Abstract
In this study, [3H]GR205171 (3(S)-(2-methoxy-5-(5-trifluoromethyltetrazol-1-yl)-phenylmethylamino)-2(S)-phenylpiperidine), a potent and selective NK1 receptor antagonist, was characterised in autoradiographic studies in gerbil brain and in binding experiments on homogenates from gerbil and human brain cortex and striatum.
In autoradiographic studies in gerbil brain, highest levels of [3H]GR205171 binding sites were observed in caudate putamen, nucleus accumbens, medial and cortical nuclei of the amygdala and intermediate levels were detected in the hypothalamus, basolateral amygdala, septum, and cortex.
Saturation experiments in homogenates of brain striatum from gerbil showed that [3H]GR205171 binds to a single receptor population with a pKd value of 10.8±0.2 and a Bmax value of 607±40 fmol mg−1. A lower number of NK1 receptor sites was found in cortex, where a Bmax of 94±6 fmol mg−1 protein was obtained. Saturation experiments performed on homogenates from brain striatum of two human subjects and brain cortex of three human subjects showed that [3H]GR205171 binds with pKd values not different from gerbil and Bmax values ranging from 318±51 to 432±27 fmol mg−1 protein in striatum and from 59±1 to 74±21 fmol mg−1 protein in cortex. The natural ligand [3H]Substance P (SP) bound with sub-nanomolar affinity to 15 and 6% sites compared to [3H]GR205171 in gerbil and human striatum, respectively.
In competition binding experiments, GR205171 and the NK1 receptor antagonists aprepitant (MK-869), L-733,060 and NKP-608 bound with similar pKi values in gerbil and human striatum, irrespective of the use of [3H]GR205171 or [3H]SP as radioligand. The following rank order was found in terms of pKi values: GR205171>aprepitant⩾L-733,060>NKP-608. In homologous displacement experiments in gerbil and human striatum, SP showed nanomolar affinity, whereas in [3H]GR205171 competition experiments SP bound with pIC50 values in the micromolar range and Hill slopes significantly lower than one.
It is concluded that the similarities of [3H]GR205171 binding characteristics and pharmacology between gerbil and human in cortex and striatum support the use of gerbil in preclinical models to study the effects of NK1 receptor antagonists in the central nervous system.
Keywords: GR205171, substance P, NK1 receptor, binding, gerbil brain, human brain
Introduction
The tachykinin receptors are G protein-coupled receptors characterized by seven trans-membrane domains. They are classified in neurokinin type 1 (NK1), 2 (NK2), and 3 (NK3) receptors at which the endogenous neuropeptides substance P (SP), Neurokinin A and Neurokinin B bind with highest affinity, respectively. NK1 receptors are widely distributed in both the central and peripheral nervous system, where the undecapeptide SP functions as a neurotransmitter and neuromodulator in a variety of physiological processes such as neuronal excitation, vasodilatation and smooth muscle contraction (Quartara & Maggi, 1998). Over recent years, interest in central NK1 receptors has increased based on reports on the therapeutic potential for NK1 receptor antagonists in psychiatric disorders. The first clinical evidence of the ability of NK1 receptor antagonists to alleviate depression and anxiety came from a double blind study with aprepitant (MK-869), which showed efficacy similar to the selective serotonin reuptake inhibitor paroxetine but without the side-effects of this class of molecules (Kramer et al., 1998). Following that study, other two independent clinical trials with two different NK1 receptor antagonists (L-759274 and CP-122,721) showed antidepressant effects in depressed patients. However, the recent negative findings with aprepitant in a depression phase III study made controversial the efficacy of NK1 receptor antagonist in depression (Herpfer & Lieb, 2005) and rendered crucial the identification of new potent and brain penetrant NK1 receptor antagonists to further explore the antidepressant and anxiolytic activity of NK1 receptor antagonists. The preclinical assessment of the efficacy of NK1 receptor antagonists has been partially hindered by the low pharmacological homology between human and rat or mouse NK1 receptors. NK1 receptor antagonists which display high affinity at human receptors, such as CP-96,345 and GR205171 show lower affinity in mouse and rat (Beresford et al., 1991; Zocchi et al., 2003). Vice versa, antagonists with high affinity at rat or mouse receptors, such as RP67580, show a lower affinity in human (Fong et al., 1992).
Guinea pig and gerbil are the preclinical species most commonly used to test efficacy of NK1 receptor antagonists since they share higher homology to human in terms of NK1 receptor pharmacology as compared to other rodents (Beresford et al., 1991; Saria, 1999). Over recent years, a number of different models have been developed in gerbil to assess brain penetrant and anxiolytic and antidepressant-like properties of NK1 receptor antagonists such as the agonist-induced foot tapping (Bristow & Young, 1994; Rupniak & Williams, 1994), the social interaction test (Cheeta et al., 2001), the elevated plus maze test (Varty et al., 2002), the fear conditioning test (Rupniak et al., 2003a) and the tail suspension test (Varty et al., 2003).
