Abstract
Type 2A serine/threonine protein phosphatases (PP2A) are important components in the reversible protein phosphorylation events in plants and other organisms. PP2A proteins are oligomeric complexes constituted by a catalytic subunit and several regulatory subunits that modulate the activity of these phosphatases. The analysis of the complete genome of Arabidopsis allowed us to characterize four novel genes, AtB′ε, AtB′ζ, AtB′η, and AtB′θ, belonging to the PP2A B′ regulatory subunit family. Because four genes of this type had been described previously, this family is composed of eight members. Reverse transcriptase-polymerase chain reaction experiments showed that AtB′ε mRNAs are present in all Arabidopsis tissues analyzed, and their levels do not respond significantly to heat stress. Expressed sequence tags corresponding to AtB′ζ, AtB′η, and AtB′θ have been identified, indicating that the new genes are actively transcribed. The genomic organization of this family of PP2A regulatory subunits is reported, as well as its chromosomal location. An extensive survey of the family has been carried out in plants, characterizing B′ subunits in a number of different species, and performing a phylogenetic study that included several B′ regulatory proteins from animals. Our results indicate that the animal and plant proteins have evolved independently, that there is a relationship between the number of B′ isoforms and the complexity of the organism, and that there are at least three main subfamilies of regulatory subunits in plants, which we have named α, η, and κ.
Reversible protein phosphorylation is widely accepted as a major mechanism for the control of biological processes in eukaryotic cells. In plants, reversible protein phosphorylation is involved in processes such as hormonal, pathogenic, or environmental stress responses (Mumby and Walter, 1993; Smith and Walker, 1993; Garbers et al., 1996; Schöntal, 1998; Janssens and Goris, 2001). In this context, Ser/Thr protein phosphatases (PPs) are important regulatory components of many signal transduction pathways (Ingebritsen and Cohen, 1983a; Schöntal, 1998). Several Ser/Thr phosphatases, grouped into different categories, have been identified in a variety of plant species. Specifically, homologs of the 1, 2A, and 2C types of animal PPs have been described in plants (Rodríguez, 1998; Lin et al., 1999; Meek et al., 1999). All these types of PPs are distinguished by their different sensitivity to inhibitors and their divalent cation requirements, and are structurally different (for review, see Mumby and Walter, 1993).
Type 2A phosphatases (PP2A) are oligomeric enzymes with no obvious requirements for ions or cofactors, and are implicated in a variety of cellular processes (Mumby and Walter, 1993; Janssens and Goris, 2001). In general, the native forms of PP2A proteins exist as oligomeric complexes, constituted by a catalytic subunit (PP2Ac), and one or more regulatory subunits named A and B. Thus, PP2A proteins can be heterodimers, consisting of a PP2Ac catalytic subunit and a type A regulatory subunit, or heterotrimers that contain an additional regulatory subunit of the B type. PP2Ac subunits are highly conserved in all organisms analyzed, and their activity, specificity, and subcellular localization depend on the association of this subunit with different A and B regulatory subunits (Hendrix et al., 1993b; Strack et al., 1998). The A regulatory subunit has a molecular mass of 65 kD, and consists of 15 imperfect repeats of 38 to 43 amino acids, through which it interacts with the PP2Ac catalytic subunit and the B regulatory subunit (Groves et al., 1999). Type B regulatory subunits of PP2A are very diverse, and can be clustered into at least three distinct groups including the 55-kD B, the 52- to 74-kD B′, and the 72- to 130-kD B′′ subunit families (Rundle et al., 1995; Corum et al., 1996; Csortos et al., 1996; McCright et al., 1996a). Each family is composed of several members with the exception of the B′′ subunit family (Hendrix et al., 1993a).
Homologs to all PP2A subunits have been described in plants. In Arabidopsis, the catalytic subunit of PP2 (PP2Ac) is encoded by at least five genes, each of which appears to be expressed in all tissues albeit at different levels (Ariño et al., 1993; Casamayor et al., 1994; Pérez-Callejón et al., 1998). Regarding the regulatory subunits, three genes encoding the A 65-kD subunit (Slabas et al., 1994), two genes encoding the B subunit (Rundle et al., 1995; Corum et al., 1996), and one gene encoding the B′′ regulatory subunit (Sato et al., 1997) have been identified. In the last years, four isoforms of the B′ regulatory subunit of PP2A have been described in Arabidopsis, named AtB′α, AtB′β, AtB′γ (Latorre et al., 1997), and AtB′δ (Haynes et al., 1999). However, Southern-blot analyses of genomic DNA indicated that at least another gene encoding a fifth B′ isoform could be present in this plant (Haynes et al., 1999). Five genes encoding B′ regulatory subunits (or PR56) also have been described in humans (Homo sapiens) that produce at least seven isoforms (McCright and Virshup, 1995; McCright et al., 1996a; Tehrani et al., 1996). Similarly, five genes encoding at least eight isotypes of B′ exist in rabbits (Oryctologus cuniculus; Csortos et al., 1996; Zolnierowicz et al., 1996).
All four AtB′ genes described so far are expressed in all Arabidopsis organs and encode very similar proteins, the central core of the B′ subunits being the most conserved region (Latorre et al., 1997; Haynes et al., 1999). It is interesting to mention that transcripts from one of these genes, AtB′γ, seem to accumulate in response to heat stress (Haynes et al., 1999), suggesting that PP2A heterotrimers containing this subunit could be involved in stress response mechanisms in plants. This has been proposed for the RTS1 (or SCS1) protein, the yeast (Saccharomyces cerevisiae) homolog of the B′ subunit. RTS1 was isolated as suppressor of mutant alleles of the ROX3 and Hsp60 genes. The ROX3 protein seems to be involved in the global stress response pathway (Evangelista et al., 1996) and Hsp60 is a mitochondrial heat shock protein (Shu and Hallberg, 1995). On the other hand, two of the B′ subunits in Arabidopsis, AtB′α and AtB′β, contain putative nuclear targeting sequences, suggesting that the different isoforms could function to target PP2A to unique subcellular locations (Latorre et al., 1997). This idea has also been proposed for the human B′ subunits, where different subcellular locations were found for α, β, and ε subunits located in the cytoplasm, and γ and δ in the nucleus (McCright et al., 1996b; Zhao et al., 1997).
Our group has contributed to the European Union Sequencing on Arabidopsis chromosome 3 (Salanoubat et al., 2000) as part of the worldwide Arabidopsis Genome Initiative (AGI) that has sequenced the complete genome of Arabidopsis (AGI, 2000). In the frame of this collaboration, we have characterized in this work four additional members of the PP2A B′ regulatory subunit family in Arabidopsis (AtB′ε, AtB′ζ, AtB′η, and AtB′θ) and have analyzed their genomic organization. We have also performed an extensive study of this family in plants, describing several new members coding for B′ subunits in rice (Oryza sativa), tomato (Lycopersicon esculentum), corn (Zea mays), medic barrel (Medicago truncatula), potato (Solanum tuberosum), soybean (Glycine max), etc. A structural and phylogenetic analysis of the new proteins is also carried out.
RESULTS
Identification and Characterization of AtB′ε, a Gene Encoding a New Isoform of the PP2A B′ Regulatory Subunit Family in Arabidopsis
As a result of our participation in the AGI, the bacterial artificial chromosome (BAC) clone T15C9 was sequenced and analyzed to identify putative coding regions. One of these regions was of particular interest due to its similarity to the B′ regulatory subunits of PP2A. The open reading frame (ORF) was composed of two exons of 1,188 and 306 nucleotides, separated by an intron of 82 nucleotides. The predicted protein encoded by this gene was 497 amino acids long, with a molecular mass of 57.5 kD. Similarity searches in protein databases showed that this protein was highly homologous to the B′ regulatory subunits of PP2A from Arabidopsis (Latorre et al., 1997; Haynes et al., 1999) and other organisms. The striking similarity with respect to the Arabidopsis B′ regulatory subunits (AtB′ α, β, γ, and δ), which ranged from 53.1% to 77.9% at the protein level, led us to consider this protein as a new member of the B′ family of regulatory subunits of PP2A. We named the hypothetical gene AtB′ε, following the previous nomenclature (Latorre et al., 1997; Haynes et al., 1999).
To know whether AtB′ε was normally expressed in any stage of the Arabidopsis development, we used the DNA sequence corresponding to the hypothetical protein to perform a BLAST similarity search against all the available Arabidopsis expressed sequence tag (EST) databases. The searches yielded no cDNA clones identical to AtB′ε, so we decided to perform a reverse transcriptase (RT)-PCR analysis to determine the expression pattern of the gene (see “Materials and Methods”). The primers were designed specifically for AtB′ε, avoiding cross amplification of sequences from the other members of the B′ family. The results show that the AtB′ε is an active gene and that the transcripts accumulate in all organs analyzed: leaves, seeds, stems, and flowers (Fig. 1A). This is in accordance with the expression patterns reported for other Arabidopsis PP2A B′ regulatory subunits (Latorre et al., 1997; Haynes et al., 1999). The experimental evidences obtained for AtB′ε confirm that it is subject to transcription and, therefore, can be considered as an active gene.
