Ca2+]c and wall [Ca2+] remains a viable possibility and Arabidopsis seedlings expressing aequorin in the wall are currently being used to probe this possibility.
MATERIALS AND METHODS
Plant Material
Transgenic Arabidopsis (http://plantpath.wisc.edu/∼afb/protocol.html) of biotype background Columbia-0 expressing cytosolic apoaequorin under the control of the cauliflower mosaic virus promoter 35S were used for [Ca2+]c measurements (Knight et al., 1997). Five hundred to 1,000 vapor-sterilized seeds (http://www.cropsci.uiuc.edu/faculty/bent/additions/vapster.html) were sown on sterile 1.2% (w/v) agar (no. A1296, Sigma-Aldrich, St. Louis) in 5-cm-diameter petri dishes (Bibby Sterilin Ltd., Stone, Staffs, UK). Agar was supplemented with 0.5× Murashige and Skoog medium (no. M0222, Duchefa, Haarlem, The Netherlands), 1% (w/v) Suc (no. 10274, BDH, Poole, UK) and adjusted with NaOH to a pH of 5.8. The plates were sealed with porous tape (12.5-mm surgical tape, Micropore, St. Paul), incubated at 4°C in the dark for 2 to 4 d for vernalization, and subsequently cultivated in the growth room at 21°C with 16-h photoperiod. To give a stand-alone support for vertical cultivation, plates were clipped with 5-cm fold-back clips. Whole plates with seedlings grown on the agar surface were used for experiments 2 to 6 d after germination where not stated otherwise.
Reconstitution of Aequorin
Reconstitution of aequorin with CTZ was performed in vivo essentially as described previously (Knight et al., 1997). However, cp-CTZ (no. C-14260, Molecular Probes, Eugene, OR) was used instead of native CTZ (Prolume Ltd., Pittsburgh; http://www.nanolight.com/nanofuel.htm). In brief, seedlings grown vertically on agar surface were covered with 2.5 mL of solution for 4 to 6 h. Therefore, cp-CTZ was dissolved first in methanol to give a 1 mm stock solution and then added to water to give a 10 μm solution for reconstitution. Calibration curves for luminescence against Ca2+ are shown in Figure 9A.
[Ca2+]c Measurements
For [Ca2+]c measurements, whole plates were mounted vertically on a purpose-built rotatable stage (Fig. 1) in a light-tight sample housing in front of a PMT (model 9829A, Electron Tubes Ltd., Middlesex, UK). The stage could be rotated by a rubber timing belt, either by hand or by a motor.
To avoid mechanical stimulation by external vibration and by the built-in cooler fan, the Peltier PMT housing (FACT50, Electron Tubes Ltd.) was replaced by a lab-made dry ice cooling housing. The use of solid CO2 also improved noise reduction by cooling the PMT down to −60°C. The luminometer chamber was constantly aerated with ambient air (19.5°C ± 1.0°C) to avoid anoxic stress and cooling of the specimen. Aequorin luminescence counts were integrated every 5 or 12 s. For reasons discussed below, relative luminescence is given in all curves instead of [Ca2+]c.
Treatment with Inhibitors
TIBA (no. T5910, Sigma-Aldrich) and NPA (no. N0067, TCI, http://www.tciamerica.com) were dissolved in dimethyl sulfoxide to give a 100 mm stock solution and then added together with cp-CTZ (see above) to water to give 100 μm final concentration in reconstitution medium. Medium (2.5 mL) was used to overlay young seedlings in a petri dish for 4 h.
Treatment with Auxin
For auxin treatment, 4-d-old seedlings grown on vertical agar were used, reconstituted for 4 to 6 h with cp-CTZ (no. C-14260, Molecular Probes), and placed in a perfusion cuvette as described by Plieth et al. (1999a). The cuvette was constantly perfused with aerated, buffered standard medium containing 0.1 mm each KCl, CaCl2, and MgCl2, and 1.0 mm MES/NaOH at pH of 5.7. Treatment of auxin was achieved by switching auxin containing standard medium into the perfusion stream. The latter was prepared from 1 m stock of IAA-methyl ester (no. I9770, Sigma-Aldrich) dissolved in dimethyl sulfoxide.