Autoradiographic and in situ mRNA hybridisation studies performed in mammalian brain showed that NK1 receptors are most abundant in striatum, nucleus accumbens, hippocampus, raphe nuclei and medulla oblongata, whereas expression in cortex is moderate (Saria, 1999; Caberlotto et al., 2003; Rigby et al., 2005). However, no data are available to compare the NK1 receptor binding pharmacology and expression between human and gerbil brain despite the large use of the latter in preclinical models.
In this study, autoradiographic experiments with the NK1 receptor antagonist [3H]GR205171 (Gardner et al., 1996) were performed in brain slices of gerbil to determine NK1 receptor distribution. Moreover, [3H]GR205171 saturation binding experiments were performed on homogenates from cortex and striatum of gerbil and human in order to assess the relative amount of NK1 receptor sites. [3H]SP saturation experiments were also performed on striatum from both gerbil and human in order to determine the amount of agonist compared to antagonist binding sites. Finally, [3H]SP and [3H]GR205171 displacement binding experiments by known NK1 receptor antagonists such as aprepitant, NKP-608 and L-733,060 (Seabrook et al., 1996; Kramer et al., 1998; Vassout et al., 2000) were performed in gerbil and human striatum.
Methods
Materials
GR205171, aprepitant and NKP-608 were synthesised in GSK Chemical Department, Verona, Italy. [3H]GR205171 (3.07 TBq mmol−1), di-trifluoroacetic acid salt was synthesised in GSK Chemical Department and labelled with [3H] by Amersham International Plc., Little Chalfont, Buckinghamshire, U.K. [3H]SP (1–2 TBq mmol−1) was purchased from Amersham Biosciences, Freiburg, Germany. Leupeptin, Bacitracin, Pefabloc, Pepstatin, Chymostatin, Phosphoramidon, Bovine serum albumin (BSA), L-733,060 and SP were purchased from Sigma, Milan, Italy. All drugs to be tested in filtration binding assays were diluted primarily in dimethyl sulfoxide (DMSO) and further diluted in the assay buffer to give a final DMSO concentration of 1%.
[3H]GR205171 autoradiography in gerbil brain
Animal manipulations were performed according to Italian law (art. 7, Legislative Decree No. 116, 27 January 1992), which acknowledged the European Directive 86/609/EEC, and to GlaxoSmithKline policy on the care and use of laboratory animals and related codes of practice. Mongolian gerbils (60–70 g, Charles River, Sulzfeld, Germany) were killed by decapitation and brains were quickly removed and frozen in isopentane at −30°C. Coronal brain sections were thawed, air dried and preincubated for 15 min at room temperature in Tris-HCl (50 mM, pH 7.4) containing 0.02% BSA. Sections were then incubated for 90 min at room temperature in the same buffer containing MnCl2 (3 mM), Bacitracin (0.04 mg ml−1), Leupeptin (0.004 mg ml−1), Chymostatin (0.002 mg ml−1), and 0.2 nM [3H]GR205171. Nonspecific binding was determined in the presence of 100 nM unlabeled GR205171 in the incubation buffer. At the end of the incubation, sections were washed four times (60 s each) in ice-cold Tris-HCl (50 mM, pH 7.4) containing 0.02% BSA, briefly dipped in ice-cold distilled water and then dried under a stream of cool air, and exposed for one week to imaging plates (Fuji Photo Film, Tokyo, Japan).
Quantification of autoradiographic results
Films from the autoradiography experiments were scanned using a BAS-5000 Bio-Imaging Analyzer (Fuji Photo Film, Tokyo, Japan) and quantified using an image analysis software system (AIS 4.0, Imaging Research Inc., St Catharines, Ontario, Canada). Regional densities of binding were determined with reference with tritium standards (RPA 510, Amersham, Freiburg, Germany) on the same film and converted to fmol mg−1 tissue.
Preparation of brain homogenates from human and gerbil
Brain cortex and striatum from mongolian gerbils (pools of five animals for each preparation) were dissected immediately after animal decapitations. The following brain samples from a total of four human donors were obtained under approved ethical guidelines and homogenates were prepared separately: cortex and striatum from subject A, male, aged 58 years, died of a cardio-respiratory failure (post-mortem interval 23 h); striatum from subject B, male aged 17 years, died of brain tumor (post-mortem interval 8 h); cortex from subject C, female aged 60 years, died of acute tracheobronchitis (post-mortem interval 7 h 30 min); cortex from subject D, female aged 93 years, died of natural causes (post-mortem interval not known). Brain homogenates were prepared as follows: fresh (gerbil) or frozen (human) tissues were weighed, crumbled and homogenised in 10 volumes of membrane-preparation buffer (HEPES 50 mM pH 7.4, Leupeptin 0.1 mM, Bacitracin 40 μg ml−1, EDTA 1 mM, Pefabloc 0.2 mM, Pepstatin 2 μM). The homogenate was then centrifuged at 48,000 × g for 20 min, and the pellet was washed once more by resuspension in 10 volumes of membrane preparation buffer and centrifugation again at 48,000 × g for 20 min. The final pellet was resuspended in 7–10 volumes of membrane preparation buffer and frozen at −80°C until use. Protein concentration was determined by the Bio-Rad Protein assay (Milan, Italy) using BSA as the standard.
