Abstract
The rostral ventrolateral medulla (RVLM) is an essential vasomotor center in the brainstem which participates in maintaining resting levels of arterial pressure and for regulating baroreflex activity. We have demonstrated that microinjections of adrenomedullin (ADM), a vasoactive neuropeptide, into the RVLM cause increased resting mean arterial pressure (MAP) and heart rate (HR). However, the effect of ADM on baroreflex function remains unclear.
The purposes of the present study were to investigate the effect of ADM in the RVLM on the regulation of baroreflex activity and to identify the underlying mechanisms. Baroreflex curves were generated with intravenous injections of multiple doses of phenylephrine and nitroprusside. The upper and lower plateaus, reflex range, MAP at the midpoint of HR range (MAP50), and gain were evaluated before and after various microinjections were made into the RVLM of urethane-anesthetized rats.
Microinjections of ADM decreased the upper plateau, reflex range, and gain, and increased MAP50, indicating that ADM in the RVLM impairs baroreflex function.
ADM22–52, a putative ADM receptor antagonist, significantly increased the baroreflex gain and upper plateau, demonstrating that endogenous ADM tonically inhibits the baroreflex. Coinjections of ADM22–52 with ADM blocked the ADM-induced baroreflex responses.
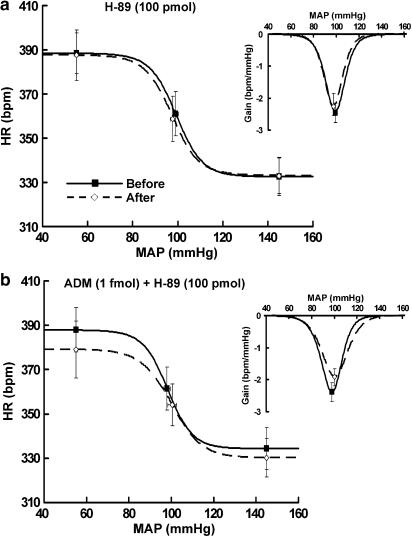
ADM's effect was abolished with H-89, a protein kinase A (PKA) inhibitor.
Our results show that ADM in the RVLM exerts an inhibitory effect on baroreflex activity via an ADM receptor-mediated mechanism, and that activation of PKA is involved in this event.
Keywords: Adrenomedullin, blood pressure, baroreflex control, neuropeptides, sympathetic nervous system
Introduction
Adrenomedullin (ADM), a vasoactive neuropeptide, has attracted a great deal of attention because of its potent peripheral vasodilatory actions (Kitamura et al., 1993; Shimekake et al., 1995; Terata et al., 2000; Champion et al., 2003). Indeed, recent studies have shown that subcutaneous administration of ADM attenuates high blood pressure in salt-sensitive hypertensive rats (Yoshihara et al., 2005), and that intravenous injections or inhalation of ADM ameliorate pulmonary hypertension in humans (Nagaya & Kangawa, 2004). Besides its effects in the periphery, ADM also modulates mean arterial pressure (MAP) and heart rate (HR) through central mechanisms. Thus, ADM administered into the paraventricular nucleus (PVN) of the hypothalamus causes a transient depressor effect (Smith & Ferguson, 2001; Xu & Krukoff, 2004b), whereas elevations in MAP and/or HR are evoked by intracerebroventricular (i.c.v.) injections of ADM (Takahashi et al., 1994; Saita et al., 1998; Samson et al., 1998; Matsumura et al., 1999; Shan & Krukoff, 2001b), or when ADM is microinjected into the area postrema (AP) (Allen et al., 1997) or the rostral ventrolateral medulla (RVLM) (Xu & Krukoff, 2004a) in the brainstem. In addition, it has been shown that i.c.v. injections of ADM regulate the baroreflex (Matsumura et al., 1999; Taylor et al., 2003), a central reflex vital to maintaining normal MAP (Malpas, 2004; Dampney et al., 2005). However, ADM's effects on the baroreflex in specific regions in the brain remain to be elucidated.
The RVLM is an essential component of the neural circuit that controls baroreflex function (Dampney et al., 2003a, 2003b; Milner & Pickel, 2003). When MAP is altered, information about changes in pressure originating from baroreceptors in the aortic arch and carotid sinus is relayed to the nucleus tractus solitarius (NTS) of the brainstem. Sensory neurons in the NTS then project to neurons in the caudal ventrolateral medulla (CVLM), which, in turn, project to presympathetic neurons in the RVLM. RVLM presympathetic neurons modulate not only HR but also sympathetic vasomotor discharge and thus peripheral resistance; as a result of these effects, normal levels of MAP can be re-established (Dampney et al., 2003a, 2003b; Milner & Pickel, 2003).
We have shown that ADM receptors are abundant in the RVLM (Stachniak & Krukoff, 2003) and that ADM-producing neurons are found in other autonomic nuclei that project to the RVLM such as the PVN, NTS, and AP (Serrano et al., 2000; Shan & Krukoff, 2001a). Furthermore, in a previous study we demonstrated that microinjections of ADM into the RVLM induce robust and long-lasting increases in both resting MAP and HR levels, suggesting that ADM exerts a sympathoexcitatory effect in the RVLM to influence cardiovascular functions (Xu & Krukoff, 2004a). However, it is not known whether ADM in the RLVM also contributes to the regulation of baroreflex function, particularly because the RVLM regulates the homeostasis of arterial pressure by both baroreflex-dependent and baroreflex-independent mechanisms (Dampney et al., 2003b; Schreihofer et al., 2005).
The aim of this study was to determine whether ADM in the RVLM modulates baroreflex control of HR, and, if so, to identify the mechanisms for this modulation. Thus, baroreflex curves were generated using a pharmacological pressor agent (phenylephrine, PHEN) and a depressor agent (nitroprusside, NP) before and after ADM and/or its antagonist were microinjected into the RVLM of anesthetized rats. Furthermore, since the cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA) pathway has been shown to mediate ADM's actions in vitro (Drake et al., 1999; Spampinato et al., 1999; Xu & Krukoff, 2005), we coinjected a PKA inhibitor (H-89) with ADM to determine whether PKA is required for ADM's effects on baroreflex activity.
