Abstract
There is direct chemical evidence that L-β,β-dimethylcysteine (L-penicillamine (L-PEN)) is a scavenger of peroxynitrite. The aim of this study was to determine whether L-PEN attenuates the haemodynamic responses elicited by peroxynitrite in pentobarbital-anaesthetized rats.
Peroxynitrite (1–20 μmol kg−1, i.v.) elicited dose-dependent reductions in mean arterial blood pressure (MAP) and mesenteric and hindquarter vascular resistances.
L-PEN (2 mmol kg−1, i.v.) elicited relatively minor but significant increases in MAP and vascular resistances. The initial reductions in MAP and vascular resistances elicited by peroxynitrite were not diminished after administration of L-PEN whereas they were much shorter in duration. As such, the total reductions in MAP and vascular resistances were markedly reduced by L-PEN.
The finding that L-PEN (2 mmol kg−1, i.v.) did not affect the hypotensive or vasodilator responses elicited of the ATP-dependent potassium-channel agonist, cromakalim (3–18 μg kg−1, i.v.), suggests that this dose of L-PEN is not a nonselective inhibitor of vasodilation.
These findings suggest that L-PEN may effectively scavenge peroxynitrite in vivo and/or interfere with the mechanisms by which peroxynitrite elicits its vasodilator responses.
Keywords: Peroxynitrite, nitric oxide (NO); L-penicillamine; haemodynamics; anaesthetized rats
Introduction
There is now considerable in vitro evidence that peroxynitrite, formed by the reaction of nitric oxide with superoxide anion (Beckman et al., 1990; Huie & Padmaja, 1993), is synthesized in a variety of cell types (see Benkusky et al., 1998; 1999). Endogenous peroxynitrite exerts effects on a numerous biological systems (see Benkusky et al., 1998; 1999) by mechanisms involving the oxidation of protein and nonprotein sulphydryls (Radi et al., 1991a) and membrane phospholipids (Radi et al., 1991b), and nitration of protein-associated tyrosines and other phenolic residues (Beckman et al., 1992; Ischiropoulos et al., 1992; 1995; Gow et al., 1996).
Peroxynitrite relaxes isolated coronary (Liu et al., 1994; Villa et al., 1994), pulmonary (Wu et al., 1994) and cerebral (Wei et al., 1996) arteries by mechanisms including the activation of ATP-dependent K+ channels (K+ATP-channels) (Wei et al., 1996) and generation of S-nitrosothiols (Moro et al., 1994, 1995; Wu et al., 1994). The mechanisms by which peroxynitrite activates K+ATP-channels are not known. However, it is unlikely that the oxidant properties of peroxynitrite are involved since oxidants reduce K+ATP-channel activity (Islam et al., 1993; Han et al., 1996). Systemic injections of peroxynitrite elicit pronounced falls in mean arterial blood pressure (MAP) and vascular resistances in pentobarbital-anaesthetized rats (Kooy & Lewis, 1996a; Benkusky et al., 1998; 1999; Graves et al., 1998; 2005a, 2005b). The peroxynitrite-induced vasodilator responses in these rats are markedly attenuated by the K+ATP-channel blocker, glibenclamide (Graves et al., 2005a) whereas they are not attenuated by a dose of L-β,β-dimethyl-cysteine (L-penicillamine, L-PEN, 500 μmol kg−1, i.v.), which markedly reduces the vasodilator responses elicited by the S-nitrosothiol, L-S-nitrosocysteine (Graves et al., 1998). Systemic injections of a 500 or 1000 μmol kg−1 doses of L-PEN elicited immediate and short-lived falls in MAP and vascular resistances in pentobarbital-anaesthetized rats (Graves et al., 1998). Resting haemodynamic parameters returned to preinjection values within 5–7 min and remained at these values for at least 90 min after injection of the 500 μmol kg−1 dose. However, resting mesenteric (MR) and hindquarter vascular resistances (HQR) gradually increased to levels above preinjection values (+51±13 and +35±11%, respectively) after recovery from the initial effects of the 1000 μmol kg−1 dose. These vasoconstrictor effects reached their plateau levels within 25–30 min and were sustained for at least 90 min (Graves et al., 1998). The mechanisms by which L-PEN exerts its initial vasodilator actions have not been determined although these effects may be related to the ability of L-PEN to act as a reducing agent (see Graves et al., 1998). The mechanisms responsible for the delayed vasoconstrictor effects of L-PEN have not been fully elucidated but may involve the blockade of the recognition sites by which endothelium-derived L-S-nitrosocysteine exerts its vasodilator actions (see Graves et al., 1998). The above findings suggest that the vasodilator actions of peroxynitrite in vivo involve activation of K+ATP-channels but not the formation of circulating S-nitrosothiols although it is possible that peroxynitrite initiates the formation of an S-nitrosothiol such as S-nitrosoglutathione whose actions are not blocked by L-PEN.
The increased production of peroxynitrite occurs in animal models of endotoxemia and acute lung injury and is implicated in the pathophysiology of human inflammatory diseases (see Benkusky et al., 1998, 1999). Tachyphylaxis rapidly develops to the vasorelaxant effects of peroxynitrite upon application to rat coronary arteries (Villa et al., 1994) or upon systemic injection in anaesthetized rats (Kooy et al., 1996; Kooy & Lewis, 1996a; Benkusky et al., 1998; Graves et al., 2005b). The changes in vascular tone elicited by cromakalim (Graves et al., 2005b), S-nitrosothiols (Villa et al., 1994) and some G protein-coupled receptor agonists (Benkusky et al., 1998; 1999) are impaired after the development of tachyphylaxis to peroxynitrite. The deleterious effects of peroxynitrite may involve the oxidation and/or nitration of functionally important amino-acid residues in K+ATP-channels (Nelson et al., 1990), S-nitrosothiol recognition sites (Hoque & Lewis, 1999) and G protein-coupled receptors (Harden, 1983).
As mentioned above, the vasodilator actions of peroxynitrite were not affected by the prior injection of a 500 μmol kg−1 dose of L-PEN (Graves et al., 1998). However, Althaus et al. (1994) provided chemical evidence that L-PEN directly scavenges peroxynitrite. More specifically, Althaus et al. (1994) demonstrated that L-PEN directly reacts with peroxynitrite to form a single S-nitro-L-PEN adduct. The development of compounds that scavenge peroxynitrite in vivo has important implications in the treatment of inflammatory states that generate peroxynitrite (see Benkusky et al., 1998; 1999). In pilot studies, we determined that a 1 mmol kg−1 dose of L-PEN partially attenuated the haemodynamic actions of peroxynitrite in pentobarbital-anaesthetized rats. As such, the objectives of this study were to determine the effect of a 2 mmol kg−1 dose of L-PEN on the haemodynamic actions of peroxynitrite and to establish that this effect of L-PEN was not due to the inhibition of K+ATP-channels. The hypothesis of this study was that the 2 mmol kg−1 dose of L-PEN would attenuate the haemodynamic actions of peroxynitrite by directly scavenging this compound in the blood.