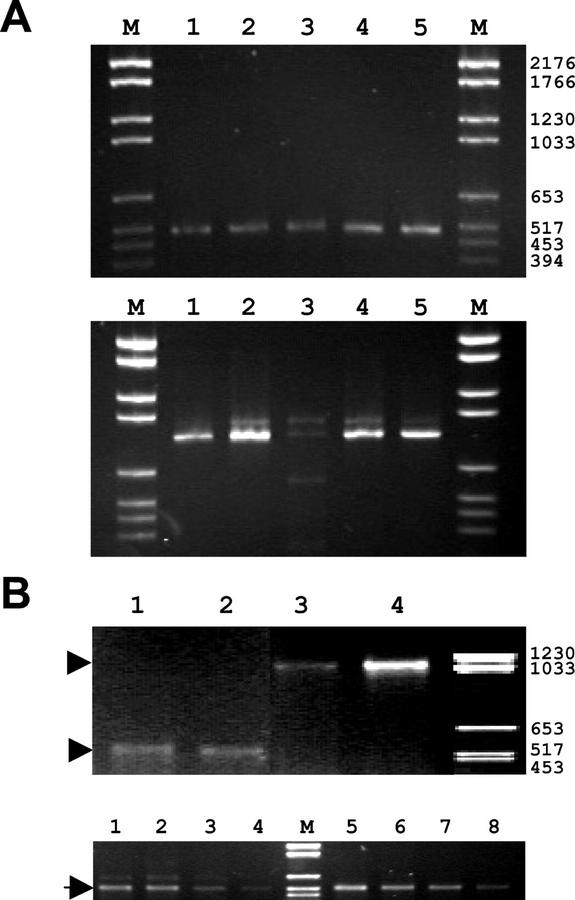
Figure 1.
RT-PCR experiments with AtB′ε. A, Expression of the AtB′ε gene in different Arabidopsis organs. Total RNA was extracted from leaves 4.5 weeks old (lane 1), leaves 7 weeks old (lane 2), seeds (lane 3), stems (lane 4), and flowers (lane 5). Lane M is the DNA size marker, and numbers indicate size of the DNA fragments in nucleotides. Upper, Results of RT-PCR using oligo(dT)- and AtB′ε-specific primers to amplify AtB′ε transcripts. Lower, Results of the RT-PCR using oligo(dT) and actin-1 primers as control. Equal amounts of both RT-PCR reactions (with AtB′ε-specific primers and actin-specific primers) were loaded for each tissue. B, Expression of the AtB′ε gene after heat shock conditions. Lower, rRNA amplification using 6:10, 7:10, 8:10, and 9:10 ratio competimers to amplify RNA from non-stressed plants (lanes 1–4) or heat-shocked plants (lanes 5–8). The intensity of the bands on the gel decreases as the ratio of competimers increases, indicating that the PCR is in a quantitative range. Upper, Expression of AtB′ε and AtB′γ at 23°C (lanes 1 and 3, respectively), and after heat shock, with 2 h at 37°C (lanes 2 and 4, respectively). M is the DNA molecular mass marker VI (Roche 1062590); numbers indicate size of the DNA fragments in bp.
It had been reported that the AtB′γ isoform appears to be involved in the heat stress response because one of the mRNAs derived from this gene (1.5 kb) accumulates in Arabidopsis seedlings after 2 h of heat shock at 37°C (Latorre et al., 1997). On the contrary, transcripts from the AtB′α, AtB′β, and AtB′δ genes do not respond to such treatment (Latorre et al., 1997; Haynes et al., 1999). To determine whether AtB′ε expression changes in response to heat stress, we performed a semiquantitative RT-PCR analysis using two different RNA samples, extracted from adult Arabidopsis plants grown in standard conditions and from plants subjected to heat stress at 37°C for 2 h. As a control for the heat shock response, we performed the same RT-PCR experiments using a set of specific primers to amplify AtB′γ mRNAs. We established that the PCR reactions were in a semiquantitative range performing a series of control reactions with ribosomal RNA with the presence of competimers that inhibit amplification at different ratios. As the ratio of competimer increases, the intensity of the band on the gel decreases (Fig. 1B, lower), which indicates that, in the same conditions, the variations observed in the experiment reactions are not an artifact. This way, it can be observed that, although there is a clear response to heat stress in AtB′γ expression, AtB′ε mRNA levels do not significantly vary under heat shock conditions (Fig. 1B, upper).
Identification of Three Additional Members of the PP2A B′ Regulatory Subunit Family
Besides the four previously described AtB′ isoforms, the BLAST searches performed to characterize AtB′ε, in the Arabidopsis databases, produced three additional predicted proteins that showed a high degree of conservation with respect to the B′PP2A regulatory subunits and AtB′ε itself. Both the scores and e values showed a clear cutoff, separating AtB′α, AtB′β, AtB′γ, AtB′δ, AtB′ε, and the three new proteins from the other ones produced by the BLAST search. The three predicted proteins were the result of the annotation of the complete genome of Arabidopsis performed by the AGI, and their accession numbers were BAB02360, BAB01065, and AAG09562. The high degree of similarity obtained allowed us to consider the novel predicted proteins as members of the B′PP2A regulatory subunit family and, following the previous nomenclature, we named them AtB′ζ, AtB′η, and AtB′θ, respectively.
In an attempt to identify additional members of this family that had not been already described at the protein level, we analyzed the whole Arabidopsis genome, dynamically translated in all reading frames, in a TBLASTN search, using the eight proteins as queries. The searches did not yield additional new members, and no new DNA sequences, apart from the previously characterized so far, produced proteins with a significant similarity. The exhaustive analyses performed, both at the protein and DNA level, allowed us to discard the existence of additional genes producing PP2A B′ regulatory subunits in the genome of Arabidopsis. Therefore, although genomic Southern-blot analyses had suggested that five genes encoding B′ isoforms were present in Arabidopsis (Latorre et al., 1997), the analysis of the complete genome of the plant revealed that this family of regulatory proteins is composed of eight members.
To know whether any of the three new members of this Arabidopsis gene family are normally expressed in any stage of the Arabidopsis development, we used the DNA sequence corresponding to the hypothetical B′ proteins we had identified to perform a BLAST similarity search against all the available Arabidopsis EST databases. cDNA clones corresponding to AtB′ζ, AtB′η, and AtB′θ were identified (Table I), confirming that these hypothetical genes are subject to transcription and, therefore, can be considered as active genes.
Table I.
Genomic organization of the Arabidopsis and rice genes
| Gene Name and Accession No. | No. of Exons | 5′ URa Lengthc | CR1b Lengthc | Intron Lengthc | CR2 Lengthc | 3′ UR Lengthc | ORF Lengthc | ESTs Found |
|---|---|---|---|---|---|---|---|---|
| AtB′α, U73526 | 2 | 284 | 1,161 | 592 | 324 | 447 | 1,485 | AV522466 and AW004404 |
| AtB′β, U73527 | 2 | 360 | 1,161 | 530 | 336 | 664 | 1,497 | AA395160, T43172, and AV561236 |
| AtB′γ, U73528 | 3 | 414 | 1,191 | 73 | 375 | 587 | 1,566 | BE520334, AI999159, AA042311, BE520336, AV547536, AA597610, and AA597613 |
| AtB′δ, AF0623396 | 2 | 393 | 1,128 | 95 | 303 | 588 | 1,431 | AI993739, AV562733, BE521372, BE521374, BE521373, AV558379, N97014, and N96717 |
| AtB′ε, AJ276037 | 2d | NDe | 1,188 | 82 | 306 | ND | 1,494 | Not found |
| AtB′ζ, BAB02360 | 2d | ND | 1,254 | 94 | 384 | ND | 1,638 | BE524995 |
| AtB′η, BAB01065 | 4 | 326 | 1,227 | 690 | 303 | ND | 1,530 | AV548590 |
| AtB′θ, AAG09562 | 2d | ND | 1,164 | 200 | 312 | ND | 1,476 | BE524785 and AI996905 |
| OsB′ζ, AJ312314 | 2 | ND | 1,194 | 1,644 | 360 | 427 | 1,664 | AU092036, AU092032, D22058, D22057, and D22055 |
| OsB′η, CAC09487 | 2 | ND | 1,173 | 1,389 | 357 | 303 | 1,530 | AU032466, AU057748, AU057747, and AU032467 |
| OsB′θ, AJ312315 | 2d | ND | 1,206 | 1,228 | 345 | ND | 1,551 | Not found |
| OsB′κ, AJ312313 | 2 | ND | 1,164 | 404 | 369 | 384 | 1,533 | AJ272423, BE040415, AU085794, and C27982 |
| OsBλ, BAB07976 | 2d | ND | 1,125 | 1,238 | 387 | ND | 1,512 | Not found |
Untranslated region.