Methods
Animals
A total of 55 male Sprague–Dawley rats (250–350 g), purchased from the Biological Animal Center, University of Alberta, were used in this study. They were housed in a 12 : 12 h light/dark cycle at 22°C, and given free access to standard lab chow and water. All experimental protocols were approved by the local Animal Welfare Committee.
Surgery
Rats were instrumented for MAP/HR recording, intravenous (i.v.) injections, and RVLM microinjections as described previously (Xu & Krukoff, 2004a, 2004b). Briefly, rats were anesthetized with urethane (intraperitoneal, 1.75 g kg−1, Sigma Chemical Co, Oakville, ON, Canada). Body temperature was monitored with a rectal thermometer and maintained at 37°C with a heating pad. The left femoral artery was cannulated using PE50 tubing (Becton Dickinson & Co., Sparks, MD, U.S.A.) for measurement of MAP and HR, and the left femoral vein was cannulated using PE10 tubing (Becton Dickinson & Co.) for i.v. injections. The rats were then placed onto a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, U.S.A.) with the toothbar set at −3.3 mm and a microinjection guide cannula (C315G; Plastics One, Wallingford, CT, U.S.A.) was inserted into the left RVLM (3.5–3.8 mm posterior, 1.9–2.1 mm lateral, and 0.7–0.9 mm dorsal to the interaural zero) (Paxinos & Watson, 1986; Xu & Krukoff, 2004a).
Measurement of MAP and HR
The arterial tubing, filled with heparinized saline, was connected to a pressure transducer (Abbott Laboratories, North Chicago, IL, U.S.A.). Digitalized arterial pressure and HR data were acquired and analyzed using WinDaq Acquisition & Playback Software (Dataq Instruments, Akron, OH, U.S.A.). After a 30 min stabilization period, the MAP and HR were measured for 10 min to obtain basal levels of MAP and HR.
Microinjections into the RVLM
Unilateral RVLM injections were performed as previously described (Xu & Krukoff, 2004a). A syringe filled with saline solution, containing a variety of pharmacological agents or combinations thereof, was connected via PE10 tubing to an internal cannula (C315I; Plastics One), which is 1 mm longer than the guide cannula. With the internal cannula inserted into the guide cannula, 100 nl solution was injected into the left RVLM with an electronic infusion pump (Harvard Apparatus, Inc. Holliston, MA, U.S.A.). All microinjections were made over 1 min. To demonstrate the effect of ADM in the RVLM on the baroreflex, ADM (American Peptide Company, Inc., Sunnyvale, CA, U.S.A.) was injected into the RVLM at subpressor doses (0.1 and 1 fmol), which were determined based on our previous study (Xu & Krukoff, 2004a) and on preliminary experiments. Animals that received injections of saline into the RVLM or of 1 fmol ADM outside the RVLM were used as controls. An ADM receptor antagonist, ADM22–52 (0.1 and 1 fmol; American Peptide Company, Inc.), was injected into the RVLM in order to investigate the effects of endogenous ADM on the baroreflex. To determine whether ADM's effect is receptor-mediated, 1 fmol ADM was coinjected with 0.1 fmol ADM22–52. A PKA inhibitor, H-89 (100 pmol; Sigma) (Chan et al., 2003), was coinjected with 1 fmol ADM to evaluate the importance of PKA activity in ADM's effect.
Evaluation of baroreflex activity
The baroreflex control of HR was evaluated before and after RVLM microinjections as previously described by others (Wong et al., 1993; Miki et al., 2003). In baroreflex test trials, blood pressure was increased or decreased by i.v. bolus injections of PHEN (0.5, 1.5, 2, and 2.5 μg; Sigma) or NP (1, 3, 4, 5 μg; Sigma), respectively. Different doses of PHEN and NP were delivered in random order and two subsequent injections were separated by a 5-min interval to ensure that MAP and HR returned to the baselines before the next injection. In most experiments, the baroreflex test was performed before and during the first hour (from 20 to 60 min) after RVLM microinjections. In the investigation of the time course of ADM's effect on the baroreflex, additional baroreflex tests were carried out during the second hour (from 60 to 120 min) and third hour (from 120 to 180 min) after RVLM microinjections.
For each baroreflex test, PHEN- and NP-induced responses were recorded and HR and MAP data were averaged over each 1 mmHg bin of MAP. Using SigmaPlot (Systat Software Inc., Point Richmond, CA, U.S.A.), HR and MAP data were fitted to a logistic sigmoid function (Kent et al., 1972):
where A and D are the upper and lower plateaus of the baroreflex curve and represent the maximal and minimal levels of HR, respectively. C is MAP at the midpoint of the HR range (MAP50), which represents the set-point of the baroreflex. B is the gain coefficient. The reflex range, threshold blood pressure (Pthr), saturation blood pressure (Psat), the first derivative of the curve, and maximal gain were then calculated according to the following equations (Miki et al., 2003):
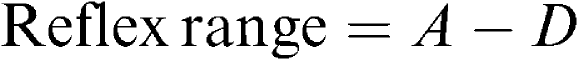 |
The reflex range is the difference between the upper plateau and the lower plateau. Pthr and Psat are the MAP values at which HR was within 5% of maximum or minimum response, respectively. The first derivative shows the baroreflex gain at different MAP levels, while the maximal gain is defined as the gain value when MAP is equal to MAP50.
Brain histology
As previously described (Xu & Krukoff, 2004a), 1% Evans blue (Sigma) was microinjected into the RVLM via the same guide cannula at the end of each experiment. The rats were decapitated, and brains were removed and fixed in 4% paraformaldehyde for 48–72 h. The brains were then frozen and coronal brainstem sections (40 μm) were cut using a cryostat (−20°C). The sections were thaw-mounted onto slides, stained with neutral red (Allied Chemical Co., New York, NY, U.S.A.), and observed under a microscope. Accurate RVLM microinjections were confirmed when Evans blue was present within the RVLM.