Methods
Surgical procedures
All studies were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised in 1996. The protocols were approved by our Institutional Animal Care and Use Committees of the University of Iowa and the University of Georgia. Male Sprague–Dawley rats (250–350 g) were anaesthetized with pentobarbital (50 mg kg−1, i.p.). A catheter was placed in a femoral vein to give drugs and a catheter was placed in a femoral artery to record MAP. Doses of pentobarbital (3–5 mg, i.v.) were given to maintain anaesthesia as necessary. A midline laparotomy was performed and miniature pulse Doppler flow probes were placed around the superior mesenteric artery and the lower abdominal aorta to measure mesenteric and hindquarter blood flow velocities, respectively, and to determine MR and HQR (Benkusky et al., 1998; 1999). Vascular resistances were calculated by the formula, Resistance=MAP divided by blood flow velocity. It should be noted that the derived vascular resistance values were estimates rather than exact values since actual blood flow values and venous pressures were not determined. The Doppler technique, including the reliability of the method for estimation of flow velocity and determination of percent changes in vascular resistances has been described by Haywood et al. (1981).
The body temperatures of all rats were maintained at 37°C during surgery and experimentation via a rectal thermometer connected to a thermostatically controlled heating pad. The rats breathed room air supplemented with a low flow rate (5 ml min−1) of 95% O2–5% CO2 via a face mask. Supplementation with 95% O2–5% CO2 maintains blood pH (7.23–7.26, 7.24±0.01), blood pO2 (93–99 mmHg, 97±2) and blood pCO2 (34–38 mmHg, 36±2) values in pentobarbital-anaesthetized rats at levels similar to those of conscious rats (see Whalen et al., 1999). Blood pH and blood gases were determined 30 min after injection of saline (n=3) or L-PEN (n=3). The values fell within the ranges described above. More specifically, pH, pO2 and pCO2 values in the saline-treated rats were 7.23±0.01, 96±2 mmHg and 34±2 mmHg, respectively. The pH, pO2 and pCO2 values in the L-PEN-treated rats were 7.22±0.01, 95±2 and 35±2 mmHg, respectively.
Experimental protocols
Some rats received two identical series of peroxynitrite injections before (first series) and after (second series) administration of either L-PEN or saline. The first injection of peroxynitrite in the second series of peroxynitrite was given at 60 min, that is, 30 min after the injection of L-PEN. The subsequent injections of peroxynitrite in the second series of injections were given at 65, 70, 75 and 80 min, respectively. In one group of rats (n=6), bolus injections of peroxynitrite (1–20 μmol kg−1) were given at 5 min intervals. The 5 min interval ensured that responses elicited by each injection had subsided fully before another injection was given. At 10 min after the last injection of peroxynitrite was given, the rats received a bolus injection of L-PEN (2 mmol kg−1). The first injection of peroxynitrite was given 25–30 min after the injection of L-PEN to allow L-PEN to be fully distributed within the cardiovascular system. An example of the sequence of injections in a rat that received injections of peroxynitrite before and after injection of L-PEN is shown in Table 1. As can be seen, the five injections of peroxynitrite were given at times 0, 5, 10, 15 and 20 min, respectively. The injection of L-PEN was given at 30 min which was 10 min after the last injection of peroxynitrite. The first injection of peroxynitrite was given at 60 min, which was 30 min after the injection of L-PEN. The subsequent injections of peroxynitrite were given at 65, 70, 75 and 80 min. In three rats in the above group, the order of injection of peroxynitrite before and after injection of L-PEN was 1, 2.5, 5, 10 and 20 μmol kg−1. In the other three rats, the order of injection was 20, 10, 5, 2.5 and 1 μmol kg−1.
Table 1.
Injection protocol in a rat that received bolus injections of peroxynitrite before and after administration of L-PEN
| Time (min) | Injection | Dose |
|---|---|---|
| 0 | Injection 1 of peroxynitrite | 1 μmol kg−1 |
| 5 | Injection 2 of peroxynitrite | 2.5 μmol kg−1 |
| 10 | Injection 3 of peroxynitrite | 5 μmol kg−1 |
| 15 | Injection 4 of peroxynitrite | 10 μmol kg−1 |
| 20 | Injection 5 of peroxynitrite | 20 μmol kg−1 |
| 30 | Bolus injection of L-PEN | 2 mmol kg−1 |
| 60 | Injection 1 of peroxynitrite | 1 μmol kg−1 |
| 65 | Injection 2 of peroxynitrite | 2.5 μmol kg−1 |
| 70 | Injection 3 of peroxynitrite | 5 μmol kg−1 |
| 75 | Injection 4 of peroxynitrite | 10 μmol kg−1 |
| 80 | Injection 5 of peroxynitrite | 20 μmol kg−1 |
In other rats (n=6), injections of peroxynitrite (1–20 μmol kg−1) were given 5 min apart before and beginning 25–30 min after a bolus injection of saline. The order of injection of peroxynitrite was identical to that in the rats that received L-PEN. In other rats, injections of the K+ATP-channel agonist, cromakalim (3–18 μg kg−1) (see Nelson et al., 1990), were given before and beginning 25–30 min after injection of saline (n=6) or L-PEN (2 mmol kg−1, n=6). The injections of cromakalim were given 5–10 min apart to allow the effects of each injection to subside completely before another injection was given. In three rats in each group, the order of injection of cromakalim was 3, 9 and 18 μg kg−1. In the other three rats, the order of injection was 18, 9 and 3 μg kg−1. In other groups of rats, bolus injections of decomposed peroxynitrite (d-peroxynitrite; equal volumes to those of 5, 10 and 20 μmol kg−1 of peroxynitrite) were given at 5 min intervals before and beginning 25–30 min after injection of L-PEN (2 mmol kg−1, n=6) or saline (n=6). The order of injection in three rats of each group consisted of equal volumes to those of 5, 10 and 20 μmol kg−1 of peroxynitrite. In the other three rats, the order consisted of equal volumes to those of 20, 10 and 5 μmol kg−1 of peroxynitrite. The injections of peroxynitrite (1–20 μmol kg−1) did not cause obvious distress to the pentobarbital-anaesthetized rats and no deaths were directly attributable to these injections of peroxynitrite. In addition, the injections of peroxynitrite and cromakalim elicited minor changes in heart rate in the pentobarbital-anaesthetized rats and so these data were not reported.
Drugs
Cromakalim and L-PEN were obtained from Sigma (St Louis, MO, U.S.A.). Sterile saline and sodium pentobarbital were obtained from Abbott (Chicago, IL, U.S.A.). All compounds were dissolved and/or diluted in sterile saline except for cromakalim, which was dissolved in 5% DMSO in saline (see Jasmin & Proschek, 1996). The rats received minimal amounts of DMSO. More specifically, the vehicle solution (5% DMSO in saline) and solution of cromakalim in 5% DMSO was prepared such that the appropriate dose of cromakalim was given as 10 μl per 100 g of body weight. Accordingly, a 300 g rat would have received 30 μl of the solution of cromakalim in DMSO or 30 μl of the vehicle. Peroxynitrite was synthesized in a quench flow reactor (see Beckman et al., 1990). In brief, solutions of NaNO2 and 0.6 M HCl/0.7 M H2O2 were vacuum suctioned into a tee-junction and mixed in glass tubing. The acid catalysed reaction of nitrous acid with hydrogen peroxide to form peroxynitrous acid was quenched by adding 1.5 M NaOH into a second tee-junction at the end of the glass tubing. Excess hydrogen peroxide was removed by adding hydrated manganese dioxide, which was subsequently removed by filtration. Solutions of peroxynitrite were stored at −70°C. Prior to each study, the concentration of peroxynitrite was determined spectrophotometrically (ɛ302=1670 M−1 cm−1) to be 120–130 mM (Beckman et al., 1990). The major contaminant of the peroxynitrite solutions synthesized in the above manner is nitrite, which is typically 20% of the stock peroxynitrite concentration (see Beckman et al., 1990). All compounds were dissolved and/or diluted in sterile saline except for cromakalim, which was dissolved in 5% DMSO in saline (see Jasmin & Proschek, 1996). Solutions of d-peroxynitrite were prepared by leaving peroxynitrite solutions in sealed containers in the light at room temperature for 3 weeks (Benkusky et al., 1998). The major contaminant of the decomposed peroxynitrite solutions prepared in the above manner is nitrite, which is typically 30% of the stock peroxynitrite concentration (see Beckman et al., 1990).