Coding region.
In bp.
Theoretically predicted.
Not determined.
Genomic Organization of the AtB′ Regulatory Subunit Family
The sequencing of the complete genome of Arabidopsis allowed us to determine the genomic organization of the eight PP2A B′ regulatory subunit genes as well as their chromosomal position. We did these determinations for the previously described genes, AtB′α, AtB′β, AtB′γ, and AtB′δ, only characterized at the cDNA level, as well as for the ones we describe here for the first time, to our knowledge, AtB′ε, AtB′ζ, AtB′η, and AtB′θ.
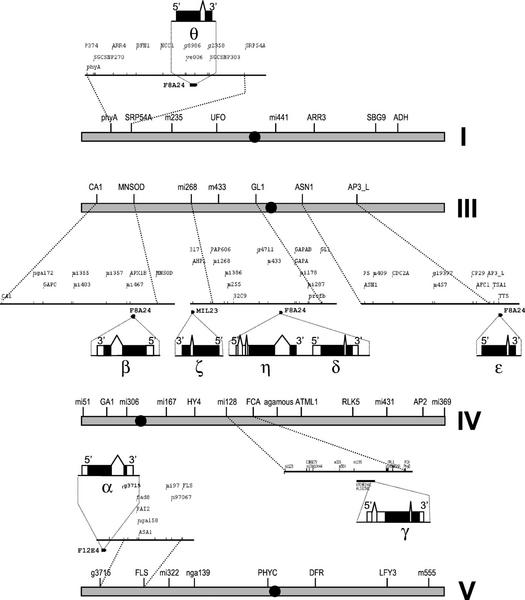
For the first group of genes, we performed a BLASTN search against the Arabidopsis genome database, using the cDNA sequences as a query, to find the genomic clones in which the genes were located. The chromosomal position of such clones was determined using the Map Viewer Tool at The Arabidopsis Information Resource (http://www.Arabidopsis.org/servlets/mapper). For the genes we describe here, this information was available in the GenBank annotation entries. The results are displayed in Table II, where the chromosome and genomic clones of the genes are shown. In summary, five of the genes (AtB′β, AtB′δ, AtB′ζ, AtB′ε, and AtB′η) are located on chromosome III, the three remaining ones, AtB′α, AtB′γ, and AtB′θ, being placed on chromosomes V, IV, and I, respectively. Only chromosome II presents no genes related to this family. It is noticeable that AtB′δ and AtB′η are adjacent genes, separated by less than 800 bp, which opens interesting questions about their evolutionary origin that will be discussed below. The chromosomal position of the genomic clones that contain the eight genes is shown in Figure 2.
Table II.
Location in the AtB′ genes
| Gene | Chrom.a | G.C.b | Acc. No.c |
|---|---|---|---|
| AtB′α | V | F12E4 | AL162751 |
| AtB′β | III | F8A24 | AC015985 |
| AtB′γ | IV | ESSAI FCA | Z97338 |
| AtB′δ | III | MPE11 | AB023041 |
| AtB′ε | III | T15C9 | AL132970 |
| AtB′ζ | III | MIL23 | AB019232 |
| AtB′η | III | MPE11 | AB023041 |
| AtB′θ | I | T6J4 | AC011810 |
Chromosome no.
Genomic BAC clone where the gene is isolated.
GenBank accession no.
Figure 2.
Genomic organization of the AtB′ genes on the Arabidopsis chromosomes. Bars marked with roman numerals represent Arabidopsis chromosomes (I and III–V), with several genetic markers indicated for each one. The centromere regions are shown as black circles. Blowups of the regions between two genetic markers containing the genomic clones with the AtB′ genes are shown, an arrow indicating the exact position of the clones. The intron-exon structure of the eight genes is also illustrated, with each gene shown as a blowup of the genomic clones where they are located. Black boxes indicate coding regions and white boxes represent untranslated ones.
The comparison of the cDNA sequence from AtB′α, AtB′β, AtB′δ, and AtB′γ against the sequence of their corresponding genomic clones allowed us to determine the intron-exon structure of the four genes, which is described here for the first time, to our knowledge. We also identified ESTs corresponding to all the previously described genes (Table I), but the partial sequences of the cDNA clones did not reveal any new exon different from those described before, although this cannot be completely discarded until the sequences of the new cDNAs are completely determined. The structure of the four new genes presented in this work was determined using prediction programs as GenScan, and, in some cases, partially confirmed by the EST sequences. Table I summarizes the data obtained, which are graphically depicted in Figure 2.
The eight genes present ORFs of similar size, and the differences are mostly produced at the variable 5′ and 3′ variable regions. All genes except AtB′λ and AtB′η present a simple exon-intron structure, with two coding exons separated by an intron of variable size. All the genes display exons of similar size, with the first one being approximately one-half the size of the second, whereas the intron length is more variable. The presence of an intron in the 5′-untranslated region of AtB′λ was reported by Haynes et al. (1999). On the other hand, the sequence of the EST found for AtB′η revealed the existence of two non-coding small exons preceding the coding ones, indicating that this gene is composed of at least four exons. The existence of non-coding exons in the four newly predicted genes will be determined when complete cDNAs are sequenced and the 5′ region of the genes is analyzed. The similar structure of the genes speaks about a common origin.
The PP2A B′ Subunit Family in Rice
We considered it of interest to study the family of B′ regulatory subunits of PP2A in another plant genome, although the Arabidopsis genome is the only one that has been completed so far. One of the most advanced genome projects is The International Rice Genome Sequencing Project, that, up to now, has released 78.8 Mb of DNA sequence to the DNA Data Bank of Japan (http://www.ddbj.nig.ac.jp/). On the other hand, the Monsanto Rice Genome Sequencing project (http://www.rice-research.org/) has produced sequence from 3,391 BACs distributed across the genome of rice cv Nipponbare (the same cultivar used by The International Rice Genome Sequencing Project) that constitute a “draft” dataset consisting of 52,202 contigs, corresponding to 259 Mb of assembled sequence data, which represents approximately 70% of the total genome size.
Thus, we decided to analyze the partially sequenced genome of rice, and we compared the eight Arabidopsis proteins against the nonredundant DNA Data Bank of Japan and Rice Research Organization nucleotide sequence databases in a TBLASTN search. This search yielded five genomic clones that contained sequences similar to the B′ family two from GenBank, and three from the Rice Research Organization (Table I). The proteins we found in the GenBank clones had been already annotated and correspond to entries CAC09487 and BAB07976, respectively, although in the first case the predicted polypeptide is much shorter than the one we obtained, probably due to a mistake in the automatic annotation process of the clone. The genomic clones from the Rice Research Organization were analyzed with the GenScan program, and three ORFs coding for three proteins of 510, 517, and 516 amino acids were predicted. Comparative analysis with respect to the Arabidopsis proteins showed a high degree of conservation between them, with similarities ranging from 58.6% to 77.5%.
To determine whether the five predicted genes were transcriptionally active, a BLAST search was performed on the EST databases, producing several cDNA clones identical to three of the predicted genes. The comparison of the genomic and cDNA sequences allowed us to confirm the intron-exon structure of these three genes, as well as to determine the length of the untranslated regions. The structure determined for the rice genes is similar to that of the Arabidopsis ones, with ORFs about 1,200 bp long, composed of two exons separated by a single intron. No EST clones were found for the other two predicted genes. All these data are summarized in Table I.
Based on the degree of conservation with respect to Arabidopsis, we named the rice proteins OsB′ζ (accession no. AJ312314), OsB′η (accession no. CAC09487), and OsB′θ (accession no. AJ312315). For the two remaining proteins, it was not possible to determine an ortholog with the Arabidopsis B′ isoforms, so we decided to name them OsB′κ and OsB′λ, following the existing nomenclature.