Statistical analyses
For each treatment group (N⩾4), averaged parameters from the baroreflex curves before and after RVLM microinjections were expressed as mean±s.e.m. Effects of various RVLM microinjections on the baroreflex parameters and differences among groups were assessed using one-way or two-way ANOVA analysis followed by the Student–Newman–Keuls test for post hoc comparisons of individual means. P<0.05 indicated statistical significance.
Results
Microinjection accuracy
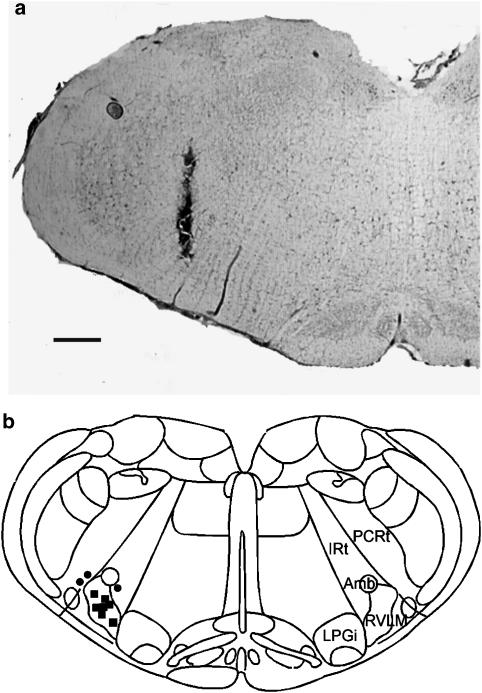
Microinjections were accurately placed within the RVLM in about 70% of rats. Figure 1a shows a representative photomicrograph indicating the termination site of an injection that is considered to be within the RVLM, and Figure 1b summarizes the termination sites of microinjections in 10 rats.
Figure 1.
(a) A brightfield photomicrograph showing an injection that is considered to be within the RVLM. The scale bar=500 μm. (b) Schematic representation of sites of microinjections in 10 rats that received microinjections. Each square represents an injection that is considered to be within the RVLM; each circle represents an injection that is considered to be outside the RVLM. Amb, ambiguous nucleus; IRt, intermediate reticular nucleus; LPGi, lateral paragigantocellular nucleus; PCRt, parvocellular reticular nucleus; RVLM, rostral ventrolateral medulla. The drawing is modified from Paxinos & Watson (1986).
Effects of microinjections on resting MAP and HR
The baseline values of MAP and HR were 92±8 mmHg and 355±16 b.p.m., respectively. Microinjections of saline or ADM alone, and ADM22–52 alone or in combination with ADM, had no significant effects on resting MAP or HR. Microinjections of H-89, alone or in combination with ADM, induced significant increases in resting levels of MAP (23±7 or 28±10 mmHg) and HR (54±15 or 55±16 b.p.m.), respectively. However, these effects lasted fewer than 20 min and MAP was allowed to return to baseline values before the baroreflex tests were initiated.
Exogenous ADM in the RLVM inhibits baroreflex control of HR
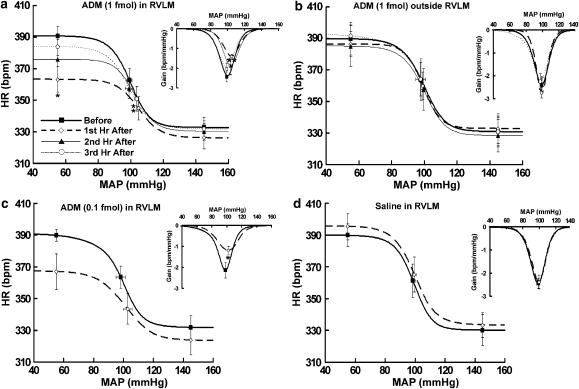
To determine the effect of exogenous ADM on the baroreflex control of HR, we microinjected ADM into the RVLM at a subpressor dose (1 fmol) (Xu & Krukoff, 2004a), and the baroreflex activities were compared before and during the first, second, and third hour after the microinjections. As shown in Figure 2a and Table 1, during the first hour after RVLM microinjections of ADM, the upper plateau of the baroreflex curves was significantly decreased from 390.8±5.9 to 363.2±8.1 b.p.m. (P<0.05), whereas the lower plateau was not affected. As a result, the reflex range was significantly reduced from 58.0±2.4 to 37.2±1.4 b.p.m. (P<0.01). In addition, ADM significantly decreased the maximal gain from −2.4±0.3 to −1.4±0.1 b.p.m. mmHg−1 (P<0.01), increased MAP50 from 99.1±1.5 to 104.8±0.4 mmHg (P<0.01), and increased Psat from 111.1±0.8 to 118.3±1.0 mmHg (P<0.01), whereas Pthr was not affected. These data indicate that exogenous ADM in the RVLM impaired the baroreflex control of HR. Furthermore, we found that the inhibitory effect of ADM on the baroreflex was time-dependent and reversible because the upper plateau, reflex range, gain, MAP50, and Psat gradually returned to baseline levels during the second and the third hours. Microinjections of ADM (1 fmol) outside the RVLM did not induce any significant changes in the baroreflex (Figure 2b and Table 1), indicating that the effects of ADM were specific to the RVLM.
Figure 2.
(a, b) Average baroreflex curves generated before and during the first, second, and third hour after ADM (1 fmol) was microinjected into the RVLM (a, n=6) or outside of RVLM (b, n=4). (c, d) Average baroreflex curves generated before and during the first hour after 0.1 fmol ADM (a, n=6) or saline (b, n=4) was microinjected into the RVLM. Insets, average gains of baroreflex control of HR as a function of MAP. Symbols represent the upper plateau, set-point, and lower plateau of baroreflex curves, respectively. *P<0.05 and **P<0.01 versus measurements before microinjections.