Binding experiments with [3H]GR205171 and [3H]SP
In saturation experiments, increasing concentrations of [3H]GR205171 (0.01–1 nM) or [3H]SP (0.02–5 nM) were incubated with homogenates from striatum (20–50 μg well−1 for [3H]GR205171, 200–500 μg well−1 for [3H]-SP) or cortex (100 μg well−1) for 60 min at room temperature in a final volume of 400 μl of 50 mM HEPES, pH 7.4, 3 mM MnCl2, 0.02% BSA, 2 μg ml−1 Leupeptin, 20 μg ml−1 Bacitracin, and 0.5 μM Phosphoramidon. Nonspecific binding was determined in the presence of 1 μM GR205171. Reactions were stopped by filtration over GF/C filters presoaked in 0.5% polyetylenimmine followed by three washings with cold 0.9% NaCl.
In competition binding experiments, 0.2 nM [3H]GR205171 or 0.6 nM [3H]SP were incubated with homogenates of gerbil or human (subject A) striatum as aforementioned, in the presence of increasing concentration of displacing compounds (1 pM–1 μM GR205171, L-733,060, aprepitant and NKP-608, 10 pM–10 μM SP).
Data analysis
Radioligand binding data were analysed by nonlinear regression analysis using GraphPad Prism 4.0 (GraphPad Software, CA, U.S.A.). Determination of KD and Bmax of [3H]GR205171 and [3H]SP was assessed by elaborating saturation experiments using one site binding (hyperbola) equation. Curve fitting from competition-binding experiments was determined by using one site competition equation after checking with F test (P<0.05) that Hill slope in the four parameter logistic equation was not statistically different from 1.0. In this condition, IC50 values were converted to Ki using the Cheng–Prusoff equation (Cheng & Prusoff, 1973). Results are expressed as mean pKi±s.e.m.
Results
[3H]GR205171 autoradiography on gerbil brain
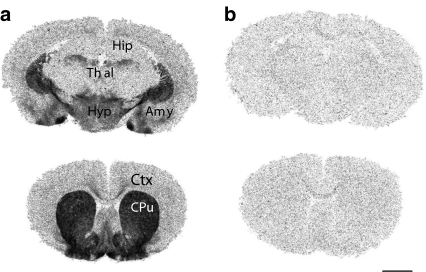
The distribution of [3H]GR205171 binding in gerbil brain was heterogeneous (Figure 1). The highest levels of binding sites were observed in caudate putamen, nucleus accumbens, medial and cortical nuclei of the amygdala. Intermediate levels were detected in the hypothalamus, basolateral amygdala, septum, and cortex, while low levels were observed in hippocampus and thalamus. The number of receptor sites (fmol mg−1 tissue) labelled by 0.2 nM [3H]GR205171 were 69.6±2.8 in striatum, 8.5±0.5 in cortex, 171.6±6.9 in medial amygdala, 30.1±2.0 in basolateral amygdala, 8.2±0.7 in thalamus, and 34.3±1.5 in hypothalamus (n=3).
Figure 1.
(a) Representative autoradiographic localisation of [3H]GR205171 binding sites at two gerbil brain levels. Brain regions containing substantial levels of binding include caudate putamen (Cpu), nucleus acccumbens, amygdala (Amy) and hypothalamus (Hyp). Medium/low levels of binding were detected in cortex (Ctx), hippocampus (Hip), thalamus (Thal) and septum. (b) Nonspecific binding in the presence of 100 nM unlabeled GR205171. Scale bar: 0.25 cm.