Statistics
The data are shown as mean±s.e.m. and were analysed by repeated measures analysis of variance (ANOVA) (Winer, 1971) followed by Student's modified t-test with the Bonferroni correction for multiple comparisons between means using the error mean square (EMS) terms from the ANOVAs (Wallenstein et al., 1980). The single s.e.m. term on each dose–response curve was derived from the formula, s.e.m=(EMS/n)1/2, where EMS is the EMS term from the ANOVA and n is the number of rats (Wallenstein et al., 1980). A value of P<0.05 denoted statistical significance.
Results
Effects of L-PEN on resting haemodynamic parameters
Resting haemodynamic values in rats that received an injection of saline or L-PEN (2 mmol kg−1) and the injections of peroxynitrite are summarized in Table 2. All values are the mean±s.e.m of the resting values recorded immediately before each of the five injections of peroxynitrite, before and after injection of saline or L-PEN. The injection of saline did not alter resting parameters (P>0.05, for all comparisons). Moreover, the peroxynitrite-induced responses subsided completely such that all preinjection values were similar to one another before and after injection of saline (P>0.05, for all comparisons). Prior to injection of L-PEN, the peroxynitrite-induced responses subsided completely such that all preinjection values were similar to one another (P>0.05, for all comparisons). Resting MAP and vascular resistances were elevated after injection of L-PEN (P<0.05, for all comparisons). Again, the peroxynitrite-induced responses subsided completely such that all preinjection values were similar to one another (P>0.05, for all comparisons). The changes in resting parameters after injection of saline or L-PEN in the rats that received injections of cromakalim were similar to those shown in Table 2 (P>0.05, for all comparisons).
Table 2.
Resting haemodynamic parameters recorded before and after the administration of saline or L-penicillamine in rats that received peroxynitrite
| Treatment | Parameter | Pre | Post | % Change |
|---|---|---|---|---|
| Saline | MAP, mmHg | 117±5 | 114±5 | −2±4 |
| MR, mmHg kHz−1 | 46±5 | 45±5 | −2±5 | |
| HQR, mmHg kHz−1 | 72±5 | 71±6 | −1±4 | |
| L-Penicillamine | MAP, mmHg | 118±3 | 137±4 | +16±4* |
| MR, mmHg kHz−1 | 43±8 | 54±9 | +25±7* | |
| HQR, mmHg kHz−1 | 65±9 | 83±12 | +33±10* |
MAP=mean arterial blood pressure. MR=mesenteric vascular resistance. HQR=hindquarter vascular resistance.
All values are the mean±s.e.m. of the resting values recorded immediately before each of the five injections of peroxynitrite, prior to and after administration of saline or L-penicillamine (2 mmol kg−1, i.v.). Post-treatment values were recorded between 25 and 50 min after administration of saline or L-PEN, the time over which the five injections of peroxynitrite were given. There were six rats in each group. *P<0.05, significant change from Pre.
Effects of L-PEN on the haemodynamic actions of peroxynitrite
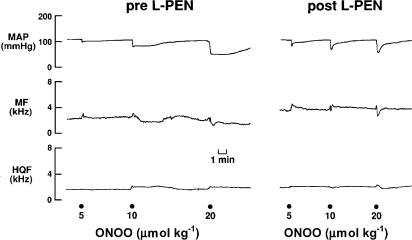
An example of the responses elicited by peroxynitrite (5, 10 and 20 μmol kg−1) before and after injection of L-PEN are shown in Figure 1. Peroxynitrite elicited dose-dependent falls in MAP that were associated with nonsignificant changes in hindquarter and mesenteric blood flow velocities.
Figure 1.
Typical examples of the effects of peroxynitrite (peroxynitrite, 5–20 μmol kg−1, i.v.) on MAP, and MF and HQF blood flow velocities in a pentobarbital-anaesthetized rat before and after administration of L-penicillamine (2 mmol kg−1, i.v.). Points of injection of peroxynitrite are shown by the large dots on the bottom of the figure.
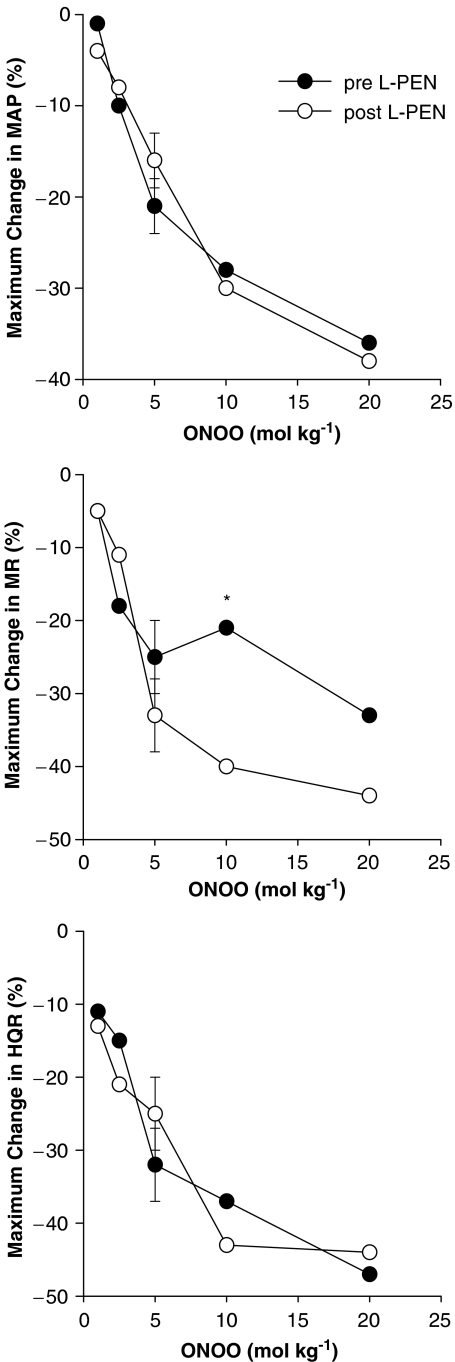
These changes in MAP and blood flows translated into dose-dependent reductions in vascular resistances (vasodilator responses). The initial peroxynitrite-induced responses, which reached maximum by 5–10 s, were not affected by L-PEN, whereas the durations of the responses were markedly diminished. The initial peak falls in MAP and vascular resistances (i.e., those recorded at 5–10 s) elicited by peroxynitrite (1–20 μmol kg−1) before and after injection of L-PEN are summarized in Figure 2. Peroxynitrite elicited dose-dependent falls in MAP and vascular resistances. The magnitude of these initial responses were not affected by L-PEN except for the fall in MR elicited by the 10 μmol kg−1 dose of peroxynitrite which was exaggerated after injection of L-PEN.
