It is widely accepted now that enhanced peroxynitrite (ONOO−) formation contributes to oxidative and nitrosative stress in a variety of cardiovascular and other pathologies (see for reviews: Ferdinandy & Schulz (2001, 2003), Denicola & Radi (2005)). Therefore, targeting ONOO− directly by ONOO− decomposition catalysts and ONOO− scavengers or indirectly by inhibitors of downstream targets of peroxynitrite such as poli(ADP-ribose)-polimerase or matrix metalloproteinases are exciting new strategies for cytoprotection (Salvemini et al., 1998; Ferdinandy et al., 2000; Virag et al., 2003; Giricz et al., 2006). In contrast, increasing evidence suggests that physiological levels of ONOO− may act as a regulator of several physiological functions (Ferdinandy & Schulz, 2001; 2003; Herold & Fago, 2005; Ji et al., 2006). However, still very little is known about the physiological roles of endogenous peroxynitrite formation, possibly due to the number of technical limitations of detecting low, physiological levels of ONOO− in biological systems (Tarpey & Fridovich, 2001; Daiber et al., 2003).
ONOO− is a powerful oxidant species, which can be formed in vivo by the nonenzymatic reaction of nitric oxide (NO) and superoxide anion at an extremely rapid rate limited only by diffusion (Figure 1). At physiological pH, ONOO− is protonated to form peroxynitrous acid which rapidly decomposes forming highly reactive oxidant species especially in the presence of CO2 (see for review Szabó, 1996). Unfortunately, due to its very short half life at physiological pH, endogenous formation of ONOO− cannot be directly detected in biological systems (Tarpey & Fridovich, 2001; Alvarez & Radi, 2003; Daiber et al., 2003). Although nitration of tyrosine residues is being recognized as a marker for ONOO− formation, the specificity and sensitivity of nitrotyrosine formation, especially in case of physiological rate of ONOO− production, is not sufficient (van der Vliet et al., 1995; Ferdinandy & Schulz, 2001; 2003; Tarpey & Fridovich, 2001). Nitrotyrosine can be formed by ONOO−-independent pathways as well, for example, via the actions of peroxidases in the presence of nitrite (Eiserich et al., 1998). Moreover, the exogenous administration of ONOO− in experimental settings (e.g. via the blood) does not accurately reflect the effects of endogenous generation of ONOO− within the cells. Exogenous ONOO− rapidly reacts with plasma proteins and thiols to form the NO donor S-nitrosothiols (see for review Ferdinandy & Schulz (2001)). Thus, ONOO− is likely to be detoxified before it has a chance to reach tissues downstream of the injection site, let alone the intracellular compartment (Ishida et al., 1999). As NO itself is a cardioprotective and antioxidant molecule (Wink et al., 1993; Rubbo et al., 1996; Ferdinandy & Schulz, 2003) tissue protection may be seen when exogenous ONOO− is administered intravenously (Lefer et al., 1997; Nossuli et al., 1997; 1998). Exogenously applied ONOO−, however, may show toxic effects when it does not have the opportunity to combine with sulphydryl groups or other antioxidant defenses before reaching its cellular targets. This is dependent upon the concentration of ONOO− and the antioxidant capacity of the cell or tissue of interest. Indeed, ONOO− has been shown to be detrimental to cellular functions when it was applied for example, in crystalloid buffer systems, in which the concentrations of extracellular antioxidants and both free and protein-bound thiols are limited (Schulz et al., 1997; Digerness et al., 1999; Ferdinandy et al., 2000).
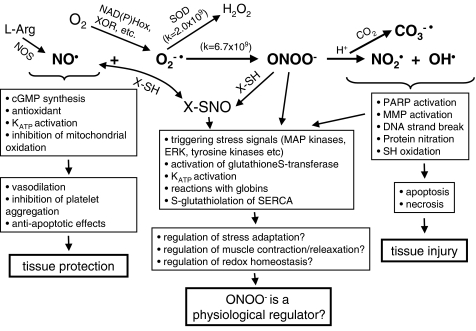
Figure 1.