Saturation binding experiments
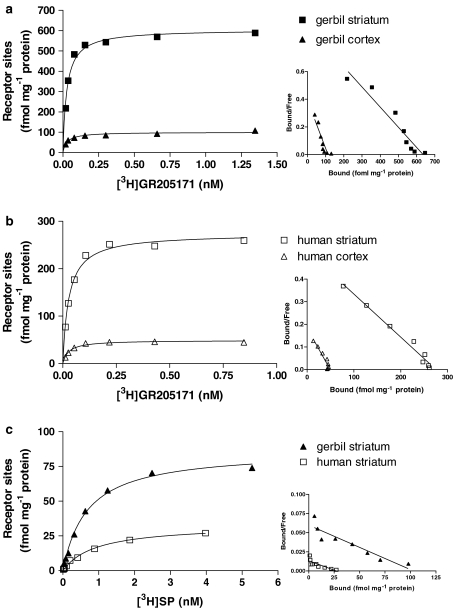
In saturation-binding experiments performed in gerbil and human striatum and cortex, [3H]GR205171 bound to a single site with similar and high affinity (Figure 2a and b). Bmax values obtained for gerbil (607±40 fmol mg−1 protein in striatum and 94±6 fmol mg−1 protein in cortex) were in agreement with the number of receptor sites labelled by [3H]GR205171 in autoradiography experiments, assuming 1 mg of protein corresponds approximately to 10 mg of tissue. Human striatum and cortex showed lower Bmax values compared to gerbil (from 318±51 to 432±27 fmol mg−1 protein in striatum and from 59±1 to 74±21 fmol mg−1 protein in cortex). However, the ratio between the number of receptors in the two tissues was similar in the two species, the striatum showing approximately six fold higher receptor expression than cortex. No significant difference in Bmax and pKd for [3H]GR205171 was observed either between subjects A and B in striatum or among subjects A, C, and D in cortex (Table 1).
Figure 2.
Representative saturation binding experiments with the NK1 receptor antagonist [3H]GR205171 in gerbil (a) and human (b) striatum and cortex and with the endogenous agonist [3H]SP (c) in gerbil and human striatum. Relative Scatchard plot is shown on the right of each saturation curve. Brain samples of subject A were used in these experiments. See Methods for experimental procedures.
Table 1.
[3H]GR205171 and [3H]SP saturation experiments on brain striatum and cortex from gerbil and different human subjects
| |
|
[3H]GR205171 binding |
[3H]SP binding |
||
|---|---|---|---|---|---|
| pKd | Bmax | pKd | Bmax | ||
| Brain |
Gerbil |
10.8±0.2 |
607±40 |
9.5±0.2 |
93±20 |
| striatum |
Subject A |
10.6±0.1 |
318±51 |
9.4±0.1 |
18±2 |
| |
Subject B |
10.7±0.1 |
432±27 |
|
|
| |
|
|
|
|
|
| Brain cortex |
Gerbil |
10.5±0.2 |
94±6 |
|
|
| |
Subject A |
10.5±0.2 |
74±21 |
|
|
| |
Subject C |
10.6±0.1 |
59±1 |
|
|
| Subject D | 10.7±0.1 | 60±2 | |||
Bmax values are expressed as fmol mg−1 protein. Data presented are the mean±s.e.m. of at least three independent experiments performed in duplicate. See Methods for experimental procedures. No significant difference in [3H]GR205171 pKd and Bmax values in striatum and cortex was observed among subjects (P>0.05, t-test).
In striatum, [3H]SP bound with similar and high affinity to a single site both in human (subject A) and gerbil (Figure 2c, Table 1), with pKd values of 9.4±0.1 and 9.5±0.2, respectively. In both species, [3H]SP bound to a smaller amount of receptor sites compared with the antagonist (Bmax=93±20 in gerbil striatum, Bmax=18±2 in human striatum). The percentage of sites recognised with high affinity by [3H]SP were 6% in human and 15% in gerbil in comparison with the sites recognised by [3H]GR205171.
Competition-binding experiments
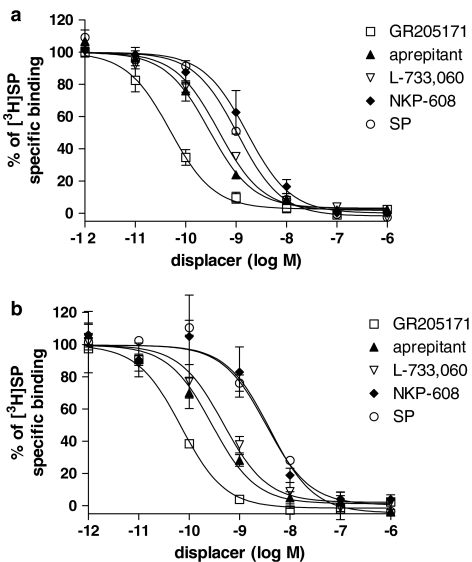
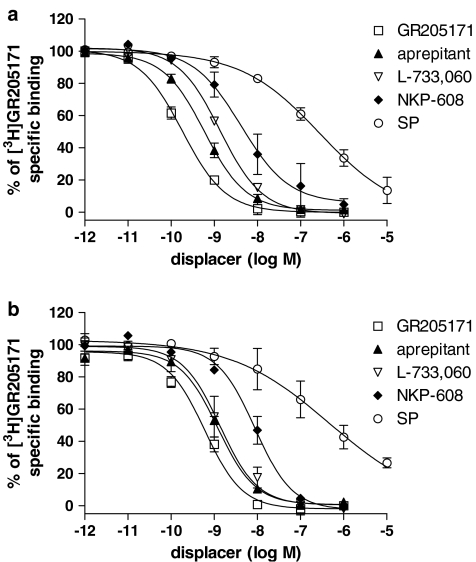
Affinity values of compounds tested in displacement experiments vs [3H]GR205171 and [3H]SP are shown in Table 2. The NK1 receptor antagonists GR205171, aprepitant, L-733,060 and NKP-608 showed similar pKi values in gerbil and human (subject A) striatum, irrespective of the radioligand used. The following rank order was found in terms of pKi values: GR205171 >aprepitant⩾L-733,060>NKP-608 (Figures 3 and 4). In homologous displacement experiments, SP showed pKi values similar to pKd obtained in [3H]SP saturation experiments (Figure 3), whereas a considerable drop in the potency was observed when tested against [3H]GR205171. SP competition curves did not fully displace [3H]GR205171 even at concentration of agonist up to 10 μM. Moreover, Hill slopes of the SP competition curves were significantly lower than one, according to a multi-site binding (Table 2, Figure 4).