Figure 2.
A summary of the maximal reductions in MAP, and MR and HQR vascular resistances produced by systemic injections of peroxynitrite (peroxynitrite, 1–20 μmol kg−1, i.v.) in pentobarbital-anaesthetized rats (n=6) before and after administration of L-penicillamine (L-PEN, 2 mmol kg−1, i.v.). All values are mean of the peroxynitrite-induced changes. The single s.e.m. values on each dose–response curve were derived from the repeated measures ANOVA (see Methods). *P<0.05, post-L-PEN versus Pre.
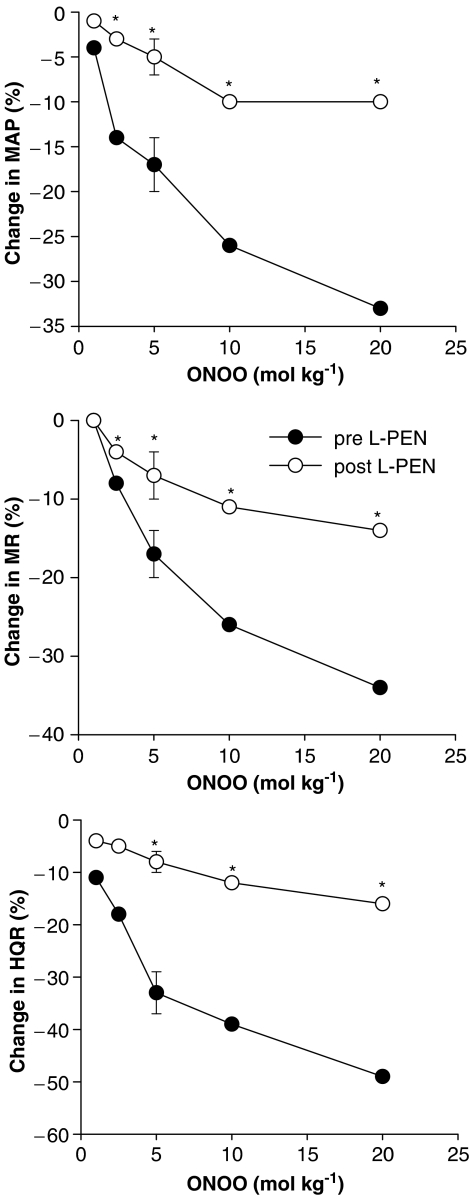
The falls in MAP and vascular resistances recorded 1 min after injection of peroxynitrite before and after injection of L-PEN are summarized in Figure 3. The haemodynamic responses elicited by peroxynitrite at this time were similar in magnitude to the initial responses recorded at 5–10 s (P>0.05 for all comparisons). The haemodynamic responses elicited by the 2.5–20 μmol kg−1 doses of peroxynitrite recorded 1 min postinjection were substantially smaller after injection of L-PEN. The post-L-PEN responses elicited by the 2.5–20 μmol kg−1 doses of peroxynitrite were also smaller than the postsaline responses elicited by these doses of peroxynitrite (P<0.05, for all comparisons). The haemodynamic responses elicited by peroxynitrite (1–20 μmol kg−1, i.v.) before and after injection of saline are summarized in Table 3. The depressor and vasodilator responses elicited by each dose of peroxynitrite were similar before and after administration of saline.
Figure 3.
A summary of the reductions in MAP, and MR and HQR vascular resistances recorded 1 min after injection of peroxynitrite (peroxynitrite, 1–20 μmol kg−1, i.v.) in pentobarbital-anaesthetized rats (n=6) before and after administration of L-penicillamine (L-PEN, 2 mmol kg−1, i.v.). The single s.e.m. values on each dose–response curve were derived from the repeated measures ANOVA (see Methods). *P<0.05, post-L-PEN versus Pre.
Table 3.
Haemodynamic responses elicited by peroxynitrite before and after injection of saline
| Dose of peroxynitrite (μmol kg−1, i.v.) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time | Parameter | Phase | 1 | 2.5 | 5 | 10 | 20 | s.e.m |
| 10–15 s | ΔMAP (%) | Pre | −2 | −12 | −23 | −30 | −42 | 3 |
| Post | −3 | −13 | −25 | −28 | −43 | 3 | ||
| ΔMR (%) | Pre | −4 | −18 | −24 | −26 | −33 | 3 | |
| Post | −3 | −22 | −26 | −28 | −31 | 4 | ||
| ΔHQR (%) | Pre | −8 | −15 | −32 | −39 | −43 | 4 | |
| Post | −6 | −13 | −34 | −42 | −46 | 3 | ||
| 1 min | ΔMAP (%) | Pre | −6 | −13 | −20 | −29 | −36 | 3 |
| Post | −5 | −15 | −22 | −26 | −33 | 4 | ||
| ΔMR (%) | Pre | −2 | −9 | −16 | −29 | −38 | 4 | |
| Post | −4 | −12 | −18 | −31 | −37 | 4 | ||
| ΔHQR (%) | Pre | −6 | −17 | −30 | −37 | −49 | 4 | |
| Post | −5 | −20 | −34 | −39 | −46 | 4 | ||
MAP=mean arterial blood pressure. MR=mesenteric vascular resistance. HQR=hindquarter vascular resistance.
All values are mean of the peroxynitrite-induced changes. The single s.e.m. values on each dose–response curve were derived from the repeated measures ANOVA (see Methods). The column heading ‘time' refers to the time the data were recorded after each injection of peroxynitrite. Post-treatment values were recorded between 25 and 50 min after administration of saline, the time over which the five injections of peroxynitrite were given. There were six rats in the group. Note that the haemodynamic responses elicited by each dose of peroxynitrite were similar before and after injection of saline (P>0.05, for all pre versus postsaline responses).
The total reductions in MAP (mmHg × s) elicited by peroxynitrite before and after injection of saline or L-PEN are summarized in Figure 4. The total reductions in MAP elicited by peroxynitrite were similar before and after injection of saline. The total reductions in MR and HQR were also similar before and after injection of saline (P>0.05 for all comparisons, data not shown). The total reductions in MAP elicited by the 5, 10 and 20 μmol kg−1 doses of peroxynitrite were substantially smaller after injection of L-PEN. The total vasodilation elicited by these doses of peroxynitrite was also substantially smaller after injection of L-PEN (P<0.05, for all comparisons).
Figure 4.
A summary of the total reductions in MAP produced by systemic injections of peroxynitrite (peroxynitrite, 1–20 μmol kg−1, i.v.) in pentobarbital-anaesthetized rats before and after administration of saline (n=6) or L-penicillamine (L-PEN, 2 mmol kg−1, i.v., n=6). Each value is the mean the total reductions in MAP (mmHg × s). The single s.e.m. values on each dose–response curve were derived from the repeated measures ANOVA (see Methods). *P<0.05, post-treatment versus Pre.
