Abstract
To find evidence for a connection between heat stress response, oxidative stress, and common stress tolerance, we studied the effects of elevated growth temperatures and heat stress on the activity and expression of ascorbate peroxidase (APX). We compared wild-type Arabidopsis with transgenic plants overexpressing heat shock transcription factor 3 (HSF3), which synthesize heat shock proteins and are improved in basal thermotolerance. Following heat stress, APX activity was positively affected in transgenic plants and correlated with a new thermostable isoform, APXS. This enzyme was present in addition to thermolabile cytosolic APX1, the prevalent isoform in unstressed cells. In HSF3-transgenic plants, APXS activity was detectable at normal temperature and persisted after severe heat stress at 44°C. In nontransgenic plants, APXS was undetectable at normal temperature, but could be induced by moderate heat stress. The mRNA expression profiles of known and three new Apx genes were determined using real-time PCR. Apx1 and Apx2 genes encoding cytosolic APX were heat stress and HSF dependently expressed, but only the representations of Apx2 mRNA met the criteria that suggest identity between APXS and APX2: not expressed at normal temperature in wild type, strong induction by heat stress, and HSF3-dependent expression in transgenic plants. Our data suggest that Apx2 is a novel heat shock gene and that the enzymatic activity of APX2/APXS is required to compensate heat stress-dependent decline of APX1 activity in the cytosol. The functional roles of modulations of APX expression and the interdependence of heat stress and oxidative stress response and signaling mechanisms are discussed.
There is increasing evidence for considerable interlinking between the responses to heat stress and oxidative stress. Both stresses induce pathways resulting in the expression/accumulation of heat shock proteins (HSP) in plants (Banzet et al., 1998; Dat et al., 1998; Schett et al., 1999; Lee et al., 2000) and, in fruit fly (Drosophila melanogaster), transient expression of small HSP (sHSP) decreases sensitivity of cells to heat and hydrogen peroxide stresses (Mehlen et al., 1993). On the other hand, there is also evidence that heat induces oxidative stress and/or expression of antioxidative enzymes in bacteria (Morgan et al., 1986), yeast (Davidson et al., 1996), and plants (Gong et al., 1998; Storozhenko et al., 1998; Lee et al., 1999). Thermotolerance can be generated by compounds that induce oxidative bursts, and very short heat pulses can induce bursts of superoxide and/or hydrogen peroxide (Vallelian-Bindschedler et al., 1998).
Reactive oxygen species (ROS) such as superoxide radicals, hydrogen peroxide, and hydroxyl radicals are continuously formed in aerobic organisms. Excess production of ROS causes oxidative damage of cellular components, and their involvement in a number of biotic and abiotic stresses is well documented (Bowler et al., 1992). Accumulation of hydrogen peroxide has not only negative consequences on living cells, but it is also involved in stress signaling, mediating the cellular redox status (Neill et al., 1999; Noctor et al., 2000). Ascorbate peroxidase (APX) is one of the most important antioxidant enzymes of plants that detoxifies hydrogen peroxide using ascorbate for reduction. Different isoforms are active in chloroplasts, cytosol, and microsomes.
Heat shock transcription factors (HSF) play a central role in stress-dependent and developmental expression of HSP in plants (for overview, see Schöffl et al., 1998a, 1998b). Plants appear to contain a larger number of different HSF than animals and the reason has remained unknown. Although human and animal cells express up to four different HSF, in Arabidopsis, more than 20 different Hsf genes have been identified at the DNA sequence level (Nover et al., 2001), at least eight of which are represented by cDNA clones (Schöffl and Prändl, 1999). Besides the structural differences and functional implications between different classes, there is no direct evidence for the activities and functions of individual HSF and their function in vivo. It is commonly accepted that the functions of different HSF are mediated through the expression and protective function of target genes, e.g. the genes encoding HSP. Although HSP are important for conferring stress tolerance, they cannot be the sole protective components, which are induced by heat stress. Stress-independent overexpression of HSP in transgenic plants was not sufficient to raise the basal level of thermotolerance by more than 2°C to 4°C (Lee et al., 1995; Prändl et al., 1998; Döhr et al., 2001). The levels of acquired thermotolerance, reached after a conditioning heat stress, were still significantly higher. There is also evidence that in Arabidopsis, HSF modifications and activities are regulated during the cell cycle (Reindl et al., 1997), which suggests that HSF is not only involved in the regulation of heat shock genes (encoding HSP) but may also be required under non-stress conditions. An involvement of HSF as playing a critical role in the cell defense against heat and oxidative stress has been demonstrated in yeast (Raitt et al., 2000), but not yet in plants.
There is only a very small number of reports identifying other than HSP-encoding genes whose expression in Arabidopsis is regulated by heat stress in an HSF-dependent fashion. One example is Apx1 (Storozhenko et al., 1998), linking the antioxidant pathway to heat stress-induced protection of common cellular functions. Apx1 is a member of a multigene family of APXs. In Arabidopsis, this family includes genes for two cytosolic isoforms, APX1 and APX2, microsomal enzyme APX3, chloroplastic stromal sAPX, and thylakoid-bound tAPX (Kubo et al., 1992; Santos et al., 1996; Jespersen et al., 1997; Zhang et al., 1997), which represent five of seven different types of APX proposed for higher plants from an expressed sequence tag database search (Jespersen et al., 1997). In different plants species, APX activity increases in response to a number of stresses, including drought, high light intensities, chilling, iron, and salt stress. At the level of gene expression, cytosolic Apx1 gene is induced by ozone, sulfur dioxide, excessive light (Kubo et al., 1995; Karpinski et al., 1997), and also by heat stress (Storozhenko et al., 1998). Induction by heat stress was attributed to heat shock element (HSE) sequences that are the binding site for HSF (Storozhenko et al., 1998). In contrast to Apx1, much less is known about the regulation of gene expression of the second cytosolic isoform, APX2. Using Apx2 promoter-luciferase reporter gene fusion, it has been shown that in transgenic plants, the luciferase activity is detected after exposure to excessive light treatment, probably induced by hydrogen peroxide, preferentially in the cells surrounding the vascular system (Karpinski et al., 1999). Activation of Apx2 gene expression was linked to a possibly systemic signaling by hydrogen peroxide, and it should be noted that this compound is involved the signaling of a number of environmental stress responses, including hypersensitive response, systemic acquired resistance, tolerance to chilling, and cross-tolerance to a variety to biotic and abiotic stresses (Neill et al., 1999; Scott et al., 1999; Noctor et al., 2000).
In this paper, we present evidence that heat stress triggers the expression of Apx2 gene at the mRNA level and this correlates with the appearance of a new APX isozyme, APXS, in Arabidopsis. The involvement of HSF in expression of Apx2 gene/APXS protein is indicated by the constitutive levels of mRNA and, respectively, APXS activity in HSF3-transgenic plants under non-stress conditions.
RESULTS
Heat Stress and HSF3 Overexpression Affect APX Activity
HSF3-transgenic plants show a lower threshold temperature for the expression of HSP than wild-type (WT) plants. At room temperature (22°C–24°C), these plants exhibit elevated mRNA levels of all heat shock genes tested, and significant levels of sHSP that is undetectable in WT plants under these conditions (Prändl et al., 1998). Upon closer inspection of the threshold temperature, we found that the optimum temperature for distinguishing the constitutive heat shock response of HSF3-transgenic plants from heat-induced HSP synthesis in WT was 28°C. We also noticed that HSF3-transgenic plants grown at 20°C lack sHSP mRNAs and proteins and are indistinguishable from WT plants in this respect.
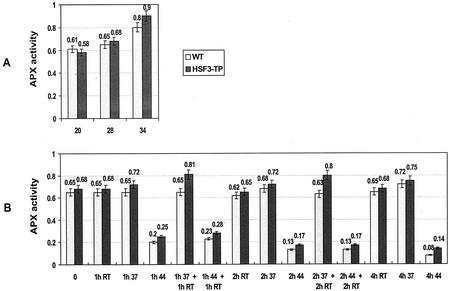
We analyzed the effects of elevated growth temperatures on APX activity in plants after 3 d of cultivation at 28°C and 34°C, respectively, compared with plants continuously grown at 20°C. In both lines, WT and HSF3-transgenic plants, the APX activities in total soluble protein extracts correlated positively with temperature (Fig. 1A). The total activity in leaves of 34°C plants was approximately 1.5 times higher than in plants grown at 20°C. The difference between WT and transgenic plants was not significant.