Calcium Sensitivity of Native Aequorin versus cp-Aequorin
We used the synthetic luminophor cp-CTZ (Shimomura et al., 1993; no. C-14260, Molecular Probes; http://www.probes.com/servlets/datatable?id=32122&item=2944) to reconstitute aequorin in vivo in Arabidopsis. The binding constant of cp-aequorin is pKCa ≈ 6.4 compared with pKCa ≈ 5.9 of native aequorin as calculated by sigmoidal fit (Fig. 8A). This means that cp-aequorin has a 3.16 times higher Ca2+-binding affinity. Hence, cp-aequorin produces about 10 times more steady-state luminescence with usual resting level of [Ca2+]c (i.e. about 50–150 nm) and is able to report tiny changes in [Ca2+]c with higher accuracy (Baum et al., 1999). The higher steady-state luminescence in unstimulated plants enabled us to detect calcium signals lower than the normal steady-state level as is the case with wind-stimulated seedlings (Figs. 2B and 3D). These “undervalues” were impossible to detect in earlier studies because of the lower signal to noise ratio produced by native aequorin.
Calculation of the Relative Luminescent Yield
We found cp-aequorin in the seedlings to be more unstable compared with native aequorin (Fig. 9B), possibly as a result of its higher sensitivity to the resting level of [Ca2+]c. Although we can calibrate cp-aequorin “in vitro” (Fig. 9A), attempts to do this “in vivo” presented major difficulties.
The normal tissue calibration for aequorin assesses the amount of aequorin at the end of the experiment and sums the total luminescence emitted during the experimental period so that luminescence values can be converted to [Ca2+]c. The calculation is based on the relative luminescence yield which is the ratio of luminescence counts obtained per second from the PMT divided by the counts still remaining in the system at each time point (Knight et al., 1997; Baum et al., 1999).
This calibration and calculation of changes in [Ca2+]c assumes that every cell responds equally. This is not even the case for cold shock (Wood et al., 2001) and is less likely for gravitropic phenomena in the root. Therefore, we depicted results in units of relative luminescence (i.e. relative luminescence yield × 10,000). Because it is evident that only a few specialized cells respond to gravistimulation (Blancaflor et al., 1998; Tasaka et al., 1999), the [Ca2+]c amplitude in these cells may well be 50 nm or even larger. With the perfusion experiments (Fig. 7), however, we attempted calibration with the assumption that all cells responded equally and simultaneously to the effector to have at least a rough estimate.
ACKNOWLEDGMENTS
We are grateful to Michael Brix (Physics Department Workshop, Kiel University, Germany) for manufacturing the luminometer sample housing and to George Steadman and Alex Harower (Institute of Cell and Molecular Biology Workshop, University of Edinburgh) for manufacturing the rotatable sample holder and the dry ice-cooling housing for the PMT. We also thank anonymous referees for helpful suggestions.
Footnotes
This work was supported by the European Community (grant no. BIO4–CT97–5080 to C.P.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.011007.
LITERATURE CITED
- Audus LJ. Geotropism. In: Wilkins MB, editor. Physiology of Plant Growth and Development. New York: McGraw-Hill; 1969. pp. 205–245. [Google Scholar]
- Baum G, Long J, Jenkins G, Trewavas AJ. Stimulation of the blue light receptor NPH1 causes a transient increase in cytosolic calcium. Proc Natl Acad Sci USA. 1999;98:13554–13559. doi: 10.1073/pnas.96.23.13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belavskaya NA. Calcium and graviperception in plants: inhibitor analysis. Int Rev Cytol. 1996;168:123–185. [Google Scholar]
- Björkman T, Cleland RE. The role of extracellular free-calcium gradients in gravitropic signalling in maize roots. Planta. 1991;185:379–384. [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S. Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol. 1998;116:213–222. doi: 10.1104/pp.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann G, Brosens F, de Schutter E. Diffusion. In: Bower JM, Bolouri H, editors. , Computational Modeling of Genetic and Biochemical Networks. Cambridge, MA: MIT Press; 2001. pp. 189–224. [Google Scholar]