Table 1.
Baroreflex parameters before and after microinjections of various pharmacological agents into the RVLM
| Treatment | Upper plateau (b.p.m.) | Lower plateau (b.p.m.) | Reflex range (b.p.m.) | MAP50 (mmHg) | GainMax (b.p.m. mmHg−1) | Pthr (mmHg) | Psat (mmHg) |
|---|---|---|---|---|---|---|---|
| Saline | |||||||
| Before | 390.1±7.3 | 330.2±9.6 | 59.9±2.8 | 98.6±1.6 | −2.4±0.2 | 86.8±2.3 | 110.5±1.4 |
| After | 390.6±8.8 | 328.1±10.7 | 62.5±5.0 | 99.5±1.6 | −2.4±0.3 | 85.5±2.9 | 113.5±2.7 |
| ADM (0.1 fmol) | |||||||
| Before | 390.8±3.7 | 331.3±7.6 | 59.4±5.9 | 98.1±3.0 | −2.1±0.4 | 83.5±6.5 | 112.6±1.3 |
| After | 367.3±11.3 | 323.4±9.1 | 43.9±6.8 | 102.1±3.0 | −1.2±0.2* | 85.7±5.0 | 118.5±2.8 |
| ADM (1 fmol) | |||||||
| Before | 390.8±5.9 | 332.7±4.2 | 58.0±2.4 | 99.1±1.5 | −2.4±0.3 | 87.1±2.5 | 111.1±0.8 |
| 1st hour after | 363.2±8.1* | 326.0±7.0 | 37.2±1.4** | 104.8±0.4** | −1.4±0.1** | 91.3±1.3 | 118.3±1.0** |
| 2nd hour after | 375.9±12.4 | 330.3±4.9 | 45.7±8.0 | 103.6±2.3 | −1.9±0.5 | 91.9±2.4 | 115.4±2.7 |
| 3rd hour after | 384.0±5.6 | 329.2±7.7 | 52.3±4.0 | 98.9±1.4 | −2.1±0.3 | 85.5±1.7 | 112.2±2.0 |
| ADM (1 fmol) outside of RVLM | |||||||
| Before | 389.9±10.3 | 330.9±8.9 | 59.0±1.8 | 99.0±1.4 | −2.4±0.3 | 86.0±2.6 | 112.1±0.7 |
| 1st hour after | 386.3±7.9 | 332.8±9.4 | 53.5±3.9 | 99.0±1.3 | −2.7±0.2 | 88.7±1.3 | 109.3±1.3 |
| 2nd hour after | 385.1±12.9 | 328.3±10.3 | 56.8±3.5 | 99.4±1.5 | −2.4±0.3 | 86.4±2.5 | 112.4±1.0 |
| 3rd hour after | 392.4±6.4 | 330.4±10.1 | 62.0±4.6 | 97.4±2.8 | −2.2±0.3 | 83.0±6.4 | 111.9±1.2 |
| ADM22–52 (0.1 fmol) | |||||||
| Before | 388.4±8.1 | 331.2±8.2 | 57.2±2.8 | 99.9±0.9 | −2.5±0.3 | 88.1±1.6 | 111.8±1.1 |
| After | 394.1±6.8 | 332.7±10.6 | 61.3±4.4 | 97.9±2.0 | −3.3±0.3 | 87.1±2.5 | 108.8±3.1 |
| ADM22–52 (1 fmol) | |||||||
| Before | 387.3±4.3 | 333.9±4.7 | 53.5±3.7 | 97.3±1.8 | −2.3±0.2 | 85.8±1.7 | 108.8±1.9 |
| After | 403.5±3.6* | 340.6±4.0 | 62.9±1.7 | 97.7±2.0 | −4.8±0.7* | 89.9±1.1 | 105.5±3.2 |
| ADM (1 fmol)+ ADM22–52 (0.1 fmol) | |||||||
| Before | 387.1±10.6 | 333.5±9.8 | 53.5±3.8 | 99.7±1.2 | −2.6±0.2 | 89.4±1.1 | 110.0±1.4 |
| After | 384.7±6.6 | 334.1±7.2 | 50.5±1.1 | 97.4±1.7 | −2.5±0.3 | 87.0±1.5 | 107.8±2.4 |
| H-89 (100 pmol) | |||||||
| Before | 388.5±9.4 | 332.5±8.6 | 55.9±2.8 | 99.0±1.3 | −2.5±0.3 | 87.4±2.3 | 110.6±1.7 |
| After | 387.6±11.3 | 332.9±8.1 | 54.7±4.0 | 97.3±1.2 | −2.3±0.4 | 84.9±2.1 | 109.6±2.3 |
| ADM (1 fmol)+H-89 (100 pmol) | |||||||
| Before | 387.9±10.0 | 334.4±9.5 | 53.6±4.5 | 98.0±1.6 | −2.4±0.3 | 86.8±2.5 | 109.3±1.7 |
| After | 379.1±12.0 | 330.1±8.6 | 49.0±5.2 | 100.5±2.0 | −1.9±0.2 | 86.7±2.3 | 114.3±2.1 |
Values are mean±s.e.m.
P<0.05 and
P<0.01 versus measurements before microinjections.
ADM at a lower dose (0.1 fmol) also inhibited the baroreflex by significantly decreasing the maximal gain from −2.1±0.4 to −1.2±0.2 b.p.m. mmHg−1 (P<0.05) (Figure 2c and Table 1). ADM (0.1 fmol) also tended to decrease the upper plateau and the reflex range, although these responses were not statistically significant (Figure 2c and Table 1). Control microinjections of saline into the RVLM had no effect on the baroreflex (Figure 2d and Table 1).