EST Analysis in Plants
The GenBank databases contain more than 1,000,000 plant ESTs, produced by large-scale cDNA sequencing projects from many different species, from green algae to the angiosperms. To obtain a broader view of the B′ regulatory subunit of PP2A family in plants, we analyzed the plant EST sequences available in the databases in an attempt to obtain cDNAs coding for proteins belonging to this family. A TBLASTN search was carried out with the eight Arabidopsis B′ isoforms as a query, and more than 200 ESTs that produced protein fragments with significant similarity were identified. The ESTs belonged to 20 plant species from five different classes, including green algae, ferns, conifers, and mono- and dicotyledons (Table III). The similarity with respect to the Arabidopsis B′ regulatory subunits presented by some protein fragments was quite striking: The EST AI489160, from tomato, produced a 223-amino acid fragment 95% similar to AtB′α; the EST BF006061, from medic barrel, produced a 197-amino acid fragment 93% similar to AtB′ζ, etc.
Table III.
ESTs coding for proteins similar to the Arabidopsis B′ regulatory subunits
Despite the partial data, it was evident that the protein fragments obtained for one species were not identical, and showed different similarity scores with respect to the Arabidopsis proteins, which suggested the existence of several isoforms. As described in “Materials and Methods,” we performed an extensive analysis of more than 100 EST sequences in an effort to identify the minimum number of B′ isoforms present in some of the species that showed related EST sequences: Chlamydomonas reinhardtii, Ceratopteris richardii, corn, potato, soybean, medic barrel, and tomato. All the ESTs available for one species were assembled, and the resulting contigs that produced nonidentical, overlapping protein fragments with significant similarity with respect to the B′ subunits were considered. Only identical EST sequences were included in a contig to ensure that each consensus corresponded to a unique cDNA. This way, we could establish that each contig corresponded to a different cDNA coding for one member of the B′ regulatory family; thus, the number of contigs represented the minimum number of PP2A B′ genes existing in one species.
Table IV summarizes the results obtained for the different species, showing the contigs from which the consensus cDNA sequences were obtained, the best similarity scores with respect to the Arabidopsis proteins, and the length of the compared fragments. It can be appreciated that most of the species contain between five and seven genes coding for these proteins in each plant species, and our hypothesis is that the final number will be very similar to that of Arabidopsis. It is noteworthy that the eight EST sequences from the green algae C. reinhardtii, form a single contig that produces a partial protein of 365 amino acids, suggesting that this species only possesses one representative of the B′ regulatory subunit family, which we named CrPP2AB′. In the case of the fern C. richardii, the three available ESTs might correspond to two different cDNAs, but it will be necessary to analyze more ESTs to confirm this result. In general terms, the degree of conservation of all the protein fragments obtained with respect to the Arabidopsis B′ isoforms is striking, with similarities ranging from 64% to 97%.
Table IV.
Analysis of the EST sequences
| Species | Consensusa | Similarityb | Lengthc |
|---|---|---|---|
| % | |||
| C. reinhardtii | Contig 1 | 74 | 365 |
| (eight ESTs) | CrPP2AB′ | ||
| C. richardii | Contig 1 | 89 | 232 |
| (three ESTs) | Contig 2 | 72 | 212 |
| Corn | Contig 1 | 81 | 105 |
| (eight ESTs) | Contig 2 | 83 | 92 |
| Contig 3 | 74 | 109 | |
| Contig 5 | 72 | 128 | |
| Contig 6 | 76 | 154 | |
| Potato | Contig 1 | 94 | 157 |
| (10 ESTs) | Contig 2 | 64 | 136 |
| Contig 3 | 86 | 132 | |
| Contig 4 | 86 | 154 | |
| Contig 5 | 90 | 223 | |
| StB′α | |||
| Contig 7 | 82 | 180 | |
| Soybean | Contig 2 | 81 | 90 |
| (31 ESTs) | Contig 3 | 74 | 175 |
| Contig 5 | 97 | 90 | |
| Contig 7 | 93 | 319 | |
| GmB′α | |||
| Contig 9 | 87 | 241 | |
| Contig 11 | 88 | 332 | |
| GmB′η | |||
| Contig 13 | 68 | 303 | |
| Medic barrel | Contig 2 | 76 | 265 |
| (31 ESTs) | Contig 3 | 90 | 248 |
| Contig 4 | 76 | 183 | |
| Contig 5 | 72 | 400 | |
| MtB′κ | |||
| Contig 6 | 91 | 201 | |
| Contig 9 | 65 | 190 | |
| Tomato | Contig 2 | 81 | 342 |
| (25 ESTs) | LeB′η | ||
| Contig 3 | 82 | 511 | |
| LeB′α | |||
| Contig 5 | 69 | 506 | |
| LeB′κ | |||
| Contig 6 | 70 | 129 | |
| Contig 7 | 84 | 236 | |
| Contig 8 | 63 | 172 |
Consensus sequence of the contig.
Best similarity score obtained in the BLAST search respect the Arabidopsis B′ subunit proteins.
Length, in amino acids, of the compared sequences.
The 25 ESTs from tomato assembled into eight contigs, six of which were clearly identified as different cDNAs. Two of the cDNAs seemed to present complete ORFs that coded for proteins of 511 and 506 amino acids, which is the average size of this type of proteins. Based on the similarity data, we named them LeB′α, and LeB′κ. On the other hand, several consensus cDNA sequences from C. reinhardtii, potato, soybean, medic barrel, and tomato produced protein fragments larger than 300 amino acids, which represent almost 60% of the average length of these proteins, so we decided to use them in a phylogenetic analysis. These partial proteins were also named based on their similarity to the previously described ones (CrPP2AB′, StB′α, GmB′α and GmB′η, MtB′κ, and LeB′η, respectively).
Comparative Analysis of the Proteins
A multiple alignment was performed with the Clustal X program, including the Arabidopsis, rice, and tomato B′ isoforms characterized so far, the partial proteins from potato, soybean, medic barrel, and C. reinhardtii, and several B′ regulatory subunits from animals: Caenorhabditis elegans (CePP2A-B′), Drosophila melanogaster (DmPP2A-B′), Xenopus laevis (XlB′ε), Oryctolagus cuniculus (γ), and humans (B56α).
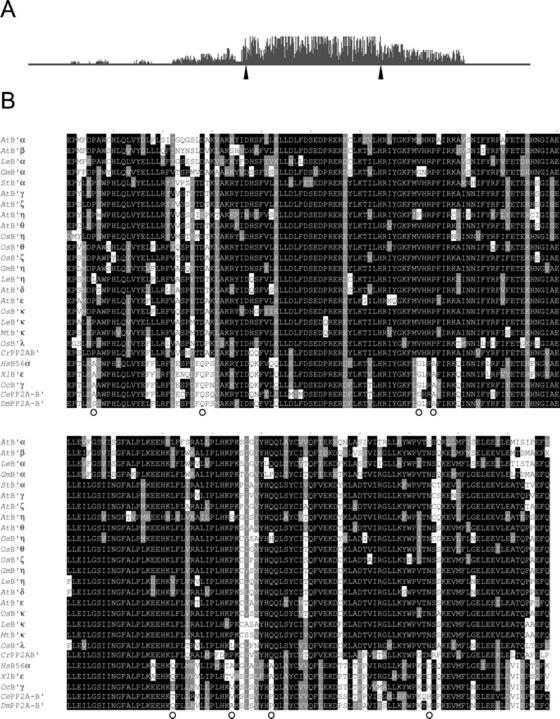
The multiple alignment of the protein sequences shows the existence of a high degree of similarity between them, with the central core regions being the most conserved, and the amino- and carboxy-terminal regions the most variable (Fig. 3). In this central region, many amino acids are identical and most of the substitutions are conservative ones. On the contrary, the amino- and carboxy-terminal regions are highly variable and they accumulate most of the size and amino acid identity changes.
Figure 3.
A, Alignment profile obtained with the Clustal X program of the B′ proteins from the species mentioned below. The height of the bars indicates the number of identical residues per position. The arrowheads indicate the region shown in the multiple alignment. B, Sequence alignment of the core region of the B′ regulatory subunits from various organisms, performed with the Clustal X software. The protein sequences included are from Arabidopsis (AtB′α, AtB′β, AtB′γ, AtB′δ, AtB′ε, AtB′ζ, AtB′γ, AtB′η, and AtB′θ), rice (OsB′ζ, OsB′η, OsB′θ, OsB′κ, and OsB′λ), tomato (LeB′α, LeB′η, and LeB′κ), soybean (GmB′α and GmB′η), medic barrel (MbB′k), potato (StB′a), C. reinhardtii (CrPP2AB′), C. elegans (CePP2A-B′, accession no. CAA98422), D. melanogaster (DmPP2A-B′, accession no. CAB86364), X. laevis (XlB′ε, accession no. AAG22076), O. cuniculus (OcB′γ, accession no. Q28651), and human (HsB56α, accession no. NM 006243). Black boxes indicate amino acid identity and gray boxes indicate conservative changes. Dashes represent gaps to maximize amino acid alignment. Circles indicate residues that are differently conserved between animals and plants.