Effects of L-PEN on the haemodynamic actions of cromakalim
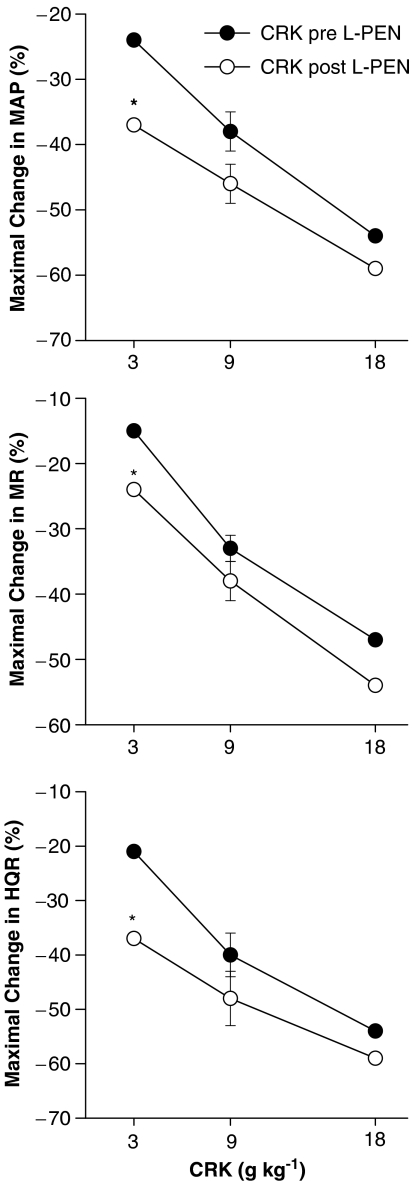
The initial maximal responses elicited by cromakalim (3–18 μg kg−1) before and after administration of L-PEN are summarized in Figure 5. Cromakalim elicited dose-dependent reductions in MAP and vascular resistances. The responses elicited by the 3 μg kg−1 dose of cromakalim were greater whereas the responses elicited by the 9 and 18 μg kg−1 doses of cromakalim were similar before and after administration of L-PEN. The haemodynamic responses produced by cromakalim were similar before and after administration of saline (P>0.05 for all comparisons, data not shown). Moreover, injections of equal volumes of vehicle used to dissolve cromakalim (5% DMSO) did not elicit significant haemodynamic responses (P>0.05 for all comparisons, data not shown).
Figure 5.
A summary of the maximal reductions in MAP, and MR and HQR vascular resistances produced by systemic injections of cromakalim (CRK, 3–18 μg kg−1, i.v.) in pentobarbital-anaesthetized rats (n=6) before and after administration of L-penicillamine (L-PEN, 2 mmol kg−1, i.v.). Each value is the mean of the percent changes in these variables. The single s.e.m. values on each dose–response curve were derived from the repeated measures ANOVA (see Methods). *P<0.05, Post-L-PEN versus Pre.
Effects of L-PEN on the haemodynamic actions of decomposed peroxynitrite
The haemodynamic responses elicited by bolus injections of d-peroxynitrite before and after administration of saline or L-PEN are summarized in Table 4. The volumes of d-peroxynitrite were those that would be given upon injection of the 5, 10 and 20 μmol kg−1 of peroxynitrite As can be seen, these injections of d-peroxynitrite elicited minor responses that were similar in magnitude before and after administration of saline or L-PEN.
Table 4.
Haemodynamic responses elicited by decomposed peroxynitrite before and after injection of saline or L-PEN
| Injection number of decomposed peroxynitrite | |||||
|---|---|---|---|---|---|
| Treatment | Parameter | Phase | 1 | 2 | 3 |
| Saline | ΔMAP (%) | Pre | −4±2 | −8±3* | −13±3* |
| Post | −3±2 | −9±3* | −14±3* | ||
| ΔMR (%) | Pre | −2±2 | −9±3* | −12±3* | |
| Post | −3±2 | −8±3* | −13±3* | ||
| ΔHQR (%) | Pre | −1±1 | −4±2 | −7±3* | |
| Post | −1±1 | −3±3 | −8±3* | ||
| L-PEN | ΔMAP (%) | Pre | −3±2 | −9±3* | −11±3* |
| Post | −1±1 | −11±3* | −14±3* | ||
| ΔMR (%) | Pre | −1±1 | −8±3* | −11±3* | |
| Post | −1±1 | −10±3* | −13±3* | ||
| ΔHQR (%) | Pre | 0±0 | −3±2 | −8±3* | |
| Post | −1±1 | −4±2 | −10±3* | ||
MR=mesenteric vascular resistance. HQR=hindquarter vascular resistance. MAP=mean arterial blood pressure.
All values are mean±s.e.m. L-PEN=L-penicillamine=L-β,β-dimethylcysteine. The volumes of injections 1, 2 and 3 of decomposed peroxynitrite were equal to those that would provide 5, 10 and 20 μmol kg−1 doses of peroxynitrite. There were six rats in each group. *P<0.05, significant response. Note that the haemodynamic responses elicited by each dose of decomposed peroxynitrite were similar before and after administration of saline or L-PEN (P>0.05, for all pre versus postsaline responses).
Discussion
The vasodilator actions of peroxynitrite in pentobarbital-anaesthetized rats are markedly attenuated after administration of the KATP-channel blocker, glibenclamide (Graves et al., 2005a). More specifically, glibenclamide did not markedly affect the initial vasodilator action of peroxynitrite whereas it markedly reduced the duration of these responses (Graves et al., 2005a). These findings suggest that peroxynitrite dilates resistance arteries in vivo by KATP-channel-dependent and KATP-channel-independent mechanisms. Although the mechanisms by which peroxynitrite activates K+ATP-channels are not known, it is unlikely that its oxidant properties are involved since oxidants reduce K+ATP-channel activity (Islam et al., 1993; Han et al., 1996). The precise mechanisms by which peroxynitrite elicits glibenclamide-insensitive vasodilation in vivo have not been established. On the basis of existing in vitro evidence, these mechanisms may involve the generation of blood-borne S-nitrosothiols, the oxidation and or nitration of amino acid residues in functional proteins regulating vascular tone, an increase in intracellular cGMP in resistance vessels, and transient oxidation and impairment of α-adrenoceptor function (see Moro et al., 1994; 1995; Wu et al., 1994) Benkusky et al., 1998; 1999). There is considerable in vitro evidence that exogenous application of peroxynitrite affects cardiac function and coronary artery tone (see Ferdinandy & Schulz, 2001; Liu & Gutterman, 2002). Although direct measurements were not performed, it is possible that the systemic injections of peroxynitrite directly affected cardiac function and coronary artery tone via changes in receptor and/or ion-channel function. It should be noted that the present studies were performed in pentobarbital anaesthetized rather than in conscious rats. Accordingly, it is possible that the mechanisms by which peroxynitrite elicits cardiovascular responses and the mechanisms by which L-PEN attenuates these responses may be affected by pentobarbital.