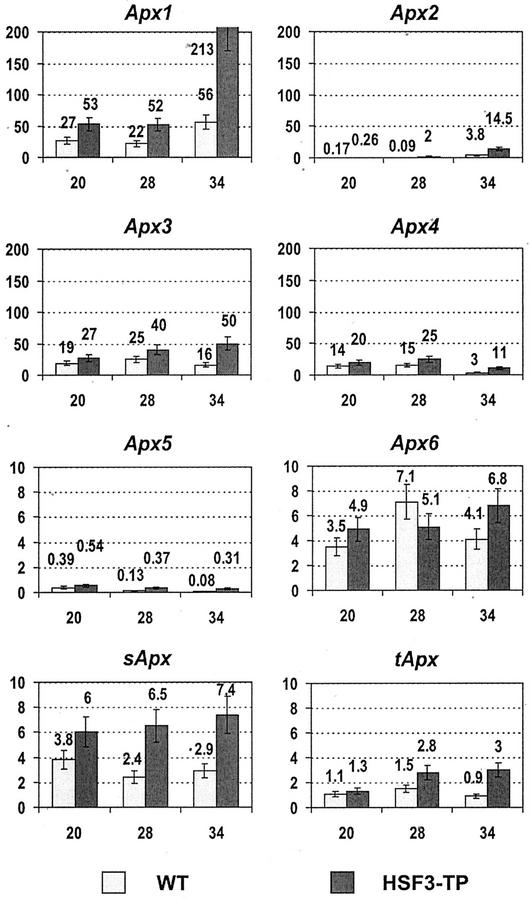
Figure 1.
Total soluble APX activities in leaves of WT and HSF3-transgenic plants (HSF3-TP) of Arabidopsis. A, After cultivation at elevated temperatures as indicated. B, After short-term heat treatments at 37°C (37) or 44°C (44) of plants precultivated at 28°C; RT, Incubation at room temperature; 0, control of fresh leaves without treatment. APX activity is expressed as micromoles of AsA oxidized per minute per milligram of protein; bars show means ± sd (n = 4).
Short-term heat stress at 37°C caused no significant differences in APX activity between WT and HSF3-transgenic plants (Fig. 1B); however, following recovery from 37°C heat stress, the APX activity increased approximately 20% in HSF3-transgenic plants, but not in WT plants. A short heat treatment at 44°C resulted in a marked decrease of APX activity after 4 h, down to 11% and 18% (compared with 100% of unstressed cells) in WT and HSF3-transgenic plants, respectively. The thermolability is characteristic for APX; the activities of other enzymes of the ascorbate-glutathione cycle (e.g. superoxide dismutase and glutathione reductase) are not affected by a prior heat stress of the leave tissue at 44°C (data not shown).
It should be noted that the measurements of APX activities in total protein extracts integrate the activities of the different APX isoenzymes. Soluble protein extracts used in our experiments contained cytosolic, microsomal, and stromal isoforms, whereas thylakoid APX should have been removed by centrifugation (Amako et al., 1994; Yamaguchi et al., 1995; Bunkelmann and Trelease, 1996). To evaluate proportion of membrane-bound APX, we examined the influence of the non-ionic detergent Triton X-100 on APX activity in extracts prepared from WT leaves cultivated at 28°C. For the same amount of plant material, the total enzymatic activity increased approximately 1.6-fold when the detergent was added prior to centrifugation. These data suggest that tAPX activity contributes approximately one-third to the total cellular APX activity in leaves of Arabidopsis, which is in good agreement with estimates reported for other species (Amako et al., 1994). Triton X-100 treatment had no negative effect on the level of APX activity if added after centrifugation to the soluble extract.
Detection of APXS, a Novel Heat Stress-Induced APX Isozyme
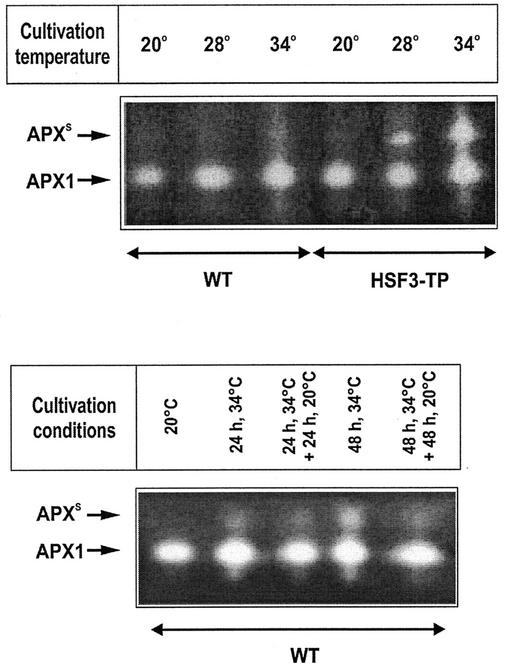
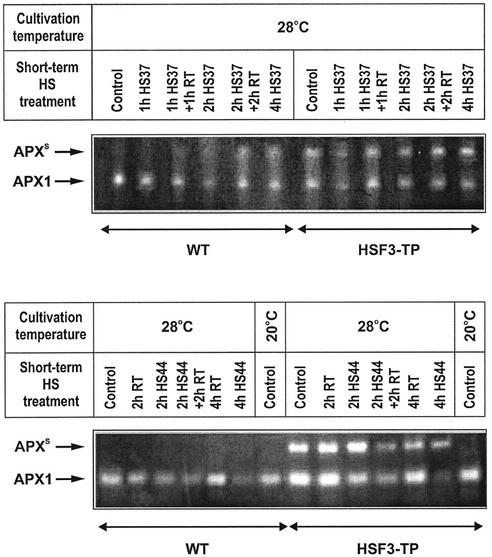
To further discriminate between the effects of elevated temperatures and heat stress on the activities of individual APX isoenzymes, we used native PAGE for separation of soluble proteins with subsequent in-gel staining of APX activity. Cultivation of plants at 28°C for 3 d or longer, compared with growth at 20°C, resulted in a profound change in the APX activity pattern in HSF3-transgenic plants, but not in WT. In WT, only one major band representing the cytosolic APX1 activity (Mittler and Zilinskas, 1993) was present at both temperatures, whereas in HSF3-transgenic plants, an additional slow-migrating band (APXS) appeared after cultivation at 28°C (Fig. 2). However, long-term cultivation (3 d) at 34°C (Fig. 2) or short-term (2 or 4 h) heat stress at 37°C (Fig. 3) led to the appearance of APXS in WT plants and to a remarkable increase of this band in HSF3-transgenic plants. When WT plants, induced for APXS by incubation at 34°C, were returned to 20°C, APXS activity was significantly reduced within 1 to 3 d (Fig. 2). Short-term recovery (1 or 2 h) from 37°C heat stress had only little effect on APXS (Fig. 3).
Figure 2.
APX isoenzyme activities in WT and HSF3-transgenic plants (HSF3-TP). Total protein extracts of leaves from plants cultivated at different temperatures and times as indicated were subjected to native PAGE followed by activity staining for APX according to Mittler and Zilinskas (1993). APXS, New slow-migrating APX isoform appearing after cultivation at elevated temperatures.
Figure 3.
Effects of short-term heat shock (HS) treatments at different temperatures on APX1 and APXS activities in leaves of WT and HSF3-transgenic plants (HSF3-TP). Plant were precultivated at 28°C or 20°C as indicated. Heat stress treatments were at 37°C (HS37) or 44°C (HS44). RT, Incubation at room temperature; Control, fresh leaves without treatments.
Heat stress at 44°C was unable to induce APXS in WT and also had negative effects on the activity of the APX1 band; after 4 h at 44°C, the band had nearly disappeared from the soluble protein extract (Fig. 3). The effect of 44°C heat treatment on APX1 was the same in HSF3-transgenic plants; however, in contrast to WT, the transgenic line showed APXS activity even after 4 h of incubation at 44°C, a condition that led to almost to a complete decline of APX1 activity (Fig. 3).
Heat- and HSF3-Dependent Changes of mRNA Levels of Apx Genes
The presence of APXS in HSF3-transgenic plants and its appearance after heat stress in WT indicated that there is a positive effect on the activity of a new thermostable isoform of APX. To test whether the appearance and induction of APXS correlates with changes in the expression of any given Apx gene, we investigated and quantified the mRNA levels of five known and three potential Apx genes of Arabidopsis. In addition to the published sequences, including the genes encoding the cytosolic APX isozymes, APX1 (Kubo et al., 1992) and APX2 (Santos et al., 1996), microsomal APX3 (Jespersen et al., 1997; Zhang et al., 1997), chloroplast stromal sAPX, and chloroplast thylakoid tAPX (Jespersen et al., 1997), we further analyzed and tested three other potential Apx genes of Arabidopsis, which we identified by database analysis. We designated these new genes Apx4, Apx5, and Apx6. The sequence of Apx6 was already available as cDNA (accession no. AV555486), suggesting expression of this gene. To test whether the new genes Apx4 and Apx5, identified as genomic sequences, are also expressed, we performed one-step reverse transcription (RT)-PCR of total RNA isolated from Arabidopsis leaf tissue using primers located near 5′- and 3′-termini on the predicted mRNA (gene-coding) sequences (Table I). PCR products were directly sequenced, and the sequences were identical with those predicted for the respective mRNAs from the genomic data (not shown). The successful amplification of cDNA representing the correctly processed forms of Apx4 and Apx5 mRNAs demonstrates that both genes are expressed in Arabidopsis.
Table I.