- Chatterjee A, Porterfield DM, Smith PS, Roux SJ. Gravity-directed calcium current in germinating spores of Ceratopteris richardii. Planta. 2000;210:607–610. doi: 10.1007/s004250050050. [DOI] [PubMed] [Google Scholar]
- Chen R, Rosen E, Masson PH. Gravitropism in higher plants. Plant Physiol. 1999;120:343–350. doi: 10.1104/pp.120.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring CA, Williams DA, Cody SH, Parish RW. Phototropism and geotropism in maize coleoptiles are spatially correlated with increases in cytosolic free calcium. Nature. 1990;345:528–530. doi: 10.1038/345528a0. [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhoff Y-D, Jürgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- Goldsmith MHM. Transport of plant growth regulators. In: Wilkins MB, editor. Physiology of Plant Growth and Development. New York: McGraw Hill; 1969. pp. 127–165. [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hejnowicz Z, Sondag C, Alt W, Sievers A. Temporal course of graviperception in intermittently stimulated cress roots. Plant Cell Environ. 1998;21:1293–1300. doi: 10.1046/j.1365-3040.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- Hoson T, Kamisaka S, Masuda Y, Yamashita M, Buchen B. Evaluation of the three-dimensional clinostat as a simulator of weightlessness. Planta. 1997;203:S187–S197. doi: 10.1007/pl00008108. [DOI] [PubMed] [Google Scholar]
- Katekar GF, Geissler AE. Auxin transport inhibitors: IV. Evidence of a common mode of action for a proposed class of auxin transport inhibitors: the phytotropins. Plant Physiol. 1980;66:1190–1195. doi: 10.1104/pp.66.6.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiegle E, Moore CA, Haseloff J, Tester J, Knight MR. Cell-type-specific calcium responses to drought, salt and cold in Arabidopsis roots. Plant J. 2000;23:267–278. doi: 10.1046/j.1365-313x.2000.00786.x. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Hertel R, Sack FD. Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta. 1989;177:198–206. [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. Recombinant aequorin methods for measurement of intracellular calcium in plants. Plant Mol Biol Manual. 1997;C4:1–22. [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Knight MR, Smith SM, Trewavas AJ. Wind-induced plant motion immediately increases cytosolic calcium. Proc Nat Acad Sci USA. 1992;89:4967–4971. doi: 10.1073/pnas.89.11.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. The optimum angle of geotropic stimulation and its relation to the starch statolith hypothesis. Physiol Plant. 1969;22:469–488. [Google Scholar]
- Lee JS, Mulkey TJ, Evans ML. Reversible loss of gravitropic sensitivity in maize roots after tip application of calcium chelators. Science. 1983a;220:1375–1377. doi: 10.1126/science.220.4604.1375. [DOI] [PubMed] [Google Scholar]
- Lee JS, Mulkey TJ, Evans ML. Gravity induce polar transport of calcium across root tips of maize. Plant Physiol. 1983b;73:874–876. doi: 10.1104/pp.73.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legue V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. Cytoplasmic free calcium in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997;114:789–800. doi: 10.1104/pp.114.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL. Molecular genetic analysis of plant gravitropism. ASGSB Bull. 1997;10:75–82. [PubMed] [Google Scholar]
- Malhó R. Coding information in plant cells: the multiple roles of Ca2+ as a second messenger. Plant Biol. 1999;1:487–494. [Google Scholar]
- Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KE, Briggs WR. Transport of IAA during gravitropism in intact maize coleoptiles. Plant Physiol. 1990;94:1763–1769. doi: 10.1104/pp.94.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera IY, Heilman I, Boss WF. Transient and sustained increases in inositol 1,4,5-trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc Nat Acad Sci USA. 1999;96:5838–5843. doi: 10.1073/pnas.96.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plieth C. Plant calcium signaling and monitoring-pros and cons and recent experimental approaches. Protoplasma. 2001;218:1–23. doi: 10.1007/BF01288356. [DOI] [PubMed] [Google Scholar]