We demonstrated that the vasodilator responses elicited by peroxynitrite were not attenuated by a dose of L-PEN (500 μmol kg−1, i.v.), which markedly reduced the vasodilator responses elicited by the S-nitrosothiol, L-S-nitrosocysteine (Graves et al., 1998). Although this suggests that the vasodilator effects elicited by systemic injections of peroxynitrite are not due to the formation of circulating S-nitrosothiols, it is distinctly possible that peroxynitrite elicits the formation of an S-nitrosothiol whose actions are not blocked by L-PEN. The finding that L-PEN did not diminish the initial peak vasodilation elicited by peroxynitrite whereas it substantially attenuated the duration of the vasodilation suggests that separate mechanisms are involved in the initiation and maintenance of peroxynitrite-induced vasodilation. Based in part on the present findings and those of Graves et al. (2005a), we speculate that the vasodilator actions of peroxynitrite may be initiated by the formation of blood-borne S-nitrosothiols whereas they may be sustained by the activation of KATP-channels.
The major finding of this study was that the hypotensive and vasodilator responses elicited by systemic injections of peroxynitrite were markedly attenuated after injection of a 2 mmol kg−1 dose of L-PEN. The initial reductions in MAP and vascular resistances elicited by peroxynitrite were unaffected whereas the durations of the responses were substantially diminished after administration of L-PEN. It is unlikely that the effects of L-PEN were due to blockade of K+ATP-channels since L-PEN did not attenuate the vasodilator effects of the K+ATP-channel opener, cromakalim. Indeed the vasodilator effects of the lowest dose of cromakalim were augmented by L-PEN. The finding that L-PEN did not diminish the haemodynamic responses elicited by cromakalim suggests that L-PEN does not interfere with all mechanisms that relax vascular smooth muscle. L-PEN may increase the activity of K+ATP-channels by virtue of its ability to reduce disulphide bonds (Althaus et al., 1994). A feasible explanation for our findings is that L-PEN directly scavenges peroxynitrite in the circulation. This would be consistent with direct evidence that L-PEN scavenges peroxynitrite in solution (Althaus et al., 1994). The findings that the minor vasodilator actions of d-peroxynitrite were not affected by L-PEN suggests that L-PEN did not scavenge or affect the actions of compounds and especially nitrates in the solutions of d-peroxynitrite (see Beckman et al., 1990).
Capillary electrophoretic analysis of the reaction between peroxynitrite and L-PEN detected a single major product, which was a nitrated rather than nitrosated form of L-PEN (Althaus et al., 1994). L-PEN is resistant to circulating enzymes including L-amino-acid oxidase and cysteine disulphydrase, which degrade cysteine (Aposhian, 1961; Levine, 1975). As such, oral (and presumably systemic) administration of L-PEN results in readily detectable amounts of this compound in blood and plasma (Levine, 1975). However, there is no direct evidence that the interaction of peroxynitrite with L-PEN forms nitrated L-PEN in the circulation or whether this product exerts haemodynamic responses. In the absence of reactive nucleophiles, peroxynitrite decomposes to the free radicals OH• and NO2• at neutral pH (Koppenol et al., 1992). Both OH• and NO2• initiate lipid peroxidation (Beckman et al., 1990; Radi et al., 1991a, 1991b). Moreover, OH• hydroxylates whereas NO2• nitrates aromatic residues (Ischiropoulos et al., 1992). Trace amounts of ferrous iron stimulates hydroxylation of salicilate and nitration of tyrosine by peroxynitrite (Ischiropoulos et al., 1992), which would further drive the decomposition of peroxynitrite. L-PEN is an effective chelator of copper, mercury and zinc and to a lesser extent, iron (see Aposhian & Aposhian, 1959; Aposhian, 1961; Levine, 1975). Accordingly, L-PEN-induced chelation of metals would be expected to diminish peroxynitrite-induced hydroxylation and nitration reactions and perhaps prolong its half-life. Consequently, it is unlikely that the ability of L-PEN to chelate metals is primarily responsible for its ability to attenuate the vasodilator effects of peroxynitrite.
The pKa for peroxynitrite is 6.8 (Radi et al., 1991b) so that at normal physiological pH of the blood, about half of the peroxynitrite molecules exist as the protonated species, peroxynitrous acid. In this form, peroxynitrous acid rapidly decomposes to hydroxyl and nitrogen dioxide radicals (Radi et al., 1991b). The exposure of thiols and aromatic compounds to peroxynitrite results in hydroxylated and/or nitrated species (see Radi et al., 1991a; Beckman et al., 1992). Whether the formation of these products occurs through direct reaction with peroxynitrite or the hydroxyl and nitrogen dioxide radicals, or both, has not been fully established (see Koppenol et al., 1992). However, Althaus et al. (1994) provided compelling evidence that peroxynitrite reacts directly with sulphydryl-containing compounds to form S-nitro products rather than S-nitrosyl products. More specifically, Althaus et al. (1994) demonstrated that L-PEN was a substantially more potent scavenger of peroxynitrite than L-cysteine and that the reaction of peroxynitrite and L-PEN yielded a single S-nitro-L-PEN adduct. Although Althaus et al. (1994) demonstrated that the sulphur atom of L-PEN does not possess greater electron density than the sulphur of cysteine, they provided evidence that the greater reactivity of L-PEN with peroxynitrite is because a greater number of L-PEN than cysteine molecules exist in a folded conformation (sulfur proximal to the carboxylate moiety).
Despite direct chemical evidence that that L-PEN is a peroxynitrite scavenger (Althaus et al., 1994), it cannot be concluded that the ability of L-PEN to attenuate the haemodynamic actions of peroxynitrite in vivo is due only to the scavenging of peroxynitrite. Indeed, the observation that the initial effects of peroxynitrite are not affected by L-PEN is evidence that this drug may not be a direct scavenger of peroxynitrite. However, it would seem possible that significant amounts of peroxynitrite may escape scavenging by L-PEN immediately upon injection and that peroxynitrite elicits vasodilation directly or indirectly through the formation of a blood adduct such as an S-nitrosothiol whose actions are not blocked by L-PEN. Moreover, since the majority of peroxynitrite would be scavenged by peroxynitrite, there would be much less peroxynitrite available to sustain the vasodilation via activation of KATP-channels. The effects of glibenclamide on peroxynitrite-induced vasodilator responses were reminiscent of those of L-PEN in that glibenclamide did not markedly affect the initial responses whereas it virtually eliminated the sustained responses. Accordingly, a possible alternate explanation for our findings is that L-PEN is not a scavenger of peroxynitrite in vivo but rather blocks the signaling mechanisms, which sustain but do not initiate peroxynitrite-induced vasorelaxation. However, this may be unlikely because the sustained effects of peroxynitrite were virtually eliminated by glibenclamide and because L-PEN does not diminish the vasodilator potency of cromakalim. It should be noted that L-PEN (2 mmol kg−1) elicited minor increases in MAP and vascular resistances. The vasodilator effects of the endothelium-derived S-nitrosothiol, L-S-nitrosocysteine (Myers et al., 1990), were markedly attenuated by a lower dose of L-PEN (0.5 mmol kg−1) (Graves et al., 1998). Accordingly, the vasoconstrictor effects of L-PEN may involve inhibition of endothelium-dependent vasodilation although other mechanisms are certainly possible. In addition, it would seem unlikely that the minor effects of L-PEN on baseline haemodynamic parameters are responsible for the loss of the peroxynitrite-induced responses.