Genomic clones containing sequences of the new presumptive Apx genes of Arabidopsis and primers used for amplification of corresponding cDNA
| Genomic Clone, Genbank Accession No. | New Presumptive Apx Genes
|
RT-PCR Primer
|
GenBank Accession No. of cDNA Obtained in This Study | ||
|---|---|---|---|---|---|
| Whole names of the genes | Abbreviations used here | Name | Sequence | ||
| AL161513 | Ascorbate peroxidase 4 | Apx4 | Apx41 | 5′-TCCTTCCTTCACCAACACAACCAAT-3′ | AF441713 |
| Apx42 | 5′-CGGTTTCACGGCTTCTTTGATACTTC-3′ | ||||
| AL1 61588 | Ascorbate peroxidase 5 | Apx5 | Apx51 | 5′-AGACCTTCGAGCTCTCATCTCTTCC-3′ | AF441714 |
| Apx52 | 5′-CTTGCCTCTTCTGCTTGCTTCATAG-3′ | ||||
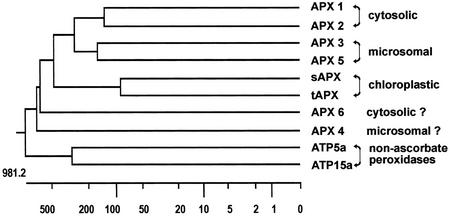
The deduced amino acid sequences of the APX proteins were compared with known APX enzymes and with sequences of non-APXs, ATP5a and ATP15a, of Arabidopsis as outgroup proteins (Fig. 4). The Apx5 sequence shows the highest degree of similarity with microsomal Apx3, particularly by the presence of similar N- and C-terminal signal sequences. Apx4 shares only little similarities with other Apx genes of Arabidopsis. A SKL motif (Subramani, 1998) localized in the C-terminal portion of APX4 suggests peroxisomal targeting, although this motif is absent in APX3 and APX5. Thus, Apx4 and Apx5 genes probably represent new microsomal isoenzymes. The putative coding region of Apx6 is shorter than the coding sequences of other Apx genes of Arabidopsis. The obvious lack of signal peptide sequences at N and C termini of the protein suggests that Apx6 represents another cytosolic isoenzyme.
Figure 4.
Protein sequences relationships within the APX family of Arabidopsis. Dendrogram was constructed applying J. Hein method with PAM250 residue weight table; presumptive N- and C-terminal signals, specific for proteins with different subcellular localization, were omitted. Protein sequences of non-APXs, ATP5a (X98809) and ATP15a (X99097), were used as outgroups.
Quantification of the mRNA levels of the eight different Apx genes was performed using real-time PCR of reverse transcripts of poly(A)+-mRNA samples of WT and HSF3-transgenic plants that had been subjected to the same temperature regimes as for the investigations of APX activities. The data were normalized with respect to the mRNA level of Act2, a housekeeping gene that is expressed at a relatively high level in Arabidopsis (An et al., 1996).
The mRNA levels of Apx genes differed markedly in WT plants grown at 20°C. Apx1, Apx3, and Apx4 mRNAs are much more abundant than mRNAs from Apx2 and Apx5 genes; mRNAs of Apx6, sApx, and tApx genes were present at intermediate levels (Fig. 5). At 20°C, the differences in mRNA levels between WT and transgenic plants were not significant.
Figure 5.
mRNA level for different Apx genes in leaves of WT and HSF3-transgenic plants (HSF3-TP) of Arabidopsis after cultivation at elevated temperatures. Poly(A)+-RNA was isolated from leaves, converted to cDNA, and subjected to real-time PCR. Relative amounts were calculated and normalized with respect to Act2 mRNA (=100%). Bars show means ± sd (n = 4–6). Note: Two different scales are used in graphs.
After long-term cultivation at elevated temperatures, the mRNAs of cytosolic Apx1 and Apx2 were significantly higher in WT and HSF3-transgenic plants at 34°C compared with 20°C (Fig. 5). In WT, the levels increased about 2-fold for APX1 and 22-fold for APX2; in HSF3-transgenic plants, Apx1 and Apx2 mRNAs increased by factors of 4 and 56, respectively. It is interesting that in HSF3-transgenic plants cultivated at 28°C, the level of Apx2 mRNA was already elevated by a factor of 8. The mRNA levels of the other six Apx genes remained unchanged or gradually decreased at elevated temperatures in WT and HSF3-transgenic plants. Only the mRNAs of Apx3 and tApx were slightly increased.
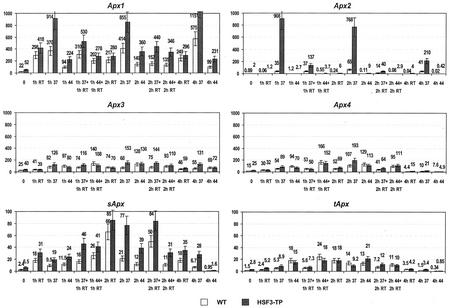
Following short-term heat treatment at 37°C, mRNA levels of Apx1 and Apx2 genes dramatically increased compared with unstressed plants (Fig. 6). The most pronounced induction is evident for Apx2 mRNA, showing a 300- to 1,000-fold increase at 37°C. The highest levels were obtained after 1 or 2 h of heat stress. After 4 h of heat stress or after heat stress followed by 1 or 2 h of recovery at room temperature, mRNA levels of Apx2 declined. Despite the heat inducibility of Apx2 mRNA, which was very similar in WT and HSF3-transgenic plants, the absolute transcript levels were very different. After 1 h of treatment at 37°C, the level in HSF3-transgenic plants was 26 times higher than in WT (35% compared with 908% with respect to Act2 mRNA standard = 100%). Similar but less pronounced was the effect of heat stress on transcript levels of the Apx1 gene (Fig. 6). There was only a 2- to 4-fold induction of mRNA levels by heat stress, and in HSF3-transgenic plants, the levels increased only by a factor of approximately 2. Changes in Apx1 and Apx2 mRNA levels after short-term heat stress at 44°C were not significant.
Figure 6.
mRNA level for different Apx genes in leaves of WT and HSF3-transgenic plants (HSF3-TP) of Arabidopsis after short-term heat treatments at 37°C (37) or 44°C (44) of plants precultivated at 28°C. RT, Incubation at room temperature; 0, control of fresh leaves without treatment. Poly(A)+-mRNA was isolated from leaves, converted to cDNA, and subjected to real-time PCR. Relative amounts were calculated and normalized with respect to Act2 mRNA (=100%). Bars show means ± sd (n = 4–6). Note: Two different scales are used in graphs.
Similar to Apx1 in WT after short-term heat stress at 37°C, the mRNA level of Apx4 was increased about two times (3-fold increase in HSF3-transgenic plants) compared with unstressed plants (Fig. 6). After heat stress at 44°C and subsequent recovery, the mRNA level increased for both microsomal enzymes (Apx3 about two times and Apx4 up to four times), and no difference was observed between WT and HSF3-transgenic plants.
The mRNA levels of sApx, tApx (Fig. 6), Apx5, Apx6 (data not shown) appear modulated to some extent by heat treatments, however, the magnitude of changes was much lower than for Apx1, Apx2, Apx3, Apx4, and differences between WT and HSF3-transgenic plants were not consistent. It should be noted that the maximum mRNA levels of chloroplastic enzymes (tAPX, sAPX) and APX5, APX6 were less than 10% of the levels reached for APX1, APX2.
APXS Is Not a Complex with HSP
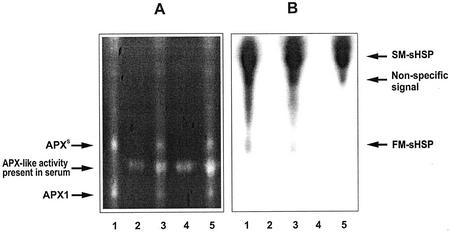
We rationalized that the appearance of APXS may be the result of HSF3-controlled expression of a heat stress-specific (thermostable) APX isozyme or, alternatively, is formed by association of an already existing, prevalent APX enzyme, e.g. APX1, with HSP chaperones stabilizing enzymatic activity. To test the chaperone hypothesis, we subjected a native gel stained for APX activity to western-blot analysis using antibodies against HSP90, HSP70, and sHSP. No differences were detectable between WT and HSF3-transgenic plants using antibodies against HSP90 and HSP70; the bands detected migrated in the native gel much slower than APX isoforms, APXS and APX1 (not shown). However, using anti-sHSP antibody, two additional bands were detected in HSF3-transgenic plants (Fig. 7), but not in WT plants (data not shown). The faster migrating band (FM-sHSP) comigrated with APXS. The comigration of APXS and sHSP suggested association and complex formation between both components. Therefore, we additionally examined whether immunological depletion of sHSP had an effect on the appearance of APXS. Anti-sHSP-antibodies, immobilized on protein A Sepharose (PAS), were used for immunoprecipitation of sHSP from total protein extracts that had been prepared from leaves of HSF3-transgenic plants grown at 28°C. Depleted extracts were tested for the presence of APXS and comigration with sHSP. Separation of sHSP-depleted extracts by native PAGE followed by APX activity staining and western-detection of sHSP showed (Fig. 7) that the FM-sHSP had been completely removed by the anti-sHSP treatment. Preimmune serum was unable to eliminate the FM-sHSP band; however, the APXS activity remained unaffected. The intensity of the APXS was approximately the same in crude extracts treated with preimmune serum and sHSP-depleted extracts. Hence, formation and activity APXS does not seem to require detectable levels of sHSP.