- Plieth C, Hansen U-P, Knight H, Knight MR. Temperature sensing by plants: the primary mechanisms of signal perception and calcium response. Plant J. 1999b;18:491–497. doi: 10.1046/j.1365-313x.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- Plieth C, Sattelmacher B, Hansen U-P. Cytoplasmic Ca++-H+-exchange buffers in green plant cells. Protoplasma. 1997;198:107–124. ; erratum Plieth C, Sattelmacher B, Hansen U-P (1997) Protoplasma 199: 223. [Google Scholar]
- Plieth C, Sattelmacher B, Hansen U-P, Knight MR. Low pH-mediated elevations in cytosolic calcium are inhibited by aluminum: a potential mechanism for aluminum toxicity. Plant J. 1999a;18:643–650. doi: 10.1046/j.1365-313x.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- Rorabaugh PA, Salisbury FB. Gravitropism in hugher plant shoots: VI. Changing sensitivity to auxin in gravistimulated soybean hypocotyls. Plant Physiol. 1989;91:1329–1338. doi: 10.1104/pp.91.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JJ. Effects of auxin transport inhibitors in gibberellins in pea. J Plant Growth Regul. 1998;17:141–146. [Google Scholar]
- Roux SJ, Serlin BS. Cellular mechanisms controlling light-stimulated gravitropism: role of calcium. CRC Crit Rev Plant Sci. 1987;5:205–236. doi: 10.1080/07352688709382240. [DOI] [PubMed] [Google Scholar]
- Sanders D, Brownlee C, Harper JF. Communicating with calcium. Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura O, Musici B, Kishi Y, Inouye S. Light-emitting properties of recombinant semisynthetic aequorins and recombinant fluorescein-conjugated aequorin for measuring cellular calcium. Cell Calcium. 1993;14:373–378. doi: 10.1016/0143-4160(93)90041-4. [DOI] [PubMed] [Google Scholar]
- Sievers A, Hejnowicz Z. How well does the clinostat mimic the effect of microgravity on plant cells and organs? ASGSB Bull. 1992;5:69–75. [PubMed] [Google Scholar]
- Sinclair W, Oliver I, Maher P, Trewavas AJ. The role of calmodulin in the gravitropic response of the Arabidopsis thaliana agr-3 mutant. Planta. 1996;199:343–351. doi: 10.1007/BF00195725. [DOI] [PubMed] [Google Scholar]
- Sinclair W, Trewavas AJ. Calcium in gravitropism: a re-examination. Planta. 1997;203:S85–S90. doi: 10.1007/pl00008120. [DOI] [PubMed] [Google Scholar]
- Slocum RD, Roux SJ. Cellular and subcellular localization of calcium in gravistimulated oat coleoptiles and its possible significance in the establishment of tropic curvature. Planta. 1983;157:481–492. doi: 10.1007/BF00396878. [DOI] [PubMed] [Google Scholar]
- Stinemetz CL, Kuzmanoff KM, Evans ML, Jarret HW. Correlation between calmodulin activity and gravitropic sensitivity in primary roots of maize. Plant Physiol. 1987;84:1337–1342. doi: 10.1104/pp.84.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaka M, Kato T, Fukaki H. The endodermis and shoot gravitropism. Trends Plant Sci. 1999;4:103–107. doi: 10.1016/s1360-1385(99)01376-x. [DOI] [PubMed] [Google Scholar]
- Trewavas AJ, Knight MR. Mechanical signalling, calcium and plant form. Plant Mol Biol. 1994;26:1329–1341. doi: 10.1007/BF00016478. [DOI] [PubMed] [Google Scholar]
- Trewavas AJ, Malhó R. Signal perception and transduction: the origin of the phenotype. Plant Cell. 1997;9:1181–1195. doi: 10.1105/tpc.9.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann D, Sievers A. Graviperception in multicellular organs. In: Haupt W, Feinlieb ME, editors. Encyclopedia of Plant Physiology, New Series: Physiology of Movements. Berlin: Springer Verlag; 1979. pp. 573–600. [Google Scholar]
- Weisenseel MH, Meyer AJ. Bioelectricity gravity and plants. Planta. 1997;203:S98–S106. doi: 10.1007/pl00008122. [DOI] [PubMed] [Google Scholar]
- Wood NT, Haley A, Viry-Moussaïd M, Johnson CH, Van der Luit A, Trewavas AJ. The calcium rhythms of different cell types oscillate with different circadian phases. Plant Physiol. 2001;125:787–796. doi: 10.1104/pp.125.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder TL, Zheng H-Q, Todd P, Staehelin LA. Amyloplast sedimentation dynamics in maize columella cells support a new model for the gravity-sensing apparatus of roots. Plant Physiol. 2001;125:1045–1060. doi: 10.1104/pp.125.2.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]