Table 2.
[3H]GR205171 and [3H]SP competition experiments on brain striatum of human (subject A) and gerbil
| |
pKi vs [3H]GR205171 |
pKi vs [3H]SP |
||
|---|---|---|---|---|
| Human | Gerbil | Human | Gerbil | |
| SP |
6.34±0.22a |
6.61±0.16a |
9.06±0.11 |
9.49±0.03 |
| |
(Hill slope=0.38±0.09) (n=4) |
(Hill slope=0.57±0.10) (n=3) |
(n=3) |
(n=3) |
| GR205171 |
10.50±0.09 |
10.79±0.02 |
10.65±0.10 |
10.48±0.10 |
| |
(n=3) |
(n=5) |
(n=4) |
(n=3) |
| L-733,060 |
9.81±0.14 |
9.94±0.15 |
9.71±0.15 |
9.77±0.04 |
| |
(n=4) |
(n=4) |
(n=4) |
(n=4) |
| Aprepitant |
10.11±0.04 |
10.34±0.07 |
10.11±0.20 |
10.06±0.12 |
| |
(n=4) |
(n=4) |
(n=4) |
(n=4) |
| NKP-608 |
9.31±0.13 |
9.34±0.28 |
9.09±0.14 |
9.30±0.23 |
| (n=3) | (n=3) | (n=3) | (n=3) | |
n represents the number of independent experiments. See Methods for experimental procedures.
pIC50 values.
Figure 3.
Inhibition of [3H]SP binding by GR205171, aprepitant (MK-869), L-733,060, NKP-608 and SP in homogenates prepared from gerbil (a) and human (b) striatum. Results are expressed as % of specific radioligand binding against the log concentration of each compound and values are mean±s.e.m. These data were taken from three independent experiments. Brain striatum of subject A was used in these experiments.
Figure 4.
Inhibition of [3H]GR205171 binding by GR205171, aprepitant (MK-869), L-733,060, NKP-608 and SP in homogenates prepared from gerbil (a) and human (b) striatum. Results are expressed as % of specific radioligand binding against the log concentration of each compound and values are mean±s.e.m. These data were taken from three independent experiments. Brain striatum of subject A was used in these experiments.
Discussion
This study was undertaken with the goals to compare the levels of NK1 receptors in brain cortex and striatum of gerbil and human by using the radiolabelled selective NK1 receptor antagonist, [3H]GR205171 and the endogenous ligand, [3H]SP. Moreover, displacement binding experiments of the two radioligands by standard NK1 receptor antagonists and SP were carried out in both species. Indeed, a high pharmacological homology between gerbil and human NK1 receptors was already proposed by Beresford et al. (1991), although it derived from the test of a single NK1 receptor antagonist. Over recent years gerbil has also become the elective species by many groups to assess the central activity of novel NK1 receptor antagonists (Cheeta et al., 2001; Duffy et al., 2002), even though a more extensive comparison of receptor binding properties and pharmacology of the NK1 receptors in the brain between gerbil and human was still lacking.
GR205171 is a potent and selective nonpeptide NK1 receptor antagonist which is widely used as reference NK1 receptor antagonist both in in vitro and in vivo studies (Gardner et al., 1996; Rupniak et al., 2001). Furthermore, [11C]GR205171 and its [18F]-analogue have been extensively used in positron emission tomography (PET) experiments in rhesus monkey and human, respectively, to determine receptor occupancy of NK1 receptor antagonists at potential therapeutic doses (Bergstrom et al., 2000; Hargreaves, 2002; Zamuner et al., 2002).
The distribution of [3H]GR205171 binding sites in gerbil brain found in the present study was particularly high in caudate putamen, nucleus accumbens, medial and cortical nuclei of the amygdala. It was intermediate in the hypothalamus, basolateral amygdala, septum and cortex, and low in hippocampus and thalamus. These data closely matched the distribution of NK1 receptors labeled by [125I]-SP, as reported by Duffy et al. (2002) and more recently by Rigby et al. (2005) in gerbil brain. Furthermore, the high level of [3H]GR205171specific binding in striatum endorses the findings of PET experiments with [11C]-GR205171 and the [18F]-analogue in rhesus monkey and human, where this region shows the most intense radioligand uptake and thus is considered the preferred region for the assessment of NK1 receptor blockade by brain-penetrant NK1 receptor antagonists.