Figure 7.
APXS isoenzyme activity in immunodepleted extracts of leaves from HSF3-transgenic plants of Arabidopsis. A, APX gel activity staining; B, western detection of sHSP. Lane 1, Protein extracts (control); lane 2, preimmune serum; lane 3, protein extracts, depleted by preimmune serum; lane 4, anti-sHSP antiserum; lane 5, protein extracts, depleted by anti-sHSP antiserum. SM-sHSP, Slow-migrating sHSP band.
Effects of Temperature on Ascorbic Acid (AsA)/ Dehydroascorbic Acid (DHA) Levels
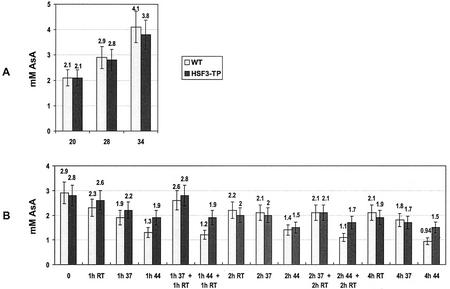
Long-term cultivation at elevated temperatures of WT and HSF3-transgenic plants resulted in a gradual increase (by a factor of 2 at 34°C) in the content of AsA in leaves (Fig. 8A); there was no difference between WT and HSF3-transgenic plants. On the other hand, after short-term treatments at 37°C, AsA levels declined in both lines (Fig. 8B). However, this decline was not only dependent on heat treatment because the controls incubated at room temperature also showed a decline, suggesting that the incubation of cut leaves in buffer caused a depletion of AsA. However, in WT, incubation at 44°C resulted in heat stress-dependent decrease of AsA content that is statistically significant.
Figure 8.
AsA content in leaves of WT and HSF3-transgenic plants (HSF3-TP) of Arabidopsis. A, After cultivation at elevated temperatures; B, after short-term heat treatments at 37°C (37) or 44°C (44) of plants precultivated at 28°C. RT, Incubation at room temperature; 0, control of fresh leaves without treatment. Bars show means ± sd (n = 4).
In WT and HSF-transgenic plants, the foliar concentration of DHA was approximately 0.3 ± 0.1 mm, irrespective of heat treatment. Considering the changes in concentration of AsA, the ratio of AsA/(AsA + DHA), reflecting intracellular redox state, increased during long-term acclimation to elevated temperature (0.81 at 20°C; 0.89 at 34°C) and declined after severe heat stress (0.65 after 4 h of treatment at 44°C in WT).
In summary, cultivation of Arabidopsis plants at elevated but non-stress temperatures led to the increase of APX enzymatic activity and of foliar concentration of its substrate, AsA. This suggests that the activation of AsA-dependent antioxidation system may be a preadaptive reaction to an enhanced production of ROS under severe stress. Heat stress-dependent decline of AsA after severe short-term heat stress applied in the darkness may reflect the lack of light-dependent photosynthetic electron transport that is required for regeneration of AsA from DHA in chloroplasts (Noctor et al., 2000).
DISCUSSION
Heat Induction of Apx Genes
It has been shown that mRNA levels of pea (Pisum sativum; Mittler and Zilinskas, 1992) and Arabidopsis (Storozhenko et al., 1998) Apx1 genes are induced by heat stress and oxidative stress, and there is evidence that in Arabidopsis, heat induction of Apx1 requires an HSE sequence present in the promoter upstream region of Apx1, which was shown to bind recombinant tomato (Lycopersicon esculentum) HSF1 in vitro (Storozhenko et al., 1998).
Our investigations of the expression of genes of the Apx family in Arabidopsis (five were previously described and three were newly identified by our present analysis), representing all seven types of known Apx genes, confirm the heat inducibility of Apx1 mRNA, but show a much stronger induction of Apx2 mRNA by heat stress. The expression of Apx2 correlates with the appearance of a new thermostable isoform APXS and both are coordinately up-regulated in HSF3-transgenic plants. In addition to the effects of moderate and severe heat stress (37°C and 44°C), we also evaluated the mRNA levels after long-term incubations at elevated temperatures (28°C and 34°C), and all data were compared between non-transgenic WT and HSF3-transgenic lines. It became clear that Apx2/APXS expression/activity follows a pattern of HSF-dependent gene expression that is a typical characteristic of heat shock genes in HSF3-transgenic plants.
mRNA Expression Profiles and Classification of Apx Genes
Our data suggest that based on the following criteria, the members of the Apx gene family can be subdivided into four groups: (1) genes encoding cytosolic APX1 and APX2, which are expressed at higher levels at elevated temperatures, are strongly induced by heat stress at 37°C but not at 44°C; Apx2 mRNA levels show the strongest dependence on enhancement of all Apx genes in HSF3-transgenic plants; (2) genes of microsomal APX3 and APX4 whose mRNA levels are increased after short-term heat stress at 37°C, but in contrast to Apx1/2 levels at 44°C are higher; no induction was observed after long-term cultivation at 34°C; (3) Apx5, which appears to be expressed at only very low levels at all temperatures tested; and (4) genes for chloroplastic tAPX, sAPX, and APX6, whose mRNAs show only little variation with growth temperatures and no clear response to heat stress; there are consistently higher levels of sApx mRNA levels present in HSF3-transgenic plants compared with WT.
HSF-Dependent Expression of Apx Genes
According to this classification, only the genes encoding cytosolic APX1 and APX2 are clearly identified as HSF-dependently expressed. The Apx2 mRNA level is already about 10-fold higher at 28°C and 140-fold higher at 34°C in HSF3-transgenic plants, 20 and four times higher, respectively, than in WT. Apx1 mRNA is only 2-fold higher in WT but 4-fold in HSF3-transgenic plants at 34°C compared with WT. We propose that this effect is due to a higher efficiency of transcription and/or higher stability of mRNA at the elevated temperatures. At 34°C, a low level of sHSP expression can be observed (Lohmann and F. Schöffl, unpublished data), suggesting that this is the threshold temperature of initiating a heat shock response in WT. The expression reaches the maximum at 37°C, and there is no expression of sHSP observed at 44°C. In HSF3-transgenic plants, this threshold temperature for HSP expression is lowered to about 25°C. Hence, only the mRNA levels of Apx1/Apx2 match the profile of HSF-dependent induction. It requires at least 3 d of cultivation at 28°C in HSF3-TP or 34°C in WT to reach constitutive expression of HSP. These are the same conditions that induce Apx2 expression and APXS enzymatic activity.
Heat stress treatments that are optimal for the induction of a heat shock response (1–2 h at 37°C) had a positive effect on the mRNA levels of all Apx genes tested; however, the effects differed qualitatively and quantitatively for different genes, and in a few cases, there were dramatic (Apx2) or noticeable (Apx1, Apx3, and Apx4) differences between WT and HSF3-transgenic plants. Apx2 mRNA is induced 750 and 640 times after 1 and 2 h, respectively, at 37° in HSF3-transgenic plants, and the level drops to 175-fold after 4 h. Very similar are the induction kinetics of Apx2 mRNA in WT; however, the maximum levels are only about 10% of the levels induced in HSF3-transgenic plants. These kinetics of a transient induction of mRNAs are a signature of the heat shock response. Another criterion of heat shock gene expression, the rapid decline of heat-induced mRNA during recovery, is only met by Apx1 and Apx2. Heat stress at 37°C followed by the recovery at room temperature for the same period of time resulted in about a 50% reduction of Apx1 and Apx4 mRNAs and about 90% reduction of Apx2 mRNA. A rapid reduction of Hsp-mRNA during recovery, with a half-life of approximately 1 h, has been originally described for soybean (Glycine max; Schöffl and Key, 1982). This decline was attributed to the rapid decay of sHsp-mRNA at normal temperature without replenishing the pool by transcription. The mRNA levels of all other Apx genes tested show, at maximum, an approximately 2-fold induction at 37°C, but subsequent recovery at room temperature had only very little, if any, effect. This is an indication that the modulations of the cellular mRNA levels of these genes result from molecular mechanisms that differ from transcriptional induction and decline of typical heat shock gene mRNA.
Inspections of the DNA sequences in the upstream promoter regions of Arabidopsis Apx1 (Storozhenko et al., 1998) and Apx2 genes (Santos et al., 1996) identified HSE-like sequences that were verified for Apx1 as HSF-dependent regulatory elements by mutational analysis, transgenic expression, and HSF binding and footprinting (Storozhenko et al., 1998). The HSE sequences present in Apx1/2 promoter regions deviate from the consensus motif nGAAnnTTCnnGAAn, but it is quite normal that other than canonical consensus sequences are functional in animals (Lardans et al., 2001) and in plants (Schöffl et al., 1989; Barros et al., 1992). It should be noted that in the promoter upstream regions of Apx3 and Apx5, HSE-like elements are present, each one showing at least one nucleotide deviation from the canonical consensus sequence. Due to the atypical pattern of induction and expression by heat stress and the lack of enhanced expression in HSF3-transgenic plants, we propose that these elements are not true sites for HSF binding and transcriptional regulation of Apx3 and Apx5.