The multiple alignment also shows differences between the proteins from plant and animal species. This way, eight positions (indicated with circles in Fig. 3) display a different conserved amino acid depending on the origin of the protein: All the animal proteins display one residue, whereas the plant ones present a different conserved amino acid.
Relationship Analysis
Because the Arabidopsis genome is completely characterized and all the B′ regulatory subunits have been identified in this species, we found it interesting to perform the relationship analysis in this plant and, posteriorly, to extend it to the remaining species. The genetic distance between the AtB′ proteins was calculated using the poisson method (Table V) and the distances obtained were of the same order between all the proteins, confirming that the ones described in this paper belong to the B′ subunit family. The presence of three pairs of very close proteins can be observed that present the lowest distance values (AtB′α and AtB′β, AtB′γ and AtB′ζ, and AtB′η and AtB′θ), suggesting that an event of gene duplication might be involved in their origin. It is intriguing that AtB′δ and AtB′η, which are located in tandem on chromosome III, present a high genetic distance (d = 0, 244), which indicates that, if there was any, the duplication event happened long ago. AtB′δ, as well as AtB′ε, are the most distant proteins, with similar distance values with respect to the remaining members of the family, which might indicate that they diverged very early during the evolution of the family.
Table V.
Genetic distances between the AtB′ isoforms estimated with the poisson correction
| AtB′α | AtB′β | AtB′γ | AtB′δ | AtB′ε | AtB′ζ | AtB′η | AtB′θ | |
|---|---|---|---|---|---|---|---|---|
| AtB′α | – | |||||||
| AtB′β | 0.157 | – | ||||||
| AtB′γ | 0.461 | 0.403 | – | |||||
| AtB′δ | 0.544 | 0.518 | 0.454 | – | ||||
| AtB′ε | 0.444 | 0.427 | 0.548 | 0.656 | – | |||
| AtB′ζ | 0.486 | 0.430 | 0.152 | 0.444 | 0.575 | – | ||
| AtB′η | 0.482 | 0.437 | 0.324 | 0.420 | 0.548 | 0.352 | – | |
| AtB′θ | 0.496 | 0.464 | 0.358 | 0.447 | 0.544 | 0.365 | 0.124 | – |
| HsB56α | 0.718 | 0.682 | 0.740 | 0.750 | 0.754 | 0.754 | 0.695 | 0.736 |
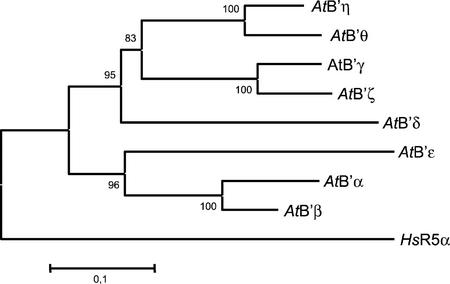
A phylogenetic tree was constructed using the neighbor joining method, based on the distance matrix previously calculated (Fig. 4). It can be appreciated that the eight proteins cluster into two groups, one formed by the pairs AtB′γ and AtB′ζ, and AtB′η and AtB′θ, plus AtB′δ, and the second composed of the cluster AtB′α and AtB′β, plus AtB′ε. The topology of the tree reflects the relationships between the proteins established by the genetic distances, presenting three clearly differentiated clusters (composed of the B′ isoforms α and β, γ and ζ, and η and θ), confirmed by bootstrap values of 100.
Figure 4.
Unrooted phylogeny of the eight isoforms of the Arabidopsis B′ regulatory subunit family. The neighbor-joining tree was obtained from an alignment using Clustal X and Molecular Evolutionary Genetic Analysis programs. The HsR5α protein from humans was used as an outgroup. The bootstrap values, shown at the nodes, are percentages for 1,000 replications. Tree branches are proportional to genetic distances.
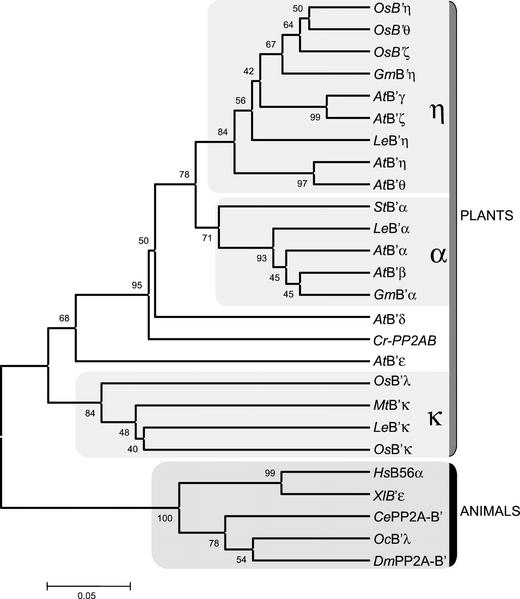
A similar analysis was performed for all the plant protein sequences used in the alignment: The genetic distances were calculated with the poisson correction distance and a phylogenetic tree was constructed with the unweighted pair group method analysis (UPGMA) method (Fig. 5), using the bootstrap test with 1,000 iterations. It can be observed that the animal and plant proteins form two separated clusters, supported by a bootstrap value of 100. On the other hand, all the plant sequences group in three separated clusters, which are supported by the bootstrap values obtained and by the fact that trees constructed with the neighbor joining and maximum parsimony methods present an identical topology (data not shown). This clustering suggests the existence of three main subfamilies of B′ regulatory subunits, which we have designated as α, η, and κ. The protein from the green algae, CrPP2AB′, as well as AtB′δ and AtB′ε, appear in separated branches, which, in the first case, can reflect the evolutionary distance between the unicellular green algae and the complex higher plants. In the case of AtB′δ and AtB′ε, it could not be discarded that close relationships can be found in other species when more sequence data are available.
Figure 5.
Phylogenetic tree based on the multiple alignment in Figure 1, constructed with the UPGMA method, with the genetic distances calculated by the poisson correction for proteins. The gray boxes indicate the main clusters obtained. The plant and animal protein sequences separate into two clusters. Most of the plant protein sequences group in three clusters, considered B′ subfamilies named α, η, and κ.
DISCUSSION
The PP2A B′ Regulatory Subunit Family in Arabidopsis
In this work, we report the identification and molecular characterization of four novel Arabidopsis genes, AtB′ε, AtB′ζ, AtB′η, and AtB′θ, which encode four new isoforms of the PP2A B′ regulatory subunit family in this species. Previous studies revealed the presence of four different AtB′ isoforms in Arabidopsis: α, β, γ, and δ, but also suggested the existence of an additional B′ gene (Latorre et al., 1997; Haynes et al., 1999). The analysis of the complete genome of Arabidopsis has allowed us to identify the ε, ζ, η, and θ isoforms, and to ensure that there are no more additional members of the family. The finding of cDNAs identical to AtB′ζ, AtB′η, and AtB′θ, and the experimental evidences obtained for AtB′ε, confirm that the four predicted genes are actively transcribed and, therefore, should be considered functional genes. This way, the description of PP2A B′ regulatory subunit family in Arabidopsis is now completed, and it has been established that it is composed of eight genes that code for eight different B′ isoforms.
RT-PCR experiments show that AtB′ε mRNAs are present in all the Arabidopsis tissues analyzed. Similar results were obtained when the expression pattern of the genes encoding the α, β, γ, and δ AtB′ isoforms was analyzed (Latorre et al., 1997; Haynes et al., 1999). All of them are ubiquitously expressed, suggesting that the PP2A B′ regulatory subunits are required for basic housekeeping function in plant cells. It is interesting to mention that in some cases different levels of expression were observed in different organs (Latorre et al., 1997; Haynes et al., 1999).
It had been reported that several B′ regulatory subunits were differentially expressed in response to heat shock stress. In Arabidopsis, only mRNAs derived from the AtB′γ gene accumulate differentially after heat shock conditions. In this work, we demonstrate that AtB′ε expression levels, as previously reported for AtB′α, β, and δ genes (Latorre et al., 1997; Haynes et al., 1999), do not fluctuate in response to heat shock. Similar experiments will have to be carried out with AtB′ζ, AtB′η, and AtB′θ.