Cellular mechanisms of the actions of NO, superoxide (O2−•), and ONOO−. NO is an important cardioprotective molecule via its vasodilator, antioxidant, antiplatelet, and antineutrophil actions and it is essential for normal cellular function. However, excess NO could be detrimental if it combines with O2−• to form ONOO− which rapidly decomposes to highly reactive oxidant species leading to tissue injury. There is a critical balance between cellular concentrations of NO, O2−•, and superoxide dismutase (SOD) which physiologically favor NO production but in pathological conditions such as, for example, ischemia and reperfusion result in ONOO− formation. ONOO− might be converted to NO donors if it combines with SH-group containing molecules (X-SH) to form S-nitroso compounds (X-SNO) including S-nitrosoglutathione. S-nitrosylation and S-glutathiolation are proposed mechanisms by which ONOO− regulates protein functions. Increasing evidence suggests that physiological levels of ONOO− act as regulator of several physiological functions. MMP, matrix metalloproteinase; NOS, NO synthase; PARP, poly-ADP ribose polymerase; XOR, xanthine oxidoreductase; SERCA, sarcoplasmic reticulum Ca2+-ATPase; KATP, ATP sensitive potassium channel.
In this issue of British Journal of Pharmacology, Graves et al. (2006) show that L-β,β-dimethylcysteine (L-penicillamine), a potential ONOO− scavenger, inhibits the dose-dependent vasodilator responses to moderate doses of peroxynitrite administered repeatedly in vivo. This group has also shown recently that the vasodilator response elicited by exogenous ONOO− involves activation of ATP-sensitive potassium channels (KATP) (Graves et al., 2005b). As the glibenclamide-sensitive vasodilator response was still seen after repeated injections of increasing doses of ONOO−, when depletion of antioxidants is suspected, ONOO− may open KATP independently from generation of S-nitrosothiols (Graves et al., 1998). However, when 10 repeated injections of a high dose of ONOO− (10 μmol kg−1) were administered, a loss of KATP function has been observed (Graves et al., 2005a). Vasodilation and opening of KATP is not the only potential regulator function of ONOO−, where ONOO− is not definitely detrimental to the tissues.
Increasing evidence suggests that ONOO− may act as a regulator of various physiologic cellular functions. Endogenous ONOO− has been shown to trigger ischemic stress adaptation of the rat myocardium (Altug et al., 2000; Csonka et al., 2001; see for review: Ferdinandy & Schulz, 2003), and to activate stress response pathways such as the tyrosine kinase-dependent MAP-kinase and ERK pathways (see for review Klotz et al. (2002)). Cerioni et al. (2006) has recently demonstrated that nontoxic concentrations of peroxynitrite induced mitochondrial translocation of PKC-alpha and activated cell survival pathways in U937 cells. It has been recently shown that activation of microsomal glutathione-S-transferase-1 by peroxynitrite is mediated by nitration of tyrosine residue 92, and represents one of the few examples in which a gain in function has been associated with nitration of a specific tyrosine residue by ONOO− (Ji et al., 2006). Reactions of ONOO− with globins are suspected to play crucial roles in regulating normal physiological responses (see for review Herold & Fago, 2005). Moreover, ONOO− appears to be essential to the reversible S-glutathiolation of sarcoplasmic reticulum Ca2+-ATPase, thereby regulating muscle relaxation (Viner et al., 1999; Adachi et al., 2004). ONOO− and NO donors can stimulate myocardial contractility independently of guanylyl cyclase activation, suggesting a role for S-nitrosylation reactions in the positive inotropic effects of NO/peroxynitrite in intact hearts (Paolocci et al., 2000). S-nitrosylation and S-glutathiolation are proposed mechanisms by which ONOO− regulates protein functions, although it should be noted that the role of NO and ONOO− in these reactions is still not clear and little is known about the oxidative actions ONOO− which seems to be more important than the nitrosative effect of ONOO− (Ji et al., 1999; Viner et al., 1999; Okamoto et al., 2001; Steffen et al., 2001). Nevertheless, it is plausible to speculate that ONOO− via its oxidative and nitrosative actions plays an important role in several physiological regulatory mechanisms that is becoming increasingly clear.