Blockade of ADM receptor in the RLVM facilitates baroreflex control of HR
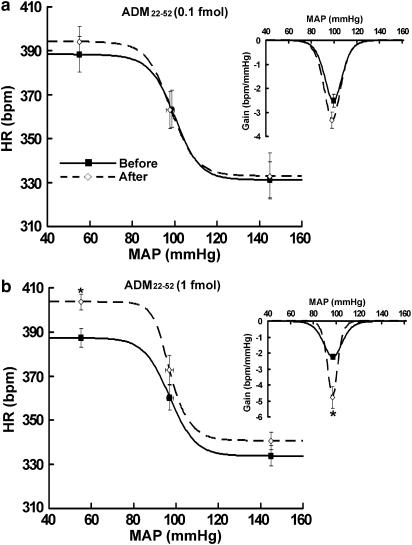
To determine whether endogenous ADM in the RVLM exerts a tonic regulatory effect on the baroreflex, we examined the effects of microinjections of a putative ADM receptor antagonist, ADM22–52, on baroreflex activity. While injections of 0.1 fmol ADM22–52 had no effects on the baroreflex (Figure 3a and Table 1), ADM22–52 at a higher dose (1 fmol) significantly increased the baroreflex gain from −2.3±0.2 to −4.8±0.7 b.p.m. mmHg−1 (P<0.05). In addition, ADM22–52 (1 fmol) significantly increased the upper plateau from 387.3±4.3 to 403.5±3.6 b.p.m. (P<0.05) and resulted in a trend towards an increased reflex range, although it was not statistically significant (Figure 3b and Table 1). ADM22–52 (1 fmol) injected outside the RVLM had no effect on baroreflex activity (data not shown).
Figure 3.
Average baroreflex curves generated before and during the first hour after 0.1 fmol ADM22–52 (a, n=4) or 1 fmol ADM22–52 (b, n=6) was microinjected into the RVLM. Insets, average gains of baroreflex control of HR as a function of MAP. Symbols represent the upper plateau, set-point, and lower plateau of baroreflex curves, respectively. *P<0.05 versus measurements before microinjections.
ADM-induced inhibition of baroreflex is mediated by ADM receptor
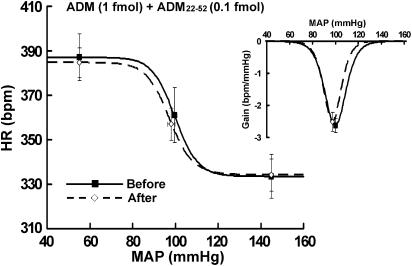
The responses in the baroreflex parameters induced by ADM (1 fmol) were completely abolished by coinjected with 0.1 fmol ADM22–52 (Figure 4 and Table 1). Similarly, coinjections of 1 fmol ADM22–52 also eliminated the ADM-induced baroreflex inhibition (data not shown). These results indicate that ADM's effect on the baroreflex is a receptor-mediated event.
Figure 4.
Average baroreflex curves generated before and during the first hour after 1 fmol ADM plus 0.1 fmol ADM22–52 (n=4) was microinjected into the RVLM. Insets, average gains of baroreflex control of HR as a function of MAP. Symbols represent the upper plateau, set-point, and lower plateau of baroreflex curves, respectively.
ADM-induced inhibition of baroreflex is dependent on PKA
As the cAMP-PKA pathway mediates some functions of ADM in vitro (Drake et al., 1999; Spampinato et al., 1999; Xu & Krukoff, 2005), we investigated whether the PKA-related signaling is required for ADM's effect on baroreflex activity. We show that the inhibition of the baroreflex induced by 1 fmol ADM was completely eliminated by coinjections of 100 pmol H-89, a PKA inhibitor, whereas H-89 alone had no significant effect on the baroreflex (Figure 5 and Table 1). These results show that PKA is required for ADM's effect on the baroreflex control of HR.
Figure 5.
Average baroreflex curves generated before and during the first hour after100 pmol H-89 (a, n=4) or 1 fmol ADM plus 100 pmol H-89 (b, n=4) was microinjected into the RVLM. Insets, average gains of baroreflex control of HR as a function of MAP. Symbols represent the upper plateau, set-point, and lower plateau of baroreflex curves, respectively.
Discussion
In the present study, we demonstrate that exogenous ADM applied in the RVLM inhibits the baroreflex control of HR by decreasing the upper plateau, reflex range, and gain of baroreflex curves and by increasing MAP50; and that this inhibition is reversible and site-specific to the RVLM. In addition, we show that blockade of ADM receptors in the RVLM increases the baroreflex gain and upper plateau, suggesting that endogenous ADM in the RVLM exerts a tonic inhibitory effect on baroreflex activity. Furthermore, our data also suggest that the PKA-associated signaling pathway is recruited by ADM to regulate baroreflex function, as a PKA inhibitor abolished ADM's effect on the baroreflex.
Few studies have focused on ADM's role in the regulation of the baroreflex. I.c.v. injections of ADM were shown to facilitate the baroreflex by increasing the upper plateau and gain (Matsumura et al., 1999), whereas blockade of central ADM with i.c.v. injections of anti-ADM serum was shown to alter the time course of the baroreflex (Taylor et al., 2003). However, the results of these studies are difficult to interpret because of the nonspecific nature of the injections. Since central ADM is known to exert different effects on resting MAP and HR depending on where it is applied (Takahashi et al., 1994; Allen et al., 1997; Saita et al., 1998; Samson et al., 1998; Matsumura et al., 1999; Shan & Krukoff, 2001b; Smith & Ferguson, 2001; Xu & Krukoff, 2004a, 2004b), it is important to study the effects of ADM in specific brain regions on the baroreflex. Interestingly, our data show that ADM in the RVLM inhibits the baroreflex and illustrate that central ADM regulates baroreflex function in a site-specific manner.