The biological effects and mechanisms of action of exogenously administered peroxynitrite may not necessarily be the same as those elicited by endogenous peroxynitrite formed under pathological conditions (see Ferdinandy & Schulz, 2001). It is also debatable whether tissue and blood concentrations of antioxidants such as thiols are sufficient to adequately detoxify peroxynitrite at the site of its endogenous formation (see Ferdinandy & Schulz, 2001). Accordingly, evidence as to the potential therapeutic efficacy of L-PEN must await studies in which the effects of this compound are evaluated in experimental models of inflammation that are known to generate peroxynitrite (see Beckman et al., 1994; Kooy et al., 1997). The possibility that L-PEN may scavenge peroxynitrite in vivo has important therapeutic implications.
The increased production of peroxynitrite is implicated in the pathophysiology of human inflammatory diseases (Beckman et al., 1994; Kooy et al., 1995, 1997) and may play a role in the pathogenesis of hypertension (Kooy & Lewis, 1996a). The deleterious effects of peroxynitrite may be due to oxidation and/or nitration of functionally important amino-acid residues in receptors, ion-channels and enzymes (see Benkusky et al., 1998; 1999). In addition, the peroxynitrite product, 3-nitro-L-tyrosine, also inhibits the haemodynamic actions of adrenoceptor agonists and angiotensin II (Kooy & Lewis, 1996b, 1996c). As such, L-PEN and perhaps N-acetyl-DL-PEN, which is more resistant to circulating enzymes that degrade L- and PEN (Aposhian, 1961; Levine, 1975), may be viable scavengers of peroxynitrite in humans. Peroxynitrite generated during inflammatory states is believed to exert deleterious effects on cardiovascular function (see Benkusky et al., 1998; 1999). However, the possibility that peroxynitrite plays a beneficial role under these conditions has not been adequately addressed. As such, it remains to be determined whether the scavenging of peroxynitrite during inflammatory states is truly beneficial and that peroxynitrite scavengers do not have harmful effects. The development of tachyphylaxis to the vasodilator actions of peroxynitrite in vivo is associated with a marked impairment of KATP-channel function (Graves et al., 2005b). The possibility that L-PEN may prevent this loss of KATP-channel function would be indicative of the efficacy of L-PEN and would suggest that the effects of peroxynitrite are indeed deleterious.
Acknowledgments
This work was supported in part by National Heart, Lung and Blood Institute Grants HL-14388 an HL-44546 (to S.J. Lewis) and to American Heart Association (Iowa Affiliate) Grant IA-97-GB-29 (to N.W. Kooy).
Abbreviations
- d-peroxynitrite
decomposed peroxynitrite
- HQF
hindquarter blood flow
- HQR
hindquarter vascular resistance
- K+ATP-channels
ATP-dependent K+ channels
- L-PEN
L-penicillamine (L-β,β-dimethylcysteine)
- MAP
mean arterial blood pressure
- MF
mesenteric blood flow
- MR
mesenteric vascular resistance
References
- ALTHAUS J.S., OIEN T.T., FICI G.J., SCHERCH H.M., SETHY V.H., VONVOIGTLANDER P.F. Structure activity relationships of peroxynitrite scavengers an approach to nitric oxide neurotoxicity. Res. Comm. Chem. Pathol. Pharmacol. 1994;83:243–254. [PubMed] [Google Scholar]
- APOSHIAN H.V. Biochemical and pharmacological properties of the metal-binding agent penicillamine. Fed. Proc. Fed. Am. Soc. Exp. Biol. 1961;20 Suppl 10:185–188. [PubMed] [Google Scholar]
- APOSHIAN H.V., APOSHIAN M.M. N-acetyl-DL-penicillamine, a new oral protective agent against the lethal effects of mercuric chloride. J. Pharmacol. Exp. Ther. 1959;126:131–135. [PubMed] [Google Scholar]
- BECKMAN J.S., BECKMAN T.W., CHEN J., MARSHALL P.A., FREEMAN B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKMAN J.S., ISCHIROPOULOS H., ZHU L., VAN DER WOERD M., SMITH C.D., CHEN J., HARRISON J., MARTIN J.C., TSAI J.H.M. Kinetics of superoxide dismutase and iron catalyzed nitration of phenolics by peroxynitrite. Arch. Biochem. Biophys. 1992;298:438–445. doi: 10.1016/0003-9861(92)90432-v. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., YE Y.Z., ANDERSON P., CHEN J., ACCAVETTI M.A., TARPEY M.M., WHITE C.R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- BENKUSKY N.A., LEWIS S.J., KOOY N.W. Attenuation of vascular relaxation after development of tachyphylaxis to peroxynitrite. Am. J. Physiol. 1998;275:H501–H508. doi: 10.1152/ajpheart.1998.275.2.H501. [DOI] [PubMed] [Google Scholar]
- BENKUSKY N.A., LEWIS S.J., KOOY N.W. Peroxynitrite-mediated attenuation of α- and β-adrenoceptor agonist-induced vascular responses in vivo. Eur. J. Pharmacol. 1999;364:151–158. doi: 10.1016/s0014-2999(98)00791-2. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., SCHULZ R. Peroxynitrite: toxic or protective in the heart? Circ. Res. 2001;88:e12–e13. doi: 10.1161/01.res.88.2.e12. [DOI] [PubMed] [Google Scholar]
- GOW A., DURAN D., THOM S.R., ISCHIROPOULOS H. Carbon dioxide enhancement of peroxynitrite-mediated protein tyrosine nitration. Arch. Biochem. Biophys. 1996;333:42–48. doi: 10.1006/abbi.1996.0362. [DOI] [PubMed] [Google Scholar]
- GRAVES J.E., LEWIS S.J., KOOY N.W. Peroxynitrite-mediated vasorelaxation: evidence against the formation of circulating S-nitrosothiols. Am. J. Physiol. 1998;274:H1001–H1008. doi: 10.1152/ajpheart.1998.274.3.H1001. [DOI] [PubMed] [Google Scholar]
- GRAVES J.E., LEWIS S.J., KOOY N.W. Role of ATP-sensitive K+-channels in the hemodynamic effects of peroxynitrite in anesthetized rats. J. Cardiovasc. Pharmacol. 2005a;46:653–659. doi: 10.1097/01.fjc.0000181715.02452.97. [DOI] [PubMed] [Google Scholar]
- GRAVES J.E., LEWIS S.J., KOOY N.W. Loss of K+ATP-channel-mediated vasodilation after induction of tachyphylaxis to peroxynitrite. J. Cardiovasc. Pharmacol. 2005b;46:646–652. doi: 10.1097/01.fjc.0000181716.79580.dd. [DOI] [PubMed] [Google Scholar]
- HAN J.E., KIM E., HO W-K, EARM Y.E. Sulfydryl redox modulates ATP-sensitive K+ channels in rabbit ventricular myocytes. Biochem. Biophys. Res. Commun. 1996;219:900–903. doi: 10.1006/bbrc.1996.0330. [DOI] [PubMed] [Google Scholar]
- HARDEN T.K. Agonist-induced desensitization of the β-adrenergic receptor-linked adenylate cyclase. Pharmacol. Rev. 1983;35:5–32. [PubMed] [Google Scholar]