The PP2A B′ Regulatory Subunit Family in Plants
In an effort to characterize the PP2A B′ regulatory subunit family in other plants, we have analyzed the genomic information available for rice. We could identify five B′ isoforms, which presented a high degree of conservation in sequence and structure with respect to the Arabidopsis B′ subunit genes and proteins. We have also performed an extensive analysis of the EST databases, which produced a large number of cDNAs coding for proteins similar to the Arabidopsis B′ regulatory subunits. The cDNAs corresponded to species from at least five different orders, including green algae (Chloroficeae), ferns (Filicopsida), conifers (Coniferopsida), monocotyledons, and dicotyledons. This fact suggests that the family of PP2A B′ regulatory subunits is present throughout the whole plant kingdom. The EST analysis also produced two complete proteins from tomato (LeB′α and LeB′η), as well as a number of large protein fragments from potato, soybean, and medic barrel. Comparison of all these protein sequences suggest a high degree of conservation of these proteins in plants, a fact that is not surprising if we consider the striking similarity found between the B′ proteins from organisms as different as Arabidopsis, yeast, insects, and vertebrates.
Analysis of the Structure of the B′ Regulatory Subunits
Sequence comparison of the plant B′ subunits shows that they contain a highly conserved central domain, and very diverged amino- and carboxy-terminal regions. The conservation of the central region is strikingly high, even when we compare the plant B′ subunits and their homologs in other organisms like D. melanogaster, C. elegans, X. laevis, rabbits, and humans. The high similarity found in the central region suggests that it could be the putative functional core of the protein that could be essential for the assembly of the B′ regulatory subunits with the other components of the PP2A complex, which are also highly conserved proteins (Depaoli-Roach et al., 1994).
On the other hand, different studies suggest that the highly variable amino- and carboxy-terminal regions of these proteins may play an important role in defining properties such as the substrate specificity and/or the cellular localization of the Ser/Thr PPs (Janssens and Goris, 2001). In fact, in some mammal isoforms of B and B′ regulatory subunits, several regions in their carboxy termini have been identified as being responsible for PP2A subcellular targeting, and also for being the subject of phosphorylation, which supports the notion that the variable regions of B′ regulatory subunits control PP2A function (Zhao et al., 1997). On the other hand, considering that reversible protein phosphorylation, which is the mechanism by which many biological functions are regulated, requires the coordinated action of protein kinases and PPs, and that only a limited number of PP catalytic subunits are present in the cell, the existence of B and B-related regulatory subunits, present in multiple isoforms, raises the possibility that a combinatorial association could generate enough PPs to counterbalance the action of the more numerous protein kinases (Depaoli-Roach et al., 1994). In the case of PP2A, heterotrimer complexes are formed by a catalytic subunit and two regulatory subunits (A and B), each with different isoforms, which can give rise to a number of different combinations that could explain how the PP2A phosphatases are involved in a variety of processes such as the initiation and termination of translation, apoptosis, or stress responses, in multiple types of eukaryotic cells (for review, see Janssens and Goris, 2001). The elucidation of the specific function of these complexes will be very important to understand the regulation of protein phosphorylation in plants.
The Number of B′ Isoforms and the Organisms' Complexity
Our analysis of the Arabidopsis genome has shown that there are eight isoforms of the B′ family. In rice, we have found that the partially sequenced genome contains five regulatory subunits and, considering that about 30% the genome remains to be sequenced, we estimate that the number of B′ isoforms will be very similar to that of Arabidopsis. The study performed on the ESTs has allowed us to identify the minimum number of genes coding for B′ regulatory subunits in the genome of the analyzed species. This way, we have found that there are at least five genes in corn, six in potato, medic barrel, and tomato and seven in soybean. Our data indicate that the number of isoforms in this species would also be on the same order than in Arabidopsis. On the contrary, the unicellular green algae, C. reinhardtii, seems to have only one B′ regulatory subunit, as is suggested by the fact that all the ESTs found are identical and form a single contig representing a unique cDNA. The difference in the number of regulatory proteins between the higher plants and C. reinhardtii is significant, and it is not biased by the number of ESTs available in the databases, which is in the same range: There are 83,453 ESTs from C. reinhardtii, 42,602 from potato, and 96,793 from corn (National Center for Biotechnology Information, dbEST release 070601).
In the animal kingdom, we find a similar situation: In humans and rabbits, five genes encoding PP2A B′ regulatory subunits have been described (McCright and Virshup, 1995; McCright et al., 1996a; Csortos et al., 1996; Tehrani et al., 1996; Zolnierowicz et al., 1996), whereas the lower eukaryote yeast possesses a single member of the family, RTS1 (Shu et al., 1997), Schizosaccharomyces pombe contains two genes, par1+ and par2+ (Jiang and Hallberg, 2000), there is only a single B′ protein in D. melanogaster (Berry and Gehring, 2000), and we have identified only two members of the family in C. elegans (C13G3.3b and W08g11.4). It appears that in plant and animal kingdoms, there is a correlation between the complexity of the organism and the number of existing B′ isoforms, so that the more complex the organism is, the larger the number of PP2A B′ isoforms.
Interestingly, plants and animals would have developed different strategies to increase the number of these regulatory subunits. The five B′ genes of human and rabbit produce seven and eight isoforms, respectively, due to alternative splicing of one of them (Csortos et al., 1996). On the contrary, there is no evidence of this mechanism in Arabidopsis: Only one transcript is derived from the AtB′α, β, and δ genes, as detected by northern blot (Latorre et al., 1997; Haynes et al., 1999), and, although three mRNAs with different sizes are derived from the AtB′γ gene, it has been suggested that the three produced identical proteins (Haynes et al., 1999). We cannot rule out the possibility of alternative splicing in all the other B′ isoforms analyzed in Arabidopsis and the other plants, although the extensive EST analysis we have performed does not provide any evidence suggesting it. This way, the eight genes found in Arabidopsis, and probably in the higher plants, would produce the same number of proteins as the five genes described in rabbits or humans. These data suggest that plants chose to increase the number of genes to obtain the necessary number of B′ regulatory subunits, whereas vertebrates, with a limited number of genes, produce a similar number of different isoforms by means of alternative splicing. However, given the level of current information regarding this hypothesis, more information will be necessary to strongly support these conclusions.
Evolutionary History
Two main conclusions can be drawn from the phylogenetic analysis (Fig. 5): First, despite the high degree of conservation, the plant and animal B′ proteins have evolved independently; and second, the B′ isoforms from plants can be subdivided into three subfamilies, which we have named α, η, and κ. The phylogenetic trees constructed with different methods (neighbor joining, UPGMA, and maximum parsimony) and significant bootstrap values support both ideas. First, all the plant proteins cluster into a group clearly separated from the animal sequences, indicating an independent evolutionary history. This separation is illustrated by the comparison of the B′ subunit core region from plants and animals, with several positions presenting a conserved amino acid in plants and a different one in animals (Fig. 3B). Second, most of the plant proteins group in three clusters, indicating that isoforms from different species are more similar between them than to the other B′ subunits from the same species. The grouping of the proteins has been shown to be consistent in all the trees constructed with different methods, suggesting the existence of at least three subfamilies, which we have named α, η, and κ. The number of subfamilies could increase if close relationships are found for AtB′δ and AtB′ε, which are left outside the main clusters, or new isoforms from other plant species are characterized. On the other hand, the position of the C. reinhardtii B′ protein may be a reflection of its primitive evolutionary position with respect to the higher plants.
Our results suggest that the family of PP2A B′ regulatory subunits appeared very early during the evolution of the eukaryotes, even before the separation of plants and animals, probably as a single-copy gene. The evolutionary process produced increasingly complex organisms, with new functions that required new regulatory subunits for PP2A; thus, new genes appeared by successive duplications of the original ancestor. This hypothesis would explain why the more complex the organism, the larger the number of genes encoding for PP2A B′ subunits. Plants and animals would have adopted different strategies to increase the number of isoforms, which in the case of animals implies alternative splicing. The evolution of the family of B′ regulatory subunits occurred in parallel in both kingdoms, starting from a common ancestor, which produced, by duplication and subsequent divergence, the different subfamilies and isoforms present nowadays in all the organisms.
MATERIALS AND METHODS
DNA Sequencing
Within the frame of an international consortium for the Arabidopsis genome sequencing, we determined the nucleotide sequence of the P1 and BAC genomic clones T15C9, F11C1, F3A4, T20E23, and T12C14, corresponding to the short arm of chromosome III (Salanoubat et al., 2000). The accession numbers for these sequences are AL132970, AL132976, AL132970, AL133363, and AL162507. Information about performance of analysis and a detailed annotation of these database entries can be viewed at the Munich Information Center for Protein Sequences (http://mips.gsf. de/). Our contribution to the sequencing of chromosome III has been the determination of the nucleotide sequence of the inserts of these five clones, which amounts to a total of 540,430 bp.