[3H]GR205171 and [3H]SP filtration binding assays on homogenates from brain cortex and striatum of gerbil and human were carried out to gain information about NK1 receptors density in the two different brain areas and to compare the pharmacology of known NK1 receptor ligands in the two different species. Saturation experiments with [3H]GR205171 showed that striatum and cortex of both gerbil and human have the same affinity values for the radioligand used. Moreover, striatum displayed more binding sites with respect to cortex by a factor of approximately six-folds in both gerbil and human. Human striatum and cortex showed lower Bmax values compared to gerbil. However, when human brain samples are used there are potential concerns that need to be taken into account such as the potential degradation of receptor proteins due to the post mortem intervals. A partial degradation of NK1 receptors may have occurred during the human post-mortem interval, causing an overall underestimation of NK1 receptor density. Nevertheless, Bmax values obtained for two subjects in striatum and three subjects in cortex revealed a similarity of NK1 receptor expression across subjects investigated.
Interestingly, a similar ratio of [3H]GR205171 binding sites in gerbil striatum with respect to cortex was also observed when the autoradiography technique was used in gerbil. In autoradiography experiments, the density of sites in striatum and cortex is in agreement with numbers of specific binding sites calculated for the corresponding brain regions in gerbil brain homogenates. Saturation binding experiments were then performed with the NK1 receptor preferred agonist SP as radioligand in striatum, one of the brain regions with the highest level of receptor expression. [3H]SP was found to bind with high affinity at six- to 18-fold lower number of a binding sites with respect to [3H]GR205171 in gerbil and human, respectively, suggesting that only a small fraction of receptors in native tissues are in the SP-preferred conformation. These data support the findings of Sagan et al. (1999) that the NK1 receptor may exist in agonist preferring conformations and accordingly to this, SP displaced [3H]GR205171 with extremely low affinity, which was not the case for GR205171, which displaced [3H]SP and [3H]GR205171 with similar potency. This latter finding further support the valuable properties of the radioligand [3H]GR205171, since it does not discriminate across NK1 receptor conformations and produces a higher signal to noise ratio with respected to [3H]SP in receptor binding experiments.
In competition binding experiments, the selective NK1 receptor antagonists GR205171, aprepitant, L-733,060 and NKP-608 showed similar affinities in human and gerbil brain striatum, irrespective of the use of [3H]GR205171 or [3H]SP as radioligand, confirming the high pharmacological homology between the gerbil and the human NK1 receptors. The pKi values obtained for GR205171, aprepitant and L-733,060 are in agreement with those found by Duffy et al. (2002) in gerbil striatum by using [125I]-SP. On the other hand, the affinity we observed for NKP-608 is intermediate between the pIC50 value of 7.9 obtained by Vassout et al. (2000) in gerbil midbrain against [3H]SP and the pKi value of 10.1 found by Rupniak et al. (2003b) at recombinant human NK1 receptors against [125I]-Tyr8-SP. Methodological differences may account for the discrepancy among the published affinities of NKP-608 and the data produced in this work.
In conclusion, the close correspondence in the NK1 receptor binding properties in gerbil and human brain supports the use of this animal species to investigate the physiology of NK1 receptors and characterise novel NK1 receptor ligands.
Acknowledgments
We thank Dr L. Caberlotto and Prof N. Bowery for useful comments.