HSF-dependent regulation of Apx1 and Apx2 genes is a first step toward the analysis of signaling pathways regulating gene expression. It is tempting to speculate that HSF may be involved not only in heat stress, but also in oxidative stress regulation of Apx expression. For Apx1, there is evidence that mutant HSE sequences have a strong negative effect on heat and also some effect on oxidative stress induction of expression. However, it cannot be excluded that alternative HSF regulators may recognize different HSE-like sequences and with different efficiencies under various stress conditions. In this context, it should be noted that more than 20 potential HSF genes were identified in Arabidopsis (Nover et al., 2001). There is also evidence that the redox status of the cell may have an influence on the activity and DNA-binding capacity of HSF (Manalo and Liu, 2001).
A New APX Isoform Is Expressed under Heat Stress Conditions
Our data show that in leaves of Arabidopsis, the total soluble APX activity increases after heat treatment at 34°C and 37°C. In HSF3-transgenic plants, the levels of APX activity are higher than in non-transgenic WT, especially during post-stress recovery. These quantitative effects correlate with the appearance of a new APX isoform, APXS, a slower migrating band identified after activity staining in native protein gels. In plants cultivated at 20°C, there is only one major isoform, the cytosolic APX1, detectable in gels (Mittler and Zilinskas, 1993). The activities of all other APX isoforms seem to be very labile even in the presence of stabilizing agents such as 5 mm AsA and 20% (w/v) sorbitol or 10% (w/v) glycerol during extraction, and are undetectable under the experimental conditions applied. In WT plants, APXS was detected after cultivation at 34°C or, alternatively, was induced by heat stress at 37°C if plants were precultured at 28°C. In HSF3-transgenic plants, APXS was also not present at 20°C, but was induced to high levels after growth at 28°C and further increased in plants grown at 34°C or heat stressed at 37°C. The activities were much higher than in WT after growth at 34°C or any other heat stress condition.
The question arose whether the APXS isoform represents a novel stress-dependent, heat-stable member of the APX family, or a stress-tolerant complex of APX1, the major enzyme present under non-stress conditions. The second possibility was taken into consideration because the increase in the intensity of APXS appeared to occur at the expenses of APX1 (Fig. 3). Could such a conversion of APX1 to APXS be the result of stress-induced conformational changes that stabilizes the activity of APX enzyme? We excluded this possibility because in HSF3-transgenic plants, APXS was already formed at 28°C, a condition that is unable to induce APXS or a heat stress response (e.g. the expression of HSP) in WT plants, but does so in HSF3-transgenic plants. This correlation between APXS activity and HSP appearance suggests that the expression of APXS and HSP is controlled by the same stimulus, in particular by HSF3 that is overexpressed in this line, or that the formation of APXS is a consequence of the constitutive level of HSP at 28°C, for example, forming a complex between APX and HSP chaperones. To date, there is evidence for the involvement of HSP90 in the formation of complexes with a number of cellular target proteins that have important functions in growth and development in animal and human cells (for overview, see Young et al., 2001) and HSP70 family proteins that are involved in the targeting of other proteins to subcellular compartments in different organisms, including plants (Lin et al., 2001). However, there is no direct experimental evidence for chaperone complex formation of any other HSP and native substrate protein under non-stress conditions in the cell. In our experiments, APXS comigrated in native gels only with sHSP, as indicated by western-blot analysis. sHSP cannot be causally related to APXS function because immunological depletion of sHSP had no detectable effect on the appearance and activity of APXS. We cannot entirely exclude the possibility that other proteins or chaperones interact with APX1 and convert it into a heat-stable complex; however, the close correlation between the appearance APXS and the induction of Apx2 mRNA by heat stress in WT and by HSF3 overexpression in transgenic plants at normal temperature suggests that APXS and APX2 are identical. Not only the heat- and HSF-dependent induction of both, but also the high levels of expression of Apx2 mRNA in HSF3 transgenic plants match very well the high levels of APXS activities in the absence of heat stress.
The conclusion that the slow migrating APXS represents the native form of APX2 is supported by the higher molecular mass of Arabidopsis APX2 protein (27,944 D) compared with APX1 (27,560 D) and the lower negative charge of APX2 (pI 6.1) versus APX1 (pI 5.9). It is unlikely that APXS represents a higher order oligomeric form of APX1 because conservation of structural features suggests that all cytosolic APX enzymes exist as dimers (Jespersen et al., 1997).
Thermostability of APX Enzymatic Activity
Of the enzymes involved in the ascorbate-glutathione cycle, the total cellular activity of APX appeared to be more sensitive to heat stress in planta compared with glutathione reductase and superoxide dismutase (J. Panchuk, R. Volkov, and F. Schöffl, unpublished data). After heat stress of leaves at 44°C, total APX activity decreased to 31% in WT and 35% in HSF3 transgenic plants in 1 h, and the activities declined to 12% and 21%, respectively, after 4 h. The thermostable APX activity remaining after severe heat stress appears to result from cytosolic APX enzymes because the intensities of cytosolic APX bands in stained native protein gel decrease less dramatically than total soluble APX activity that may also include microsomal and chloroplast localized APX enzymes. Of the two cytosolic isoforms, the heat-inducible APXS/APX2 appears to be much more thermostable than APX1. The higher level of total APX activity (after 4 h at 44°C) correlates with the higher level (intensity of band stained in native gels) of APXS in HSF3-transgenic plants. In this line, but not in WT plants, APXS had already accumulated during growth at 28°C, prior to heat stress. Under severe heat stress, the de novo synthesis of non-HSPs is largely inhibited (Key et al., 1981; Sachs and Ho, 1986), hence, the APX activities determined after 44°C treatment most probably reflect the enzymatic composition existing prior to heat stress. It should be noted that heat stress at 44°C was survived by HSF3-transgenic plants, but not by WT plants (Prändl et al., 1998), and it is tempting to speculate that besides the constitutive levels of HSP, the presence of APXS/APX2 also plays an important role in plant protection under stress.
Regulation and Functional Roles of APX
Heat-inducible transcriptional activation of cytosolic Apx genes corresponds with an increase in APX activity. Taking into consideration that production of ROS, in particular hydrogen peroxide, increases in response to abiotic stresses, including high temperature (Bartosz, 1997; Foyer et al., 1997; Smirnoff, 1998), it is conceivable that the increased expression/activity of theses enzymes is functionally linked to an increase in hydrogen peroxide concentration in the cytosol that has to be contained under heat stress. Bursts of hydrogen peroxide, induced by excess light, led to an increase in mRNA levels of Apx1 and Apx2 (Karpinski et al., 1997, 1999). The design of our experiments, showing the expression of these genes is also regulated by heat stress in a HSF-dependent fashion, suggests that overproduction of hydrogen peroxide in chloroplasts is not required because heat stress was administered in darkness. It is conceivable that hydrogen peroxide is generated during heat stress in other cellular compartments, e.g. in mitochondria or microsomes. The involvement of microsomes in heat stress response is indicated by improved heat tolerance of transgenic Arabidopsis overexpressing a microsomal Apx gene from barley (Hordeum vulgare; Shi et al., 2001).
Despite its toxic effects, it is well established that hydrogen peroxide also acts as a second messenger in the signaling induced by many abiotic and biotic stresses (Noctor and Foyer, 1998; Neill et al., 1999; Scott et al., 1999). After treatment with hydrogen peroxide, expression of sHSP (Banzet et al., 1998; Lee et al., 2000) and increased thermostability of plants was reported (Lopez-Delgado et al., 1998), indicating that hydrogen peroxide may be involved in signal transduction leading to the heat shock response. Thus, it seems possible that regarding the dual role of hydrogen peroxide, heat-inducible activation of APX isoenzyme may have also a dual function: protection against hydrogen peroxide and regulation of hydrogen peroxide-dependent signaling pathways, and it may be even involved in the regulation of the heat shock response. It has been shown that hydrogen peroxide-inducible expression of sHSP18.2 is mediated by mitogen-activated protein kinase signaling in Arabidopsis (Kovtun et al., 2000), suggesting that phosphorylation of transcription factors may play a role, as has been shown for yeast HSF under oxidative and heat stress (Liu and Thiele, 1996).
The involvement of HSF in the induction of Apx2/APXS expression indicates an interdependence between heat and oxidative stress signaling. The involvement of hydrogen peroxide in oxidative stress-dependent activation of the heat stress response in plants is not yet understood and will be the focus of future research.
MATERIALS AND METHODS
Plant Material, Cultivation, and Heat Treatments
Arabidopsis WT (ecotype Columbia 24) and HSF3-overexpressing transgenic plants, generated and described by Prändl et al. (1998), were used. Plants were routinely grown on soil in a dark/light cycle of 16/8 h at 20°C for 6 weeks, and were then kept under these conditions or the growth temperature was elevated to 28°C or 34°C for 3 d. Monitoring the expression of sHSP by western analysis, a sensitive indicator of the heat shock response, at least 3 d at 28°C was required for the derepression HSP synthesis in HSF3-transgenic plants. These conditions were not sufficient to induce the heat shock response in WT plants. For determinations of enzymatic activities, AsA/DHA content, and mRNA levels, 25 leaves of the same developmental state (from the middle of the rosette) were collected, subjected to the appropriate treatments, frozen in liquid nitrogen, and used for experiments.