The BAC insert DNAs were sequenced using a shotgun library approach, which produced a library containing about 2,000 clones with inserts in the range of 0.5 to 1.5 kb long for each BAC clone. Plasmid DNA extractions were made with the High Pure Plasmid Isolation Kit (Boehringer Mannheim/Roche, Basel). DNA sequences were obtained with an ABI PRISM 377 Automated DNA Sequencer (Perkin-Elmer Applied Biosystems, Foster City, CA), using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit and the ABI PRISM dRhodamine Terminator Cycle Sequencing Ready Reaction Kit (Perkin-Elmer Applied Biosystems). Specific primers were designed when necessary to fill gaps in the sequence. Editing and assembling of the DNA sequences were made using the STADEN software package (Staden, 1996). A total of 84,233 bp were analyzed, with an average redundancy of 7.62.
Sequence Analysis
The GenScan program (Burge and Karlin, 1997) was used to predict the presence of putative ORFs in the complete DNA sequence of the BAC insert (http://genes.mit.edu/GENSCAN.html). Databases searches looking for sequence similarities were performed using the BLAST program (Altschul et al., 1997) at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov:80/BLAST), or in specific Arabidopsis databases such as The Arabidopsis Information Resource (http://godot.ncgr.org/Blast/index.html) or the Arabidopsis Database (http://genome-www.stanford.edu/Arabidopsis/index.old.html). Multiple sequence alignments were made using the Clustal X program (Thompson et al., 1997). Analyses of the putative protein sequences deduced from the DNA were performed using different programs such as EMOTIF SEARCH (http://dna.stanford.edu/identify/), to look for functional domains in the sequences, or PSORT (http://psort.nibb.ac.jp/form.html), to identify putative signals for subcellular localization of the proteins. Genetic distances were calculated with the Poisson correction method (Nei and Chakraborty, 1976) for amino acid sequences; the phylogenetic trees were constructed with the neighbor joining (Saitou and Nei, 1987), UPGMA (Swofford and Selander, 1981), and maximum parsimony (Fitch and Farrish, 1974) methods; and the bootstrap test was carried out with 1,000 iterations. These analyses were performed with the Molecular Evolutionary Genetic Analysis platform (Sudhir et al., 1993).
Analysis of AtB′ε Expression
Arabidopsis ecotype Columbia was used in all experiments. Plants were grown under a 16-h-light/8-h-dark illumination regime in a growth cabinet at 23°C. Plants grown in standard conditions were used as control. Plant material was harvested and immediately frozen in liquid N2 at −80°C. Total RNA was extracted from leaves 4.5 weeks old, leaves 7 weeks old, seeds, stems, and flowers following a standard procedure (Prescott and Martin, 1987) and purified by LiCl precipitation. cDNAs were obtained using SuperScript II RT (Life Technologies/Gibco-BRL, Cleveland) and oligo(dT) primers.
PCR reactions were carried out using the following conditions: initial denaturation at 94°C for 5 min, followed by 40 cycles of 30 s at 94°C, 30 s at 55°C, and 30 s at 72°C. Upon completion of the last cycle, samples were incubated for 4 min at 68°C. Primers BE1 and BE2 were used to amplify AtB′ε, BG1, and BG2 for AtB′γ (Latorre et al., 1997), and AC1 and AC2 for actin-1. The sizes of the amplified fragments were 500, 1,032, and 1,131 bp, respectively. Actin transcripts were amplified to check RNA amounts in all samples.
Heat Shock Experiments
Adult plants were incubated at 37°C for 2 h for heat shock experiments. Plants grown in standard conditions were used as control. Total RNA extraction and PCR reaction were performed as indicated above. AtB′ε and AtB′γ were amplified by RT-PCR from heat-shocked and control plants' RNA.
As a control for quantitation of RNA levels in all samples, ribosomal RNA amplification with primers 18S1 and 18S2 and competimers was carried out. 3′-Modified oligonucleotides were used as competimers to amplify an approximately 500-bp fragment of the 18S rRNA. Ratios from 6:10 to 9:10 modified:unmodified primers were used.
The sequences of the oligonucleotides used in the PCR reactions are: rRNA, 18S1, 5′-TGGGATATCCTGCCAGTAGTCAT-3′ and 18S2, 5′-CTGGATCCAATTACCAGACTCAA-3′; AtB′ε, BE1, 5′-TTCTGGTAAAGTCAATGAGACG-3′ and BE2, 5′-ATCAGCCTATGCTCCTCTCTC-3′; AtB′γ, BG1, 5′-TCCTTCTGCGAATCACGAGAG-3′ and BG2, 5′-GACGAGCACTGCCTCGTTGC-3′; and actin-1, AC1, 5′-ATGATGCACCTAGAGCTG-3′ and AC2, 5′-TTCCAGGGAACATTGTGG-3′.
EST Analysis
The search for ESTs in Arabidopsis and other plant species was performed by comparing the Arabidopsis protein sequences against different EST databases dynamically translated in all reading frames in a TBLASTN search. For rice (Oryza sativa), tomato (Lycopersicon esculentum), soybean (Glycine max), medic barrel (Medicago truncatula), potato (Solanum tuberosum), corn (Zea mays), Chlamydomonas reinhardtii, and Ceratopteris richardii, the searches were limited to one species at a time to detect all the existing ESTs that presented significant similarity. The selected ESTs were assembled with the STADEN software package (Staden, 1996), with the assembling parameters such that only identical sequences were included in one contig. The resulting contigs were translated in all reading frames, and the amino acid sequences were tested in a BLASTP search, selecting those that presented a significant similarity with respect to the PP2A B′ regulatory subunits existing in the databases. Finally, the amino acid sequences were aligned with the Clustal X program (Thompson et al., 1997), and only the contigs that produced overlapping nonidentical protein fragments were considered to estimate the number of genes coding for B′ subunits.
ACKNOWLEDGMENTS
We thank Francisco Marco for his technical support and good advice about the RT-PCR experiments. We are most grateful to Ana Martinez for providing the actin oligonucleotides.
Footnotes
This work was supported by the EC (grant no. BIO4–CT98–0549) and by Dirección General de Enseñanza Superior e Investigación Científica, Spanish Ministerio de Educación y Cultura (grant no. BIO99–1320–CE).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.020004.
LITERATURE CITED
- AGI. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariño J, Pérez-Callejón E, Cunillera N, Camps M, Posas F, Ferrer A. Protein phosphatases in higher plants: multiplicity of type 2A phosphatases in Arabidopsis thaliana. Plant Mol Biol. 1993;21:475–485. doi: 10.1007/BF00028805. [DOI] [PubMed] [Google Scholar]
- Berry M, Gehring W. Phosphorylation of the SCR homeodomain determines its functional activity: essential role for protein phosphatase 2AB′. EMBO J. 2000;19:2946–2957. doi: 10.1093/emboj/19.12.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J Mol Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- Casamayor A, Pérez-Callejón E, Pujol G, Ariño J, Ferrer A. Molecular characterization of a fourth isoform of the catalytic subunit of protein phosphatase 2A from Arabidopsis thaliana. Plant Mol Biol. 1994;26:523–528. doi: 10.1007/BF00039564. [DOI] [PubMed] [Google Scholar]
- Corum JW, Hartung AJ, Stamey RT, Rundle SJ. Characterization of DNA sequences encoding a novel isoform of the 55 kDa B regulatory subunit of the type 2A protein serine/threonine phosphatase of Arabidopsis thaliana. Plant Mol Biol. 1996;31:419–427. doi: 10.1007/BF00021804. [DOI] [PubMed] [Google Scholar]
- Csortos C, Zolnierowicz S, Bako E, Durbin SD, DePaoli-Roach AA. High complexity in the expression of the B′ subunit of protein phosphatase 2A. Evidence for the existence of at least seven novel isoforms. J Biol Chem. 1996;271:2578–2588. doi: 10.1074/jbc.271.5.2578. [DOI] [PubMed] [Google Scholar]