HR responses to hypotension and hypertension are differentially regulated by the sympathetic and parasympathetic nervous systems (Stornetta et al., 1987). The parasympathetic nervous system is primarily responsible for hypertension-induced bradycardia, whereas the sympathetic and parasympathetic systems both contribute to the hypotension-induced tachycardia (Stornetta et al., 1987). Interestingly, our data show that exogenous ADM affected the upper plateau of the baroreflex curve, but left the lower plateau intact, suggesting that ADM in the RVLM regulates baroreflex control of HR only when the MAP is decreased. Therefore, although changes in sympathetic activities were not directly measured in the present study, our results suggest that ADM in the RVLM regulates the baroreflex mainly by modulating the sympathetic outflow from this nucleus. Indeed, we have previously shown that microinjections of ADM at higher doses (10 and 100 fmol) into the RVLM induce robust increases in HR as well as modest increases in MAP, indicating that ADM in the RVLM stimulates neurons that control cardiac sympathetic activity (Xu & Krukoff, 2004a).
In an attempt to identify the mechanisms underlying the effect of ADM on baroreflex activity, we first demonstrated that ADM's effect is receptor-mediated, because an ADM receptor antagonist, ADM22–52 (0.1 fmol), eliminated the ADM-induced inhibition of baroreflex. To further determine whether PKA activity is involved in ADM's effect, we blocked PKA activity in the RVLM with 100 pmol H-89. H-89 is a commonly used PKA inhibitor which has been shown at 100 pmol to effectively inhibit PKA in the NTS to block heat shock-induced cardiovascular responses (Chan et al., 2003). Our data show that coinjections of H-89 with ADM abolished the ADM-induced inhibition of the baroreflex to implicate PKA as a mediator of ADM's effect on the baroreflex, although we cannot rule out the possibility that a small part of the effect of H-89 is due to nonspecific effects on other intracellular messengers such as protein kinase G (Satake et al., 1996) and Rho kinase (Leemhuis et al., 2002). Consistent with our results, it has been shown that ADM elevates intracellular cAMP levels in various types of cells (Kitamura et al., 1993; Shimekake et al., 1995), including neural cells (Drake et al., 1999; Spampinato et al., 1999; Xu & Krukoff, 2005), and that many of ADM's actions are mediated by the cAMP-PKA pathway in vitro (Drake et al., 1999; Spampinato et al., 1999; Xu & Krukoff, 2005).
We found that blockade of ADM receptors alone with 1 fmol ADM22–52 significantly increased the baroreflex gain and upper plateau. A trend towards increased reflex range was also observed, although this difference was not statistically significant due to variation among animals. Thus, we propose that endogenous ADM in the RVLM exerts a tonic inhibitory effect on the baroreflex control of HR.
The sources of endogenous ADMergic inputs to the RVLM have not been definitively identified. However, we and others previously showed that either ADM-like immunoactivity or mRNA of prepoadrenomedullin (ADM precursor) is present in other autonomic centers including the PVN, lateral hypothalamic area, amygdala, parabrachial nucleus, NTS, and AP (Serrano et al., 2000; Shan & Krukoff, 2001a). These nuclei have also been shown to contain barosensitive neurons that project to the RVLM (Polson et al., 1995; Dampney et al., 2003b). Among these candidates, we speculate that the PVN, NTS, and AP are more likely to provide major ADMergic inputs to the RVLM to regulate cardiovascular activities because we found that expression of ADM in these regions is suppressed when arterial pressure homeostasis is challenged by physiological stressors (Shan & Krukoff, 2001a). Given that ADM in the RVLM inhibits the baroreflex control of HR, we propose that the resulting decrease in ADM production may provide animals with a protective mechanism to maintain cardiovascular homeostasis.
Whereas baroreflex activity is conventionally believed to be important primarily for moment-to-moment control of arterial pressure (Cowley, 1992), recent findings show that the baroreflex also contributes to the long-term control of arterial pressure (Malpas, 2004; Dampney et al., 2005) and that chronic impairment of baroreflex activity results in neurogenic hypertension (Thrasher, 2002). Therefore, the fact that ADM in the RVLM inhibits the baroreflex control of HR, especially by resetting MAP50 to a higher level, together with our previous finding that ADM in the RVLM induces a robust and long-lasting vasopressor effect (Xu & Krukoff, 2004a), suggests that potentiated ADMergic neurotransmission within the RVLM (e.g. elevated ADM release or increased expression of ADM receptors) will contribute to the development of hypertension. Indeed, it has been shown that in hypertensive rats, expression of ADM receptors in the brainstem is increased compared to normotensive rats (Li et al., 2004).
In conclusion, our results demonstrate that endogenous ADM in the RVLM exerts an inhibitory effect on the baroreflex control of HR through ADM receptor-mediated mechanisms. Furthermore, we suggest that activation of PKA-related signaling is involved in ADM-induced baroreflex inhibition. Together with our previous finding that ADM in the RVLM increases MAP and HR under resting conditions (Xu & Krukoff, 2004a), these data indicate that ADM in this autonomic center contributes to the control of blood pressure not only by regulating the resting MAP and HR levels but also by modulating baroreflex activity.
Acknowledgments
Y.X. is the recipient of a studentship from the Heart and Stroke Foundation of Canada. This work was supported by the Heart and Stroke Foundation of Alberta/Northwest Territories/Nunavut.