The effects of short-term heat stress were determined on 7-week-old plants, including the final cultivation for 3 d at 28°C. Leaves were collected and incubated in section incubation buffer (1 mm potassium phosphate, pH 6.0, and 1% [w/v] Suc) in a shaking water bath (60 strokes min−1) at 37°C, 44°C, or at room temperature for 1, 2, or 4 h in the dark. The effects of post-stress recovery on Apx gene expression and enzymatic activities were examined after immediate incubation of heat-shocked leaves at room temperature for 1 or 2 h, respectively, as indicated.
Protein Extraction, APX Activity Measurement, and Gel Activity Staining
One-hundred milligrams of leaf tissue was ground in liquid nitrogen, mixed with 0.5 mL of extraction buffer containing 50 mm Na-phosphate (pH 7.0), 0.25 mm EDTA, 2% (w/v) polyvinylpyrrolidone-25, 10% (w/v) glycerol, and 1 mm AsA, and centrifuged at 14,000g for 10 min at 0°C. The supernatant (soluble fraction) was collected and immediately used for the estimation of APX activity in a reaction mixture that contained 25 mm Na-phosphate (pH 7.0), 0.1 mm EDTA, 1 mm H2O2, 0.25 mm AsA, and protein extract in a total volume 1 mL. The oxidation rate of AsA was assayed photometrically (UV/Visible Spectrophotometer Ultrospec III; Pharmacia LKB, Uppsala) by monitoring the decrease in A290 after 1 min of incubation following the addition of protein extract (Nakano and Asada, 1981; Amako et al., 1994). Protein concentration was determined using a Protein Assay system (Bio-Rad, Hercules, CA).
To monitor activity patterns of APX isoenzymes, protein extracts were subjected to native PAGE (12% [w/v] acrylamide) in the presence of 2 mm AsA and 10% (w/v) glycerol at 4°C for 1.5 h with subsequent staining of APX activity as described by Mittler and Zilinskas (1993).
Considering the high instability of APX in plant cell extracts, especially in the AsA-depleted medium (Nakano and Asada, 1987; Mittler and Zilinskas, 1993; Miyake and Asada, 1996), glycerol and AsA were added to all buffers used for protein extraction, APX activity measurements, and electrophoresis. Under these conditions, APX activity remained constant in total protein extracts for up to 1 h of incubation on ice, but decreased markedly after 3 to 4 h of incubation on ice or within a few minutes when incubated at room temperature.
AsA/DHA Assay
Leaf tissue was collected in liquid nitrogen, ground, thawed in 2 n HClO4, and incubated at for 20 min on ice. After centrifugation at 13,000g for 10 min, the supernatant was neutralized to pH 5.6 with 1.25 m K2CO3. Ascorbic acid was determined immediately by measuring the decrease in A265 upon addition of 3 units of ascorbate oxidase from Cucurbita sp. (Sigma, St. Louis) as described by Foyer et al. (1983).
DHA content was determined indirectly by measuring the formation of AsA after nonenzymatic reduction by 10 mm reduced glutathione in 0.1 m MES (pH 8.5) incubated for 15 min at 25°C. A265 was measured before and after incubation (Foyer et al., 1983).
Western Blot and Immunoprecipitation
Western-blotting experiments were performed according to standard protocols (Harlow and Lane, 1988; Sambrook et al., 1989). Rabbit antibodies against small Arabidopsis HSP (anti-sHSP antibody) was prepared in our laboratory (Lee et al., 1995), and commercial mouse anti-HSP70 antibody and anti-HSP90 antibody (Stress-Gene, San Diego) were used as primary antibody in combination with anti-rabbit or anti-mouse, respectively, peroxidase antibody conjugates (Sigma) as secondary antibody. The detection was performed by monitoring fluorescence generated by Chemoluminescence Reagent Plus (PerkinElmer Life Sciences, Boston).
For immunoprecipitation, 50 μL of PAS (Sepharose 4B Fast Flow; Sigma) equilibrated with incubation buffer (0.1 m sodium phosphate, pH 8.0, and 0.1 m EDTA) was mixed with 25 μL of anti-sHSP (25 μg μL−1) or with preimmune serum and was incubated for 1 h at room temperature under slow agitation and was then washed three times with incubation buffer. To check immobilization of antibodies, aliquots of PAS were incubated with Gly-HCl buffer, pH 3.0. Aliquots of 10 μL of each washing step were collected, protein concentration was determined, and samples were analyzed by SDS-PAGE.
PAS-immobilized antibodies were incubated with native protein extracts for 30 min at 4°C under slow agitation. Supernatants were collected and analyzed by native gel electrophoresis with subsequent APX activity staining. An aliquot of the same native protein extract, not subjected for immunoprecipitation, was loaded on the same gel. After activity staining, the gel was used in western-blot analysis for the detection of HSP.
DNA Sequence Database Analysis
Database searches were performed at the National Center of Biotechnology Information server (http://www.ncbi.nlm.nih.gov) with Entrez, BLAST (Altschul et al., 1997). Three new putative genes (Apx4, 5, and 6) were identified by sequence similarities to known Apx genes of Arabidopsis and/or other dicotyledonous plants, as described by Jespersen et al. (1997). Sequence alignment and dendrogram construction was performed using J. Hein or Clustal methods with Megalign software of the DNA-Star package (version 3.0; Madison, WI).
Amplification of cDNAs of APX4 and APX5
Total RNA prepared from leaves of WT with an RNeasy kit (Qiagen, Valencia, CA) was subjected to RT-PCR using a One-Step RT-PCR kit (Qiagen). Primer pairs used for amplification (Apx41 and Apx42 or Apx51 and Apx52, see Table I) were deduced from the genomic sequences of two new putative Apx genes identified in database (Arabidopsis Sequencing Project). PCR products were electrophoretically separated in agarose gels, purified with a Gel Band Purification kit (Amersham Biosciences, Uppsala), and sequenced using the same primers in combination with the Big Dye Terminator Cycle Sequencing kit and ABI Prism 310 sequencer (Applied Biosystems, Foster City, CA).
mRNA Isolation and cDNA Preparation for Real-Time PCR
Following heat stress treatments, total RNA was isolated as described above. RNA quantity was measured spectrophotometrically and its quality was checked by agarose-formaldehyde gel electrophoresis (Sambrook et al., 1989). Only RNA without detectable degradation of 26S rRNA was used for subsequent preparation of poly(A)+-mRNA using an Oligotex kit (Qiagen). Poly(A)+-mRNA was quantified using RiboGreen RNA Quantitation reagent (Molecular Probes, Leiden, The Netherlands) and 50 ng was converted into cDNA using anchored oligoT18-primer and RNase H-minus reverse transcriptase from ThermoScript RT-PCR System (Invitrogen, Carlsbad, CA). The amount of poly(A)+-mRNA/cDNA double-stranded products obtained after RT was measured using PicoGreen dsDNA Quantitation reagent (Molecular Probes, Eugene, OR).
Primer Design and PCR Product Identity
Primer pairs for real-time PCR were designed using Primer 3 Software (http://www.genome.wi.mit.edu/cgi-bin/primer/primer3.cgi), and gene sequences are available in GenBank (see Table II for details). Gene-specific primers were chosen so that the resulting PCR product had approximately the same size of 300 bp. The quality of PCR products was visually inspected by electrophoresis, and the generation of only one single band of the expected size was taken as a criterion for specificity. The identity of PCR products was confirmed by direct DNA sequencing.
Table II.