In summary, although it is widely accepted that enhanced ONOO− formation is cytotoxic, increasing evidence suggests that physiologic levels of ONOO− contribute to regulation of normal cellular functions. However, due to the numerous limitations of ONOO− detection using the currently available techniques, the conclusions should be drawn cautiously from studies based on ONOO− measurements. The development of more sensitive techniques to detect ONOO− and/or the discovery of specific and sensitive markers for endogenous ONOO− formation at a physiological rate will definitely enhance the exploration of the physiological roles of ONOO−.
Acknowledgments
I acknowledge the support of grants from the Hungarian National Scientific Research Found (OTKA T046417), Hungarian Ministries of Health (ETT 616/2003) and of Economy and Transport (GVOP-TST0095/2004), and the National Office for Research and Technology (NKTH-RET2004, Asboth-2005).
References
- ADACHI T., WEISBROD R.M., PIMENTEL D.R., YING J., SHAROV V.S., SCHONEICH C., COHEN R.A. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- ALTUG S., DEMIRYUREK A.T., KANE K.A., KANZIK I. Evidence for the involvement of peroxynitrite in ischaemic preconditioning in rat isolated hearts. Br. J. Pharmacol. 2000;130:125–131. doi: 10.1038/sj.bjp.0703280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALVAREZ B., RADI R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids. 2003;25:295–311. doi: 10.1007/s00726-003-0018-8. [DOI] [PubMed] [Google Scholar]
- CERIONI L., PALOMBA L., BRUNE B., CANTONI O. Peroxynitrite-induced mitochondrial translocation of PKCalpha causes U937 cell survival. Biochem. Biophys. Res. Commun. 2006;339:126–131. doi: 10.1016/j.bbrc.2005.10.193. [DOI] [PubMed] [Google Scholar]
- CSONKA C., CSONT T., ONODY A., FERDINANDY P. Preconditioning decreases ischemia/reperfusion-induced peroxynitrite formation. Biochem. Biophys. Res. Commun. 2001;285:1217–1219. doi: 10.1006/bbrc.2001.5308. [DOI] [PubMed] [Google Scholar]
- DAIBER A., BACHSCHMID M., KAVAKLI C., FREIN D., WENDT M., ULLRICH V., MUNZEL T. A new pitfall in detecting biological end products of nitric oxide-nitration, nitros(yl)ation and nitrite/nitrate artefacts during freezing. Nitric Oxide. 2003;9:44–52. doi: 10.1016/j.niox.2003.08.002. [DOI] [PubMed] [Google Scholar]
- DENICOLA A., RADI R. Peroxynitrite and drug-dependent toxicity. Toxicology. 2005;208:273–288. doi: 10.1016/j.tox.2004.11.023. [DOI] [PubMed] [Google Scholar]
- DIGERNESS S.B., HARRIS K.D., KIRKLIN J.W., URTHALER F., VIERA L., BECKMAN J.S., DARLEY-USMAR V. Peroxynitrite irreversibly decreases diastolic and systolic function in cardiac muscle. Free Rad. Biol. Med. 1999;27:1386–1392. doi: 10.1016/s0891-5849(99)00184-7. [DOI] [PubMed] [Google Scholar]