- Depaoli-Roach AA, Park IK, Cerovsky V, Csortos C, Durbin SD, Kuntz MJ, Sitikov A, Tang PM, Verin A, Zolnierowicz S. Serine/threonine protein phosphatases in the control of cell function. Adv Enzyme Regul. 1994;34:199–224. doi: 10.1016/0065-2571(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Evangelista CCJ, Rodriguez TA, Limbach MP, Zitomer RS. Rox3 and Rts1function in the global stress response pathway in baker's yeast. Genetics. 1996;142:1083–1093. doi: 10.1093/genetics/142.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch WM, Farris JS. Evolutionary trees with minimum nucleotide replacements from amino acid sequences. J Mol Evol. 1974;3:263–278. doi: 10.1007/BF01796042. [DOI] [PubMed] [Google Scholar]
- Garbers C, DeLong A, Deruere J, Bernasconi P, Soll D. A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J. 1996;15:2115–2124. [PMC free article] [PubMed] [Google Scholar]
- Groves MR, Hanlon N, Turowski P, Hemmings BA, Barford D. The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs. Cell. 1999;96:99–110. doi: 10.1016/s0092-8674(00)80963-0. [DOI] [PubMed] [Google Scholar]
- Haynes JG, Hartung AJ, Hendershot JD, Passingham RS, Rundle SJ. Molecular characterization of the B′ regulatory subunit gene family of Arabidopsisprotein phosphatase 2A. Eur J Biochem. 1999;260:127–136. doi: 10.1046/j.1432-1327.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- Hendrix P, Mayer-Jackel RE, Cron P, Goris J, Hofsteenge J, Merlevede W, Hemmings BA. Structure and expression of a 72 kDa regulatory subunit of protein phosphatase 2A. Evidence for different size forms produced by alternative splicing. J Biol Chem. 1993a;268:15267–15276. [PubMed] [Google Scholar]
- Hendrix P, Turowski P, Mayer-Jaekel RE, Goris J, Hofsteenge J, Merlevede W, Hemmings BA. Analysis of subunit isoforms in protein phosphatase 2A holoenzymes from rabbit and Xenopus. J Biol Chem. 1993b;268:7330–7337. [PubMed] [Google Scholar]
- Ingebritsen TS, Cohen P. Protein phosphatases: properties and role in cellular regulation. Science. 1983a;221:331–338. doi: 10.1126/science.6306765. [DOI] [PubMed] [Google Scholar]
- Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Hallberg RL. Isolation and characterization of par1+ and par2+. Two Schizosaccharomyces pombegenes encoding B′ subunits of protein phosphatase 2A. Genetics. 2000;154:1025–1038. doi: 10.1093/genetics/154.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre KA, Harris DM, Rundle SJ. Differential expression of three Arabidopsisgenes encoding the B′ regulatory subunit of protein phosphatase 2A. Eur J Biochem. 1997;245:156–163. doi: 10.1111/j.1432-1033.1997.00156.x. [DOI] [PubMed] [Google Scholar]
- Lin Q, Buckler ES, Muse SV, Walker JC. Molecular evolution of type 1 serine/threonine protein phosphatases. Mol Phylogenet Evol. 1999;12:57–66. doi: 10.1006/mpev.1998.0560. [DOI] [PubMed] [Google Scholar]
- McCright B, Brothman AR, Virshup DM. Assignment of human protein phosphatase 2A regulatory subunit genes b56alpha, b56beta, b56gamma, b56delta, and b56epsilon (PPP2R5A-PPP2R5E), highly expressed in muscle and brain, to chromosome regions 1q41, 11q12, 3p21, 6p211, and 7p112→p12. Genomics. 1996a;36:168–170. doi: 10.1006/geno.1996.0438. [DOI] [PubMed] [Google Scholar]
- McCright B, Rivers AM, Audlin S, Virshup DM. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J Biol Chem. 1996b;271:22081–22089. doi: 10.1074/jbc.271.36.22081. [DOI] [PubMed] [Google Scholar]
- McCright B, Virshup DM. Identification of a new family of protein phosphatase 2A regulatory subunits. J Biol Chem. 1995;270:26123–26128. doi: 10.1074/jbc.270.44.26123. [DOI] [PubMed] [Google Scholar]
- Meek S, Morrice N, MacKintosh C. Microcystin affinity purification of plant protein phosphatases: PP1C, PP5 and a regulatory A-subunit of PP2A. FEBS Lett. 1999;457:494–498. doi: 10.1016/s0014-5793(99)01093-5. [DOI] [PubMed] [Google Scholar]
- Mumby MC, Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol Rev. 1993;73:673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- Nei M, Chakraborty R. Empirical relationship between the number of nucleotide substitutions and interspecific identity of amino acid sequences in some proteins. J Mol Evol. 1976;26:313–323. doi: 10.1007/BF01743627. [DOI] [PubMed] [Google Scholar]
- Pérez-Callejón E, Casamayor A, Pujol G, Camps M, Ferrer A, Ariño J. Molecular cloning and characterization of two phosphatase 2A catalytic subunit genes from Arabidopsis thaliana. Gene. 1998;209:105–112. doi: 10.1016/s0378-1119(98)00013-4. [DOI] [PubMed] [Google Scholar]
- Prescott A, Martin C. A rapid method for the quantitative assessment of levels of specific mRNAs in plants. Plant Mol Biol Rep. 1987;4:219–224. [Google Scholar]
- Rodríguez PL. Protein phosphatase 2C (PP2C) function in higher plants. Plant Mol Biol. 1998;38:919–927. doi: 10.1023/a:1006054607850. [DOI] [PubMed] [Google Scholar]
- Rundle SJ, Hartung AJ, Corum JW, O'Neill M. Characterization of a cDNA encoding the 55 kDa B regulatory subunit of Arabidopsisprotein phosphatase 2A. Plant Mol Biol. 1995;28:257–266. doi: 10.1007/BF00020245. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Salanoubat M, Lemcke K, Rieger M, Ansorge W, Unseld M, Fartmann B, Valle G, Blocker H, Perez-Alonso M, Obermaier B et al. Sequence and analysis of chromosome 3 of the plant Arabidopsis thaliana. Nature. 2000;408:820–822. doi: 10.1038/35048706. [DOI] [PubMed] [Google Scholar]
- Sato S, Kotani H, Nakamura Y, Kaneko T, Asamizu E, Fukami M, Miyajima N, Tabata S. Structural analysis of Arabidopsis thalianachromosome 5 I Sequence features of the 16 Mb regions covered by twenty physically assigned P1 clones. DNA Res. 1997;4:215–230. doi: 10.1093/dnares/4.3.215. [DOI] [PubMed] [Google Scholar]
- Schöntal AH. Role of PP2A in intracellular signal transduction pathways. Front Biosci. 1998;3:D1262–D1273. doi: 10.2741/A361. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hallberg RL. SCS1, a multicopy suppressor of hsp60-ts mutant alleles, does not encode a mitochondrially targeted protein. Mol Cell Biol. 1995;15:5618–5626. doi: 10.1128/mcb.15.10.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Yang H, Hallberg E, Hallberg RL. Molecular genetic analysis of RTS1p, a B′ regulatory subunit of Saccharomyces cerevisiaeprotein phosphatase 2A. Mol Cell Biol. 1997;17:3242–3253. doi: 10.1128/mcb.17.6.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabas AR, Fordham-Skelton AP, Fletcher D, Martinez-Rivas JM, Swinhoe R, Croy RR, Evans IM. Characterization of cDNA and genomic clones encoding homologues of the 65 kDa regulatory subunit of protein phosphatase 2A in Arabidopsis thaliana. Plant Mol Biol. 1994;26:1125–1138. doi: 10.1007/BF00040694. [DOI] [PubMed] [Google Scholar]
- Smith RD, Walker JC. Expression of multiple type 1 phosphoprotein phosphatases in Arabidopsis thaliana. Plant Mol Biol. 1993;21:307–316. doi: 10.1007/BF00019946. [DOI] [PubMed] [Google Scholar]
- Staden R. The Staden sequence analysis package. Mol Biotechnol. 1996;5:233–241. doi: 10.1007/BF02900361. [DOI] [PubMed] [Google Scholar]
- Strack S, Zaucha JA, Ebner FF, Colbran RJ, Wadzinski BE. Brain protein phosphatase 2A: developmental regulation and distinct cellular and subcellular localization by B subunits. J Comp Neurol. 1998;392:515–527. [PubMed] [Google Scholar]
- Sudhir K, Koichir T, Masatosahi N (1993) Molecular Evolutionary Genetic Analysis, Version 10. Pennsylvania State, University Park
- Swofford DL, Selander RR (1981) BIOSYS-1: A Computer Program for the Analysis of Allelic Variation in Genetics. University of Illinois, Urbana
- Tehrani MA, Mumby MC, Kamibayashi C. Identification of a novel protein phosphatase 2A regulatory subunit highly expressed in muscle. J Biol Chem. 1996;271:5164–5170. doi: 10.1074/jbc.271.9.5164. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Boguslawski G, Zitomer RS, DePaoli-Roach AA. Saccharomyces cerevisiaehomologs of mammalian B and B′ subunits of protein phosphatase 2A direct the enzyme to distinct cellular functions. J Biol Chem. 1997;272:8256–8262. doi: 10.1074/jbc.272.13.8256. [DOI] [PubMed] [Google Scholar]
- Zolnierowicz S, Van Hoof C, Andjelkovic N, Cron P, Stevens I, Merlevede W, Goris J, Hemmings BA. The variable subunit associated with protein phosphatase 2A defines a novel multimember family of regulatory subunits. Biochem J. 1996;317:187–194. doi: 10.1042/bj3170187. [DOI] [PMC free article] [PubMed] [Google Scholar]