Real-time PCR primers used for evaluation of steady-state mRNA levels of APX and actin 2 (ACT2)
| Primer
|
||||
|---|---|---|---|---|
| Protein Name and Intracellular Localization | mRNA GenBank Accession No. | Name | Sequence | Position to Stop Codon |
| APX1, cytosolic | X59600 | Apx1A | 5′-GTCCATTCGGAACAATGAGGTTTGAC-3′ | −587 bp |
| Apx1B | 5′-GTGGGCACCAGATAAAGCGACAAT-3′ | −262 bp | ||
| APX2, cytosolic | X98275 | Apx2A | 5′-TGATGTGAAGACGAAGACAGGAGGAC-3′ | −610 bp |
| Apx2B | 5′-CCCATCCGACCAAACACATCTCTTA-3′ | −305 bp | ||
| APX3, microsomal | X98003 | Apx3A | 5′-CCCAAAATCACATACGCAGACCTGTA-3′ | −603 bp |
| X98276 | Apx3B | 5′-AGTTGTCAAACTTCAGCGGCTCTTG-3′ | −306 bp | |
| APX4, microsomal | AF441713 | Apx4A | 5′-CTACTAAATCCGGGGGAGCCAATG-3′ | −659 bp |
| Apx4B | 5′-CTCTGTTGCATCACTCCTTCCAAAAT-3′ | −337 bp | ||
| APX5, microsomal | AF441714 | Apx5A | 5′-AGCTAAACCGTCCACACAACAAAGGT-3′ | −653 bp |
| Apx5B | 5′-GTCCCAAAGTGTGACCTCCAGAGAGA-3′ | −354 bp | ||
| APX6, cytosolic | AV555486 | Apx6A | 5′-TGCAAAACGAAATAAGGAAAGTGGTG-3′ | −497 bp |
| AI995973 | Apx6B | 5′-CACTCAGGGTTTCTGGAGGTAGCTTG-3′ | −255 bp | |
| sAPX, chloroplastic stromal | X98925 | ApxSA | 5′-TGCTAATGCTGGTCTTGTGAATGCTT-3′ | −625 bp |
| ApxSB | 5′-CCACTACGTTCTGGCCTAGATCTTCC-3′ | −209 bp | ||
| tAPX, chloroplastic thylakoidal | X98926 | ApxTA | 5′-CAGAATGGGACTTGATGACAAGGAAA-3′ | −604 bp |
| ApxTB | 5′-ATGCAGCCACATCTTCAGCATACTTC-3′ | −306 bp | ||
| ACT2 | U41998 | Act2A | 5′-ACCTTGCTGGACGTGACCTTACTGAT-3′ | −592 bp |
| Act2B | 5′-GTTGTCTCGTGGATTCCAGCAGCTT-3′ | −295 bp | ||
| Act2C | 5′-ATTCAGATGCCCAGAAGTCTTGTTCC-3′ | −366 bp | ||
| Act2D | 5′-ACCACCGATCCAGACACTGTACTTCC-3′ | −99 bp | ||
For monitoring the degree of potential RNA degradation, two primer pairs spanning proximal and distal parts of the mRNA with respect to the translation stop-codon of the Act2 gene were used, and efficiencies were compared by real-time PCR. Intact mRNA, converted to full-length cDNA, resulted in the amplifications of PCR products with identical numbers of the threshold cycles (measured by real-time PCR), irrespective of the use of “distal” or “proximal” primer pairs (R. Volkov and F. Schöffl, unpublished data).
Real-Time PCR and Quantification of mRNA Levels
The real-time PCR was performed in 50 μL of reaction mixture composed of cDNA and master mix (final concentrations: 1 unit of Platinum Hot-Start Taq Polymerase [Invitrogen], 50 mm KCl, 3 mm MgCl2, 20 mm Tris, pH 8.4, 300 μm each dNTPs [Sigma], and 0.5 μm gene-specific primers) using an iCycler iQ system (Bio-Rad). Amplification of PCR products was monitored via intercalation of SYBR-Green (Molecular Probes; 1:250,000 dilution of 10,000× stock solution). The following program was applied: initial polymerase activation: 95°C, 10 min; then 35 cycles at 94°C, 20 s; 60°C, 50 s; 72°C, 30 s. PCR conditions were optimized for high amplification efficiency >95% for all primer pairs used. Efficiency was determined by comparison of experimentally determined and theoretically expected threshold values in dilution series of the same cDNA using 10, 1, 0.1, or 0.01 ng per reaction.
Statistical Methods
APX activity and AsA/DHA content were measured for at least four batches of plants in four replicates each. The differences between mean values were checked for significance using t test (Engel, 1997).
In real-time PCR experiments, two concentrations of cDNA (1 and 0.1 ng) were routinely measured in parallel, and duplicate samples were run for each concentration. All experiments were repeated at least two times for cDNA prepared for two batches of plants. Using standardized conditions, deviations of threshold values were less than 1.0 cycle for independent cDNA preparations and less than 0.5 cycle for replicates of the same cDNA. Changes by a factor of 2 or more in the relative concentrations of PCR-products/steady-state mRNA levels were statistically significant according to t test (Engel, 1997). Act2 mRNA, set 100%, was used as an internal standard in all experiments. Temperature-dependent variations in the steady-state levels of Act2 mRNA were determined (R. Volkov and F. Schöffl, unpublished data), and RT-PCR products/mRNA levels of Apx genes were normalized accordingly.
Footnotes
This work was supported in part by Sonderforschungsbereich 446 University of Tübingen of the Deutsche Forschungsgemeinschaft.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.001362.
LITERATURE CITED
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman D. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amako K, Chen G, Asada K. Separate assays for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994;35:497–504. [Google Scholar]
- An Y-Q, McDowell JM, Huang S, McKinney EC, Chambliss S, Meagher RB. Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J. 1996;10:107–121. doi: 10.1046/j.1365-313x.1996.10010107.x. [DOI] [PubMed] [Google Scholar]
- Banzet N, Richaud C, Deveaux Y, Kazmaier M, Gagnon J, Triantaphlides C. Accumulation of small heat shock proteins, including mitochondrial HSP22, induced by oxidative stress and adaptive response in tomato cells. Plant J. 1998;13:519–527. doi: 10.1046/j.1365-313x.1998.00056.x. [DOI] [PubMed] [Google Scholar]
- Barros MD, Czarnecka E, Gurley WB. Mutational analysis of a plant heat shock element. Plant Mol Biol. 1992;19:665–675. doi: 10.1007/BF00026792. [DOI] [PubMed] [Google Scholar]
- Bartosz G. Oxidative stress in plants. Acta Physiol Plant. 1997;19:47–64. [Google Scholar]
- Bowler C, Van Montagu M, Inzé D. Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:83–116. [Google Scholar]
- Bunkelmann JR, Trelease RN. Ascorbate peroxidase: a prominent membrane protein in oilseed glyoxysomes. Plant Physiol. 1996;110:589–598. doi: 10.1104/pp.110.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat JF, Foyer CH, Scott IM. Changes in salicylic acid and antioxidants during induction of thermotolerance in mustard seedlings. Plant Physiol. 1998;118:1455–1461. doi: 10.1104/pp.118.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JF, Whyte B, Bissinger PH, Schiestl RH. Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5116–5121. doi: 10.1073/pnas.93.10.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döhr S, Wunderlich M, Schöffl F. Derepression of the heat shock response in transgenic tobacco expressing Arabidopsis HSF1 fusion proteins. Recent Res Dev Plant Physiol. 2001;2:67–78. [Google Scholar]
- Engel J. Signifikante Schule der schlichten Statistik. Fürth, Germany: Filander Verlag; 1997. [Google Scholar]
- Foyer C, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant. 1997;100:241–254. [Google Scholar]
- Foyer C, Rowell J, Walker D. Measurement of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta. 1983;157:239–244. doi: 10.1007/BF00405188. [DOI] [PubMed] [Google Scholar]
- Gong M, Li YJ, Chen SZ. Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J Plant Physiol. 1998;153:488–496. [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Jespersen H, Kjaersgard I, Ostergaard L, Welinder K. From sequence analysis of three novel ascorbate peroxidases from Arabidopsis thaliana to structure, function and evolution of seven types of ascorbate peroxidase. Biochem J. 1997;326:305–310. doi: 10.1042/bj3260305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell. 1997;9:627–640. doi: 10.1105/tpc.9.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S, Reynolds B, Karpinska B, Wingsle G, Creissen G, Mullineaux P. The role of hydrogen peroxide and antioxidants in systemic acclimation to photo-oxidative stress in Arabidopsis. In: Smallwood MF, Calvert CM, Bowles DJ, editors. Plant Responses to Environmental Stress. Oxford: Bios Scientific Publishers; 1999. pp. 25–32. [Google Scholar]
- Key JL, Lin CY, Chen YM. Heat shock proteins of higher plants. Proc Natl Acad Sci USA. 1981;78:3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Hikaru S, Tanaka K, Tanaka K, Kondo N. Cloning and sequencing of a cDNA encoding ascorbate peroxidase from Arabidopsis thaliana. Plant Mol Biol. 1992;18:691–701. doi: 10.1007/BF00020011. [DOI] [PubMed] [Google Scholar]
- Kubo A, Saji H, Tanaka K, Kondo N. Expression of Arabidopsis cytosolic ascorbate peroxidase gene in response to ozone or sulfur dioxide. Plant Mol Biol. 1995;29:479–489. doi: 10.1007/BF00020979. [DOI] [PubMed] [Google Scholar]
- Lardans V, Ram D, Lantner F, Ziv E, Schechter I. Differences in DNA-sequence recognition between the DNA-binding domain fragment and the full-length molecule of the heat-shock transcription factor of schistosome. Biochim Biophys Acta. 2001;1519:230–234. doi: 10.1016/s0167-4781(01)00220-2. [DOI] [PubMed] [Google Scholar]