- EISERICH J.P., HRISTOVA M., CROSS C.E., JONES A.D., FREEMAN B.A., HALLIWELL B., VAN D.V. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., SCHULZ R. Peroxynitrite: Toxic or protective in the heart. Circ. Res. 2001;88:e12–e13. doi: 10.1161/01.res.88.2.e12. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., SCHULZ R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br. J. Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERDINANDY P., DANIAL H., AMBRUS I., ROTHERY R.A., SCHULZ R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ. Res. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- GIRICZ Z., LALU M.M., CSONKA C., BENCSIK P., SCHULZ R., FERDINANDY P. Hyperlipidemia attenuates the infarct size-limiting effect of ischemic preconditioning: role of matrix metalloproteinase-2 inhibition. J. Pharmacol. Exp. Ther. 2006;316:154–161. doi: 10.1124/jpet.105.091140. [DOI] [PubMed] [Google Scholar]
- GRAVES J.E., KOOY N.W., LEWIS S.J.L-beta-beta-dimethylcysteine attenuates the haemodynamic responses elicited by systemic injections of peroxynitrite in anaesthetized rats Br. J. Pharmacol. 20061487–15.(this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAVES J.E., LEWIS S.J., KOOY N.W. Peroxynitrite-mediated vasorelaxation: evidence against the formation of circulating S-nitrosothiols. Am. J. Physiol. 1998;274:H1001–H1008. doi: 10.1152/ajpheart.1998.274.3.H1001. [DOI] [PubMed] [Google Scholar]
- GRAVES J.E., LEWIS S.J., KOOY N.W. Loss of K+ATP-channel-mediated vasodilation after induction of tachyphylaxis to peroxynitrite. J. Cardiovasc. Pharmacol. 2005a;46:646–652. doi: 10.1097/01.fjc.0000181716.79580.dd. [DOI] [PubMed] [Google Scholar]
- GRAVES J.E., LEWIS S.J., KOOY N.W. Role of ATP-sensitive K+-channels in hemodynamic effects of peroxynitrite in anesthetized rats. J. Cardiovasc. Pharmacol. 2005b;46:653–659. doi: 10.1097/01.fjc.0000181715.02452.97. [DOI] [PubMed] [Google Scholar]
- HEROLD S., FAGO A. Reactions of peroxynitrite with globin proteins and their possible physiological role. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005;142:124–129. doi: 10.1016/j.cbpb.2005.06.009. [DOI] [PubMed] [Google Scholar]
- ISHIDA H., GENKA C., NAKAZAWA H. Application of authentic peroxynitrite to biological materials. Methods Enzymol. 1999;301:402–409. doi: 10.1016/s0076-6879(99)01104-0. [DOI] [PubMed] [Google Scholar]
- JI Y., AKERBOOM T.P., SIES H., THOMAS J.A. S-nitrosylation and S-glutathiolation of protein sulfhydryls by S-nitroso glutathione. Arch. Biochem. Biophys. 1999;362:67–78. doi: 10.1006/abbi.1998.1013. [DOI] [PubMed] [Google Scholar]
- JI Y., NEVEROVA I., VAN EYK J.E., BENNETT B.M. Nitration of tyrosine 92 mediates the activation of rat microsomal glutathione S-transferase by peroxynitrite. J. Biol. Chem. 2006;281:1986–1991. doi: 10.1074/jbc.M509480200. [DOI] [PubMed] [Google Scholar]
- KLOTZ L.O., SCHROEDER P., SIES H. Peroxynitrite signaling: receptor tyrosine kinases and activation of stress-responsive pathways. Free Radic. Biol. Med. 2002;33:737–743. doi: 10.1016/s0891-5849(02)00892-4. [DOI] [PubMed] [Google Scholar]
- LEFER D.J., SCALIA R., CAMPBELL B., NOSSULI T.O., HAYWARD R., SALAMON M., GRAYSON J., LEFER A.M. Peroxynitrite inhibits leukocyte-endothelial cell interactions and protects against ischemia-reperfusion injury in rats. J. Clin. Invest. 1997;99:684–691. doi: 10.1172/JCI119212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOSSULI T.O., HAYWARD R., JENSEN D., SCALIA R., LEFER A.M. Mechanisms of cardioprotection by peroxynitrite in myocardial ischemia and reperfusion injury. Am. J. Physiol. 1998;275:H509–H519. doi: 10.1152/ajpheart.1998.275.2.H509. [DOI] [PubMed] [Google Scholar]