Abbreviations
- Bmax
receptor density
- BSA
bovine serum albumin
- DMSO
dimethyl sulphoxide
- GPCR
G-protein-coupled receptors
- GR205171
3(S)-(2-methoxy-5-(5-trifluoromethyltetrazol-1-yl)-phenylmethylamino)-2(S)-phenylpiperidine
- HEPES
4-2-(hydroxyethyl)piperazine-1-ethanesulfonic acid
- IC50
half-maximal inhibitory concentration
- L-733,060
(2S,3S)-3-((3,5-bis(trifluoromethyl)phenyl)methyloxy)-2-phenyl piperidine
- MK-869
aprepitant, 5-[[2(R)-[1(R)-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3(S)-(4-fluorophenyl)-4-morpholinyl]methyl]-2,4-dihydro-3H-1,2,4-triazol-3-one
- NKP-608
(quinoline-4-carboxilic acid [trans-(2R, 4S)-1-(3,5-bistrifluoromethyl-benzoyl)-2-(4-chloro-benzyl)-piperidin-4-yl]-amide)
- PET
positron emission tomography
- SP
Substance P
References
- BERESFORD I.J., BIRCH P.J., HAGAN R.M., IRELAND S.J. Investigation into species variants in tachykinin NK1 receptors by use of the non-peptide antagonist, CP-96,345. Br. J. Pharmacol. 1991;104:292–293. doi: 10.1111/j.1476-5381.1991.tb12423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGSTROM M., FASTH K.J., KILPATRICK G., WARD P., CABLE K.M., WIPPERMAN M.D., SUTHERLAND D.R., LANGSTROM B. Brain uptake and receptor binding of two [11C]labelled selective high affinity NK1-antagonists, GR203040 and GR205171-PET studies in rhesus monkey. Neuropharmacology. 2000;39:664–670. doi: 10.1016/s0028-3908(99)00182-3. [DOI] [PubMed] [Google Scholar]
- BRISTOW L.J., YOUNG L. Chromodacryorrhea and repetitive hind paw tapping: models of peripheral and central tachykinin NK1 receptor activation in gerbils. Eur. J. Pharmacol. 1994;253:245–252. doi: 10.1016/0014-2999(94)90198-8. [DOI] [PubMed] [Google Scholar]
- CABERLOTTO L., HURD Y.L., MURDOCK P., WAHLIN J.P., MELOTTO S., CORSI M., CARLETTI R. Neurokinin 1 receptor and relative abundance of the short and long isoforms in the human brain. Eur. J. Neurosci. 2003;17:1736–1746. doi: 10.1046/j.1460-9568.2003.02600.x. [DOI] [PubMed] [Google Scholar]
- CHEETA S., TUCCI S., SANDHU J., WILLIAMS A.R., RUPNIAK N.M., FILE S.E. Anxiolytic actions of the substance P (NK1) receptor antagonist L-760735 and the 5-HT1A agonist 8-OH-DPAT in the social interaction test in gerbils. Brain Res. 2001;915:170–175. doi: 10.1016/s0006-8993(01)02846-3. [DOI] [PubMed] [Google Scholar]
- CHENG Y., PRUSOFF W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- DUFFY R.A., VARTY G.B., MORGAN C.A., LACHOWICZ J.E. Correlation of neurokinin (NK) 1 receptor occupancy in gerbil striatum with behavioral effects of NK1 antagonists. J. Pharmacol. Exp. Ther. 2002;301:536–542. doi: 10.1124/jpet.301.2.536. [DOI] [PubMed] [Google Scholar]
- FONG T.M., YU H., STRADER C.D. Molecular basis for the species selectivity of the neurokinin-1 receptor antagonists CP-96,345 and RP67580. J. Biol. Chem. 1992;267:25668–25671. [PubMed] [Google Scholar]
- GARDNER C.J., ARMOUR D.R., BEATTIE D.T., GALE J.D., HAWCOCK A.B., KILPATRICK G.J., TWISSELL D.J., WARD P. GR205171: a novel antagonist with high affinity for the tachykinin NK1 receptor, and potent broad-spectrum anti-emetic activity. Regul. Pept. 1996;65:45–53. doi: 10.1016/0167-0115(96)00071-7. [DOI] [PubMed] [Google Scholar]
- HARGREAVES R. Imaging substance P receptors (NK1) in the living human brain using positron emission tomography. J. Clin. Psychiatry. 2002;63:18–24. [PubMed] [Google Scholar]
- HERPFER I., LIEB K. Substance P receptor antagonists in Psychiatry. Rationale for Development and therapeutic potential. CNS Drugs. 2005;19:275–293. doi: 10.2165/00023210-200519040-00001. [DOI] [PubMed] [Google Scholar]
- KRAMER M.S., CUTLER N., FEIGHNER J., SHRIVASTAVA R., CARMAN J., SRAMEK J.J., REINES S.A., LIU G., SNAVELY D., WYATT-KNOWLES E., HALE J.J., MILLS S.G., MACCOSS M., SWAIN C.J., HARRISON T., HILL R.G., HEFTI F., SCOLNICK E.M., CASCIERI M.A., CHICCHI G.G., SADOWSKI S., WILLIAMS A.R., HEWSON L., SMITH D., CARLSON E.J., HARGREAVES R.J., RUPNIAK N.M. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- QUARTARA L., MAGGI C.A. The tachykinin NK1 receptor. Part II: Distribution and pathophysiological roles. Neuropeptides. 1998;32:1–49. doi: 10.1016/s0143-4179(98)90015-4. [DOI] [PubMed] [Google Scholar]