- Lee BH, Won SH, Lee HS, Miyao M, Chung WI, Kim IJ, Jo J. Expression of the chloroplast-localized small heat shock protein by oxidative stress in rice. Gene. 2000;245:283–290. doi: 10.1016/s0378-1119(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim KY, You SH, Kwon SY, Kwak SS. Molecular characterization and expression of a cDNA encoding copper/zinc superoxide dismutase from cultured cells of cassava (Mannihot esculenta Crantz) Mol Gen Genet. 1999;262:807–814. doi: 10.1007/pl00013819. [DOI] [PubMed] [Google Scholar]
- Lee JH, Hübel A, Schöffl F. Derepression of the activity of genetically engineered heat shock factor causes constitutive synthesis of heat shock proteins and increased thermal tolerance in transgenic Arabidopsis. Plant J. 1995;8:603–612. doi: 10.1046/j.1365-313x.1995.8040603.x. [DOI] [PubMed] [Google Scholar]
- Lin B-L, Wang J-S, Liu H-C, Chen R-W, Meyer Y, Barakat A, Delseny M. Genomic analysis of the Hsp 70 superfamily in Arabidopsis thaliana. Cell Stress Chaperones. 2001;6:173–296. doi: 10.1379/1466-1268(2001)006<0201:gaoths>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XD, Thiele DJ. Oxidative stress induces heat shock factor phosphorylation and HSF-dependent activation of yeast metallothionein gene transcription. Genes Dev. 1996;10:592–603. doi: 10.1101/gad.10.5.592. [DOI] [PubMed] [Google Scholar]
- Lopez-Delgado H, Dat JF, Foyer CH, Scott IA. Induction of thermotolerance in potato microplants by acetylsalicylic acid and H2O2. J Exp Bot. 1998;49:713–720. [Google Scholar]
- Manalo DJ, Liu AY-C. Resolution, detection, and characterization of redox conformers of human HSF1. J Biol Chem. 2001;276:23554–23561. doi: 10.1074/jbc.M011300200. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Briolay J, Smith L, Diaz-Latoud C, Fabre N, Pauli D, Arrigo AP. Analysis of the resistance to heat and hydrogen peroxide stresses in COS cells transiently expressing wild type of deletion mutants of the Drosophila 27-kDa heat shock protein. Eur J Biochem. 1993;215:277–284. doi: 10.1111/j.1432-1033.1993.tb18032.x. [DOI] [PubMed] [Google Scholar]
- Mittler R, Zilinskas A. Molecular cloning and characterization of gene encoding pea cytosolic ascorbate peroxidase. J Biol Chem. 1992;267:21802–21807. [PubMed] [Google Scholar]
- Mittler R, Zilinskas A. Detection of ascorbate peroxidase activity in native gels by inhibition of the ascorbate-dependent reduction of nitroblue tetrazolium. Anal Biochem. 1993;212:540–546. doi: 10.1006/abio.1993.1366. [DOI] [PubMed] [Google Scholar]
- Miyake C, Asada K. Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate: Hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant Cell Physiol. 1996;37:423–430. [Google Scholar]
- Morgan RW, Christman MF, Jacobson FS, Stroz G, Ames BN. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci USA. 1986;83:8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano J, Asada K. Purification of ascorbate peroxidase in spinach chloroplasts: its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987;28:131–140. [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Neill S, Desikan R, Clarke A, Hancock J. H2O2 signalling in plant cells. In: Smallwood MF, Calvert CM, Bowles DJ, editors. Plant Responses to Environmental Stress. Oxford: Bios Scientific Publishers; 1999. pp. 59–64. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Noctor G, Veljovic-Jovanovic S, Foyer CH. Peroxide processing in photosynthesis: antioxidant coupling and redox signalling. Phil Trans R Soc Lond B. 2000;355:1465–1475. doi: 10.1098/rstb.2000.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L, Bharti K, Döring P, Mishra SK, Ganguli A, Scharf KD. Arabidopsis and the heat stress transcription factor world: How many heat stress transcription factors do we need? Cell Stress Chaperones. 2001;6:177–189. doi: 10.1379/1466-1268(2001)006<0177:aathst>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prändl R, Hinderhofer K, Eggers-Schumacher G, Schöffl F. HSF3, a new heat shock factor from Arabidopsis thaliana, derepresses the heat shock response and confers thermotolerance when overexpressed in transgenic plants. Mol Gen Genet. 1998;258:269–278. doi: 10.1007/s004380050731. [DOI] [PubMed] [Google Scholar]
- Raitt C, Johnson AL, Erkine AM, Makino K, Morgan B, Gross DS, Johnston HL. The Skn7 response regulator of Saccharomyces cerevisiae interacts with Hsf1 in vivo and is required for the induction of heat shock genes by oxidative stress. Mol Biol Cell. 2000;11:2335–2347. doi: 10.1091/mbc.11.7.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reindl A, Schöffl F, Schell J, Koncz C, Bako L. Phosphorylation by a cycline-dependent kinase modulates DNA binding of the Arabidopsis heat shock transcription factor HSF1 in vitro. Plant Physiol. 1997;115:93–100. doi: 10.1104/pp.115.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs MM, Ho THD. Alteration of gene expression during environmental stress in plants. Rev Plant Physiol. 1986;37:363–376. [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Santos M, Gouisseau H, Lister C, Foyer C, Creissen G, Mullineaux P. Cytosolic ascorbate peroxidase from Arabidopsis thaliana L. is encoded by a small multigene family. Planta. 1996;198:64–69. doi: 10.1007/BF00197587. [DOI] [PubMed] [Google Scholar]
- Schett G, Steiner CW, Groger M, Winkler S, Graninger W, Smolen J, Su Q, Steiner F. Activation of Fas inhibits heat induced activation of HSF1 and up-regulation of HSP70. FASEB J. 1999;13:833–842. doi: 10.1096/fasebj.13.8.833. [DOI] [PubMed] [Google Scholar]
- Schöffl F, Key JL. An analysis of mRNAs for a group of heat shock proteins of soybean using cloned cDNAs. J Mol Appl Gen. 1982;1:301–314. [PubMed] [Google Scholar]
- Schöffl F, Prändl R. Derepression of the heat shock protein synthesis in transgenic plants. In: Smallwood MF, Calvert CM, Bowles DJ, editors. Plant Responses to Environmental Stress. Oxford: Bios Scientific Publishers; 1999. pp. 65–73. [Google Scholar]
- Schöffl F, Prändl R, Reindl A. Regulation of the heat shock response. Plant Physiol. 1998a;117:1135–1141. doi: 10.1104/pp.117.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F, Prändl R, Reindl A. Molecular responses to heat stress. In: Shinozaki K, Yamaguchi-Shinozaki K, editors. Molecular Responses to Cold, Drought, Heat and Salt Stress in Higher Plants. R.G. Austin, TX: Landes; 1998b. pp. 81–98. [Google Scholar]
- Schöffl F, Rieping M, Baumann G, Bevan M, Angermüller S. The function of plant heat shock promoter elements in the regulated expression of chimeric genes in transgenic tobacco. Mol Gen Genet. 1989;217:246–253. doi: 10.1007/BF02464888. [DOI] [PubMed] [Google Scholar]
- Scott IM, Dat JF, Lopez-Delgado H, Foyer CH. Salicylic acid and hydrogen peroxide in abiotic stress signalling in plants. Plant Physiol. 1999;39:13–17. [Google Scholar]
- Shi WM, Muramato Y, Ueda A, Takabe T. Cloning of peroxisomal ascorbate peroxidase gene from barley and enhanced thermotolerance by overexpressing in Arabidopsis thaliana. Gene. 2001;273:23–27. doi: 10.1016/s0378-1119(01)00566-2. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. Plant resistance to environmental stress. Curr Opin Biotechnol. 1998;9:214–219. doi: 10.1016/s0958-1669(98)80118-3. [DOI] [PubMed] [Google Scholar]
- Storozhenko S, De Pauw P, Van Montague M, Inzè D, Kushnir S. The heat-shock element is a functional component of the Arabidopsis APX1 gene promotor. Plant Physiol. 1998;118:1005–1014. doi: 10.1104/pp.118.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S. Components involved in peroxisome import, biogenesis, proliferation, turnover and movement. Physiol Rev. 1998;78:171–188. doi: 10.1152/physrev.1998.78.1.171. [DOI] [PubMed] [Google Scholar]
- Vallelian-Bindschedler L, Schweizer P, Mosinger E, Metraux JP. Heat-induced resistance in barley to powdery mildew (Blumeria graminis f. sp. Hordei) is associated with busts of AOS. Physiol Plant Pathol. 1998;52:165–199. [Google Scholar]
- Yamaguchi K, Mori H, Nishimura M. A novel isoenzyme of ascorbate peroxidase localized on glyoxysomal and leaf peroxisomal membranes in pumpkin. Plant Cell Physiol. 1995;36:1157–1162. doi: 10.1093/oxfordjournals.pcp.a078862. [DOI] [PubMed] [Google Scholar]
- Young JC, Moarefi I, Hartl FU. Hsp 90: a specialized but essential protein-folding tool. J Cell Biol. 2001;154:267–273. doi: 10.1083/jcb.200104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang J, Nickel U, Allen R, Goodman H. Cloning and expression of an Arabidopsis gene encoding a putative peroxisomal ascorbate peroxidase. Plant Mol Biol. 1997;34:967–971. doi: 10.1023/a:1005814109732. [DOI] [PubMed] [Google Scholar]