Abbreviations
- ADM
adrenomedullin
- AP
area postrema
- cAMP
cyclic adenosine monophosphate
- CVLM
caudal ventrolateral medulla
- HR
heart rate
- MAP
mean arterial pressure
- NP
nitroprusside
- NTS
nucleus tractus solitarius
- PHEN
phenylephrine
- PKA
protein kinase A
- PVN
paraventricular nucleus
- RVLM
rostral ventrolateral medulla
References
- ALLEN M.A., SMITH P.M., FERGUSON A.V. Adrenomedullin microinjection into the area postrema increases blood pressure. Am. J. Physiol. 1997;272:R1698–R1703. doi: 10.1152/ajpregu.1997.272.6.R1698. [DOI] [PubMed] [Google Scholar]
- CHAMPION H.C., BIVALACQUA T.J., PIERCE R.L., MURPHY W.A., COY D.H., HYMAN A.L., KADOWITZ P.J. Responses to human CGRP, ADM, and PAMP in human thymic arteries. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R531–R537. doi: 10.1152/ajpregu.00337.2002. [DOI] [PubMed] [Google Scholar]
- CHAN S.H., WANG L.L., CHANG K.F., OU C.C., CHAN J.Y. Altered temporal profile of heat shock factor 1 phosphorylation and heat shock protein 70 expression induced by heat shock in nucleus tractus solitarii of spontaneously hypertensive rats. Circulation. 2003;107:339–345. doi: 10.1161/01.cir.0000044942.94957.87. [DOI] [PubMed] [Google Scholar]
- COWLEY A.W., JR Long-term control of arterial blood pressure. Physiol. Rev. 1992;72:231–300. doi: 10.1152/physrev.1992.72.1.231. [DOI] [PubMed] [Google Scholar]
- DAMPNEY R.A., HORIUCHI J., KILLINGER S., SHERIFF M.J., TAN P.S., MCDOWALL L.M. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin. Exp. Pharmacol. Physiol. 2005;32:419–425. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- DAMPNEY R.A., HORIUCHI J., TAGAWA T., FONTES M.A., POTTS P.D., POLSON J.W. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta. Physiol. Scand. 2003a;177:209–218. doi: 10.1046/j.1365-201X.2003.01070.x. [DOI] [PubMed] [Google Scholar]
- DAMPNEY R.A., POLSON J.W., POTTS P.D., HIROOKA Y., HORIUCHI J. Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell. Mol. Neurobiol. 2003b;23:597–616. doi: 10.1023/A:1025080314925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAKE W.M., AJAYI A., LOWE S.R., MIRTELLA A., BARTLETT T.J., CLARK A.J. Desensitization of CGRP and adrenomedullin receptors in SK-N-MC cells: implications for the RAMP hypothesis. Endocrinology. 1999;140:533–537. doi: 10.1210/endo.140.1.6606. [DOI] [PubMed] [Google Scholar]
- KENT B.B., DRANE J.W., BLUMENSTEIN B., MANNING J.W. A mathematical model to assess changes in the baroreceptor reflex. Cardiology. 1972;57:295–310. doi: 10.1159/000169528. [DOI] [PubMed] [Google Scholar]
- KITAMURA K., KANGAWA K., KAWAMOTO M., ICHIKI Y., NAKAMURA S., MATSUO H., ETO T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- LEEMHUIS J., BOUTILLIER S., SCHMIDT G., MEYER D.K. The protein kinase A inhibitor H89 acts on cell morphology by inhibiting Rho kinase. J. Pharmacol. Exp. Ther. 2002;300:1000–1007. doi: 10.1124/jpet.300.3.1000. [DOI] [PubMed] [Google Scholar]
- LI X., LI L., SHEN L.L., QIAN Y., CAO Y.X., ZHU D.N. Changes of adrenomedullin and its receptor components mRNAs expression in the brain stem and hypothalamus–pituitary–adrenal axis of stress-induced hypertensive rats. Sheng. Li. Xue. Bao. 2004;56:723–729. [PubMed] [Google Scholar]
- MALPAS S.C. What sets the long-term level of sympathetic nerve activity: is there a role for arterial baroreceptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R1–R12. doi: 10.1152/ajpregu.00496.2003. [DOI] [PubMed] [Google Scholar]
- MATSUMURA K., ABE I., TSUCHIHASHI T., FUJISHIMA M. Central adrenomedullin augments the baroreceptor reflex in conscious rabbits. Hypertension. 1999;33:992–997. doi: 10.1161/01.hyp.33.4.992. [DOI] [PubMed] [Google Scholar]
- MIKI K., YOSHIMOTO M., TANIMIZU M. Acute shifts of baroreflex control of renal sympathetic nerve activity induced by treadmill exercise in rats. J. Physiol. 2003;548:313–322. doi: 10.1113/jphysiol.2002.033050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILNER T.A., PICKEL V.M. Receptor targeting in medullary nuclei mediating baroreceptor reflexes. Cell Mol. Neurobiol. 2003;23:751–760. doi: 10.1023/A:1025052903538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGAYA N., KANGAWA K. Adrenomedullin in the treatment of pulmonary hypertension. Peptides. 2004;25:2013–2018. doi: 10.1016/j.peptides.2004.07.007. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates. : Orlando: Academic Press; 1986. [Google Scholar]
- POLSON J.W., POTTS P.D., LI Y.W., DAMPNEY R.A. Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla after sustained hypertension in conscious rabbits. Neuroscience. 1995;67:107–123. doi: 10.1016/0306-4522(95)00034-g. [DOI] [PubMed] [Google Scholar]
- SAITA M., SHIMOKAWA A., KUNITAKE T., KATO K., HANAMORI T., KITAMURA K., ETO T., KANNAN H. Central actions of adrenomedullin on cardiovascular parameters and sympathetic outflow in conscious rats. Am. J. Physiol. 1998;274:R979–R984. doi: 10.1152/ajpregu.1998.274.4.R979. [DOI] [PubMed] [Google Scholar]
- SAMSON W.K., MURPHY T.C., RESCH Z.T. Central mechanisms for the hypertensive effects of preproadrenomedullin-derived peptides in conscious rats. Am. J. Physiol. 1998;274:R1505–R1509. doi: 10.1152/ajpregu.1998.274.5.R1505. [DOI] [PubMed] [Google Scholar]