- RIGBY M., O'DONNELL R., RUPNIAK N.M. Species Differences in Tachykinin Receptor Distribution: Further Evidence That the Substance P (NK1) Receptor Predominates in Human Brain. J. Comarat. Neurol. 2005;490:335–353. doi: 10.1002/cne.20664. [DOI] [PubMed] [Google Scholar]
- RUPNIAK N.M., WEBB J.K., FISHER A., SMITH D., BOYCE S. The substance P (NK1) receptor antagonist L-760735 inhibits fear conditioning in gerbils. Neuropharmacology. 2003a;44:516–523. doi: 10.1016/s0028-3908(03)00023-6. [DOI] [PubMed] [Google Scholar]
- RUPNIAK N.M., CARLSON E.J., SHEPHEARD S., BENTLEY G., WILLIAMS A.R., HILL A., SWAIN C., MILLS S.G., DI SALVO J., KILBURN R., CASCIERI M.A., KURTZ M.M., TSAO K.L., GOULD S.L., CHICCHI G.G. Comparison of the functional blockade of rat substance P (NK1) receptors by GR205171, RP67580, SR140333 and NKP-608. Neuropharmacology. 2003b;45:231–241. doi: 10.1016/s0028-3908(03)00157-6. [DOI] [PubMed] [Google Scholar]
- RUPNIAK N.M., CARLSON E.J., WEBB J.K., HARRISON T., PORSOLT R.D., ROUX S., DE FELIPE C., HUNT S.P., OATES B., WHEELDON A. Comparison of the phenotype of NK1R−/− mice with pharmacological blockade of the substance P (NK1) receptor in assays for antidepressant and anxiolytic drugs. Behav. Pharmacol. 2001;12:497–508. doi: 10.1097/00008877-200111000-00011. [DOI] [PubMed] [Google Scholar]
- RUPNIAK N.M., WILLIAMS A.R. Differential inhibition of foot tapping and chromodacryorrhoea in gerbils by CNS penetrant and non-penetrant tachykinin NK1 receptor antagonists. Eur. J. Pharmacol. 1994;265:179–183. doi: 10.1016/0014-2999(94)90430-8. [DOI] [PubMed] [Google Scholar]
- SAGAN S., KAROYAN P., CHASSAING G., LAVIELLE S. Further delineation of the two binding sites (R*(n)) associated with tachykinin neurokinin-1 receptors using [3-Prolinomethionine(11)]SP analogues. J. Biol. Chem. 1999;274:23770–23776. doi: 10.1074/jbc.274.34.23770. [DOI] [PubMed] [Google Scholar]
- SARIA A. The tachykinin NK1 receptor in the brain: pharmacology and putative functions. Eur. J. Pharmacol. 1999;375:51–60. doi: 10.1016/s0014-2999(99)00259-9. [DOI] [PubMed] [Google Scholar]
- SEABROOK G.R., SHEPHEARD S.L., WILLIAMSON D.J., TYRER P., RIGBY M., CASCIERI M.A., HARRISON T., HARGREAVES R.J., HILL R.G. L-733,060, a novel tachykinin NK1 receptor antagonist; effects in [Ca2+]i mobilisation, cardiovascular and dural extravasation. Eur. J. Pharamacol. 1996;317:129–135. doi: 10.1016/s0014-2999(96)00706-6. [DOI] [PubMed] [Google Scholar]
- VARTY G.B., COHEN-WILLIAMS M.E., HUNTER J.C. The antidepressant-like effects of neurokinin NK1 receptor antagonists in a gerbil tail suspension test. Behav. Pharmacol. 2003;14:87–95. doi: 10.1097/00008877-200302000-00009. [DOI] [PubMed] [Google Scholar]
- VARTY G.B., COHEN-WILLIAMS M.E., MORGAN C.A., PYLAK U., DUFFY R.A., LACHOWICZ J.E., CAREY G.J., COFFIN V.L. The gerbil elevated plus-maze II: anxiolytic-like effects of selective neurokinin NK1 receptor antagonists. Neuropsychopharmacology. 2002;27:371–379. doi: 10.1016/S0893-133X(02)00313-5. [DOI] [PubMed] [Google Scholar]
- VASSOUT A., VEENSTRA S., HAUSER K., OFNER S., BRUGGER F., SCHILLING W., GENTSCH C. NKP608: a selective NK-1 receptor antagonist with anxiolytic-like effects in the social interaction and social exploration test in rats. Regul. Pept. 2000;96:7–16. doi: 10.1016/s0167-0115(00)00194-4. [DOI] [PubMed] [Google Scholar]
- ZAMUNER S., GOMENI R., BYE A. Estimate the time varying brain receptor occupancy in PET imaging experiments using non-linear fixed and mixed effect modeling approach. Nucl. Med. Biol. 2002;29:115–123. doi: 10.1016/s0969-8051(01)00275-x. [DOI] [PubMed] [Google Scholar]
- ZOCCHI A., VARNIER G., ARBAN R., GRIFFANTE C., ZANETTI L., BETTELINI L., MARCHI M., GERRARD P.A., CORSI M. Effects of antidepressant drugs and GR 205171, a neurokinin-1 (NK1) receptor antagonist, on the response in the forced swim test and on monoamine extracellular levels in the frontal cortex of the mouse. Neurosci Lett. 2003;345:73–76. doi: 10.1016/s0304-3940(03)00305-7. [DOI] [PubMed] [Google Scholar]