- NOSSULI T.O., HAYWARD R., SCALIA R., LEFER A.M. Peroxynitrite reduces myocardial infarct size and preserves coronary endothelium after ischemia and reperfusion in cats. Circulation. 1997;96:2317–2324. doi: 10.1161/01.cir.96.7.2317. [DOI] [PubMed] [Google Scholar]
- OKAMOTO T., AKAIKE T., SAWA T., MIYAMOTO Y., VAN DER VLIET A., MAEDA H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J. Biol. Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- PAOLOCCI N., EKELUND U.E., ISODA T., OZAKI M., VANDEGAER K., GEORGAKOPOULOS D., HARRISON R.W., KASS D.A., HARE J.M. cGMP-independent inotropic effects of nitric oxide and peroxynitrite donors: potential role for nitrosylation. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H1982–H1988. doi: 10.1152/ajpheart.2000.279.4.H1982. [DOI] [PubMed] [Google Scholar]
- RUBBO H., DARLEY-USMAR V., FREEMAN B.A. Nitric oxide regulation of tissue free radical injury. Chem. Res. Toxicol. 1996;9:809–820. doi: 10.1021/tx960037q. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., STERN M.K., CURRIE M.G., MISKO T.P. Peroxynitrite decomposition catalysts: novel therapeutics for peroxynitrite-mediated pathology. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2659–2663. doi: 10.1073/pnas.95.5.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULZ R., DODGE K.L., LOPASCHUK G.D., CLANACHAN A.S. Peroxynitrite impairs cardiac contractile function by decreasing cardiac efficiency. Am. J. Physiol. Heart Circ. Physiol. 1997;272:H1212–H1219. doi: 10.1152/ajpheart.1997.272.3.H1212. [DOI] [PubMed] [Google Scholar]
- STEFFEN M., SARKELA T.M., GYBINA A.A., STEELE T.W., TRASSETH N.J., KUEHL D., GIULIVI C. Metabolism of S-nitrosoglutathione in intact mitochondria. Biochem. J. 2001;356:395–402. doi: 10.1042/0264-6021:3560395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABÓ C. The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia-reperfusion injury. Shock. 1996;6:79–88. doi: 10.1097/00024382-199608000-00001. [DOI] [PubMed] [Google Scholar]
- TARPEY M.M., FRIDOVICH I. Methods of detection of vascular reactive species: nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ. Res. 2001;89:224–236. doi: 10.1161/hh1501.094365. [DOI] [PubMed] [Google Scholar]
- VAN DER VLIET A., EISERICH J.P., O'NEILL C.A., HALLIWELL B., CROSS C.E. Tyrosine modification by reactive nitrogen species: a closer look. Arch. Biochem. Biophys. 1995;329:341–349. doi: 10.1006/abbi.1995.1303. [DOI] [PubMed] [Google Scholar]
- VINER R.I., WILLIAMS T.D., SCHONEICH C. Peroxynitrite modification of protein thiols: oxidation, nitrosylation, and S-glutathiolation of functionally important cysteine residue(s) in the sarcoplasmic reticulum Ca-ATPase. Biochemistry. 1999;38:12408–12415. doi: 10.1021/bi9909445. [DOI] [PubMed] [Google Scholar]
- VIRAG L., SZABO E., GERGELY P., SZABO C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol. Lett. 2003;140–141:113–124. doi: 10.1016/s0378-4274(02)00508-8. [DOI] [PubMed] [Google Scholar]
- WINK D.A., HANBAUER I., KRISHNA M.C., DEGRAFF W., GAMSON J., MITCHELL J.B. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc. Natl. Acad. Sci. U.S.A. 1993;90:9813–9817. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]