- SATAKE N., FUJIMOTO S., SHIBATA S. The potentiation of nitroglycerin-induced relaxation by PKG inhibition in rat aortic rings. Gen. Pharmacol. 1996;27:701–705. doi: 10.1016/0306-3623(95)00116-6. [DOI] [PubMed] [Google Scholar]
- SCHREIHOFER A.M., ITO S., SVED A.F. Brain stem control of arterial pressure in chronic arterial baroreceptor-denervated rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R1746–R1755. doi: 10.1152/ajpregu.00307.2005. [DOI] [PubMed] [Google Scholar]
- SERRANO J., UTTENTHAL L.O., MARTINEZ A., FERNANDEZ A.P., MARTINEZ DE VELASCO J., ALONSO D., BENTURA M.L., SANTACANA M., GALLARDO J.R., MARTINEZ-MURILLO R., CUTTITTA F., RODRIGO J. Distribution of adrenomedullin-like immunoreactivity in the rat central nervous system by light and electron microscopy. Brain Res. 2000;853:245–268. doi: 10.1016/s0006-8993(99)02273-8. [DOI] [PubMed] [Google Scholar]
- SHAN J., KRUKOFF T.L. Distribution of preproadrenomedullin mRNA in the rat central nervous system and its modulation by physiological stressors. J. Comp. Neurol. 2001a;432:88–100. doi: 10.1002/cne.1090. [DOI] [PubMed] [Google Scholar]
- SHAN J., KRUKOFF T.L. Intracerebroventricular adrenomedullin stimulates the hypothalamic–pituitary–adrenal axis, the sympathetic nervous system and production of hypothalamic nitric oxide. J. Neuroendocrinol. 2001b;13:975–984. doi: 10.1046/j.1365-2826.2001.00721.x. [DOI] [PubMed] [Google Scholar]
- SHIMEKAKE Y., NAGATA K., OHTA S., KAMBAYASHI Y., TERAOKA H., KITAMURA K., ETO T., KANGAWA K., MATSUO H. Adrenomedullin stimulates two signal transduction pathways, cAMP accumulation and Ca2+ mobilization, in bovine aortic endothelial cells. J. Biol. Chem. 1995;270:4412–4417. doi: 10.1074/jbc.270.9.4412. [DOI] [PubMed] [Google Scholar]
- SMITH P.M., FERGUSON A.V. Adrenomedullin acts in the rat paraventricular nucleus to decrease blood pressure. J. Neuroendocrinol. 2001;13:467–471. doi: 10.1046/j.1365-2826.2001.00657.x. [DOI] [PubMed] [Google Scholar]
- SPAMPINATO S., FALCUCCI B., CACCIAGUERRA S., CAMPANA G., MURARI G. Characterization of a putative calcitonin receptor in IMR 32 human neuroblastoma cells. Neurosci. Lett. 1999;273:167–170. doi: 10.1016/s0304-3940(99)00661-8. [DOI] [PubMed] [Google Scholar]
- STACHNIAK T.J., KRUKOFF T.L. Receptor activity modifying protein 2 distribution in the rat central nervous system and regulation by changes in blood pressure. J. Neuroendocrinol. 2003;15:840–850. doi: 10.1046/j.1365-2826.2003.01064.x. [DOI] [PubMed] [Google Scholar]
- STORNETTA R.L., GUYENET P.G., MCCARTY R.C. Autonomic nervous system control of heart rate during baroreceptor activation in conscious and anesthetized rats. J. Auton. Nerv. Syst. 1987;20:121–127. doi: 10.1016/0165-1838(87)90109-3. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI H., WATANABE T.X., NISHIMURA M., NAKANISHI T., SAKAMOTO M., YOSHIMURA M., KOMIYAMA Y., MASUDA M., MURAKAMI T. Centrally induced vasopressor and sympathetic responses to a novel endogenous peptide, adrenomedullin, in anesthetized rats. Am. J. Hypertens. 1994;7:478–482. doi: 10.1093/ajh/7.5.478. [DOI] [PubMed] [Google Scholar]
- TAYLOR M.M., KEOWN C.A., SAMSON W.K. Involvement of the central adrenomedullin peptides in the baroreflex. Regul. Pept. 2003;112:87–93. doi: 10.1016/s0167-0115(03)00026-0. [DOI] [PubMed] [Google Scholar]
- TERATA K., MIURA H., LIU Y., LOBERIZA F., GUTTERMAN D.D. Human coronary arteriolar dilation to adrenomedullin: role of nitric oxide and K(+) channels. Am. J. Physiol. Heart. Circ. Physiol. 2000;279:H2620–H2626. doi: 10.1152/ajpheart.2000.279.6.H2620. [DOI] [PubMed] [Google Scholar]
- THRASHER T.N. Unloading arterial baroreceptors causes neurogenic hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R1044–R1053. doi: 10.1152/ajpregu.00431.2001. [DOI] [PubMed] [Google Scholar]
- WONG J., CHOU L., REID I.A. Role of AT1 receptors in the resetting of the baroreflex control of heart rate by angiotensin II in the rabbit. J. Clin. Invest. 1993;91:1516–1520. doi: 10.1172/JCI116357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU Y., KRUKOFF T.L. Adrenomedullin in the rostral ventrolateral medulla increases arterial pressure and heart rate: roles of glutamate and nitric oxide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004a;287:R729–R734. doi: 10.1152/ajpregu.00188.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU Y., KRUKOFF T.L. Decrease in arterial pressure induced by adrenomedullin in the hypothalamic paraventricular nucleus is mediated by nitric oxide and GABA. Regul. Pept. 2004b;119:21–30. doi: 10.1016/j.regpep.2003.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU Y., KRUKOFF T.L.Adrenomedullin stimulates nitric oxide release from SK-N-SH human neuroblastoma cells by modulating intracellular calcium mobilization Endocrinology 20051462295–2305.Epub 2005 Jan 27 [DOI] [PubMed] [Google Scholar]
- YOSHIHARA F., SUGA S., YASUI N., HORIO T., TOKUDOME T., NISHIKIMI T., KAWANO Y., KANGAWA K. Chronic administration of adrenomedullin attenuates the hypertension and increases renal nitric oxide synthase in Dahl salt-sensitive rats. Regul. Pept. 2005;128:7–13. doi: 10.1016/j.regpep.2004.12.028. [DOI] [PubMed] [Google Scholar]