- HAYWOOD J.R., SHAFFER R.A., FASTENOW C., FINK G.D., BRODY M.J. Regional blood flow measurement with pulsed Doppler flowmeter in conscious rat. Am. J. Physiol. 1981;241:H273–H278. doi: 10.1152/ajpheart.1981.241.2.H273. [DOI] [PubMed] [Google Scholar]
- HOQUE A., LEWIS S.J. In vivo evidence that L-S-nitrosocysteine may exert its vasodilator effects by interaction with thiol residues in the vasculature. Eur. J. Pharmacol. 1999;384:169–172. doi: 10.1016/s0014-2999(99)00686-x. [DOI] [PubMed] [Google Scholar]
- HUIE R.E., PADMAJA S. The reaction of NO with superoxide. Free Radic. Res. Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., AL-MEDHI A.B., FISHER A.B. Reactive species in rat lung injury: contribution of peroxynitrite. Am. J. Physiol. 1995;269:L158–L164. doi: 10.1152/ajplung.1995.269.2.L158. [DOI] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., ZHU L., CHEN J., TSAI J.H.M., MARTIN J.C., SMITH S.D., BECKMAN J.S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- ISLAM M.S., BERGGREN P.-O., LARSSON O. Sulfhydryl oxidation induces rapid and reversible closure of the ATP-regulated K+ channel in the pancreatic β-cell. FEBS Letts. 1993;319:128–132. doi: 10.1016/0014-5793(93)80051-u. [DOI] [PubMed] [Google Scholar]
- JASMIN G., PROSCHEK L. Prevention by cromakalim of spontaneously occurring cardiac necroses in polymyopathic hamsters. Cardiovasc. Drugs Ther. 1996;10:587–591. doi: 10.1007/BF00051001. [DOI] [PubMed] [Google Scholar]
- KOOY N.W., LEWIS S.J. Elevation in arterial blood pressure following the development of tachyphylaxis to peroxynitrite. Eur J. Pharmacol. 1996a;307:R5–R7. doi: 10.1016/0014-2999(96)00343-3. [DOI] [PubMed] [Google Scholar]
- KOOY N.W., LEWIS S.J. Nitrotyrosine attenuates the hemodynamic effects of adrenoceptor agonists in vivo: relevance to the pathophysiology of peroxynitrite. Eur. J. Pharmacol. 1996b;310:155–161. doi: 10.1016/0014-2999(96)00376-7. [DOI] [PubMed] [Google Scholar]
- KOOY N.W., LEWIS S.J. The peroxynitrite product 3-nitro-L-tyrosine attenuates the hemodynamic effects to angiotensin II in vivo. Eur. J. Pharmacol. 1996c;315:165–170. doi: 10.1016/s0014-2999(96)00623-1. [DOI] [PubMed] [Google Scholar]
- KOOY N.W., LEWIS S.J., ROYALL J.A., YE Y.Z., KELLY D.R., BECKMAN J.S. Extensive tyrosine nitration in human myocardial inflammation: Evidence for the presence of peroxynitrite. Crit. Care Med. 1997;25:812–819. doi: 10.1097/00003246-199705000-00017. [DOI] [PubMed] [Google Scholar]
- KOOY N.W., ROYALL J.A., LEWIS S.J.Peroxynitrite is a vasorelaxant which attenuates catecholamine hemodynamic responses in vivo The Biology of Nitric Oxide, Part 5 1996WA: Portland Press; 208–213.eds. Stamler, J., Gross, S., Moncada, S. & Higgs, A.E., pp. [Google Scholar]
- KOOY N.W., ROYALL JA YE Y.Z., KELLY D.R., BECKMAN J.S. Evidence for in vivo peroxynitrite production in human acute lung injury. Am. J. Resp. Crit. Care. Med. 1995;151:1250–1254. doi: 10.1164/ajrccm/151.4.1250. [DOI] [PubMed] [Google Scholar]
- KOPPENOL W.H., MORENO J.J., PRIOR W.A., ISCHIROPOULOS H., BECKMAN J.S. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem. Res. Toxicol. 1992;5:834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- LEVINE W.G.Heavy metal antagonists The Pharmacological Basis of Therapeutics 1975New York, NY: MacMillan Publishing Company; 912–923.eds. Goodman, L.S. & Gilman, A., pp. [Google Scholar]
- LIU S., BECKMAN J.S., KU D.D. Peroxynitrite, a product of superoxide and nitric oxide, produces coronary vasorelaxation in dogs. J. Pharmacol. Exp. Ther. 1994;268:1114–1121. [PubMed] [Google Scholar]
- LIU Y., GUTTERMAN D.D. Oxidative stress and potassium channel function. Clin. Exp. Pharmacol. Physiol. 2002;29:305–311. doi: 10.1046/j.1440-1681.2002.03649.x. [DOI] [PubMed] [Google Scholar]
- MORO M.A., DARLEY-USMAR V.M., GOODWIN D.A., READ N.G., ZAMORA-PINO R., FEELISCH M., RADOMSKI M.W., MONCADA S. Paradoxical fate and biological actions of peroxynitrite on human platelets. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6702–6706. doi: 10.1073/pnas.91.14.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORO M.A., DARLEY-USMAR V.M., LIZASOAIN I., SU Y., KNOWLES R.G., RADOMSKI M.W., MONCADA S. The formation of nitric oxide donors from peroxynitrite. Brit. J. Pharmacol. 1995;116:1999–2004. doi: 10.1111/j.1476-5381.1995.tb16404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS P.R., MINOR R., JR, GUERRA R., JR, BATES J.N., HARRISON D.G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resembles S-nitrosocysteine than nitric oxide. Nature. 1990;345:161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., PATLAK J.B., WORLEY J.F., STANDEN N.B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am. J. Physiol. 1990;259:C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- RADI R., BECKMAN J.S., BUSH K.M., FREEMAN B.A. Peroxynitrite oxidation of sulfhydryls: the cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991a;266:4244–4250. [PubMed] [Google Scholar]
- RADI R., BECKMAN J.S., BUSH K.M., FREEMAN B.A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991b;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- VILLA L.M., SALAS E., DARLEY-USMAR V.M., RADOMSKI M.W., MONCADA S. Peroxynitrite induces both vasodilation and impaired vascular relaxation in the isolated perfused rat heart. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12383–12387. doi: 10.1073/pnas.91.26.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLENSTEIN S., ZUCKER C.L., FLEISS J.C. Some statistical methods useful in circulation research. Circ. Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- WEI E.P., KONTOS H.A., BECKMAN J.S. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am. J. Physiol. 1996;271:H1262–H1266. doi: 10.1152/ajpheart.1996.271.3.H1262. [DOI] [PubMed] [Google Scholar]
- WINER B.J. Statistical Principles of Experimental Design. New York, NY: McGraw-Hill; 1971. pp. 752–809. [Google Scholar]
- WHALEN E.J., JOHNSON A.K., LEWIS S.J. Hemodynamic actions of systemically-injected pituitary adenylate cyclase activating polypeptide-27 in the rat. Eur. J. Pharmacol. 1999;365:205–215. doi: 10.1016/s0014-2999(98)00852-8. [DOI] [PubMed] [Google Scholar]
- WU M.K., PRITCHARD A., KAMINSKI P.M., FAYNGERSH R.P., HINTZE T.H., WOLIN M.S. Involvement of nitric oxide and nitrosothiols in relaxation of pulmonary arteries to peroxynitrite. Am. J. Physiol. 1994;266:H2108–H2114. doi: 10.1152/ajpheart.1994.266.5.H2108. [DOI] [PubMed] [Google Scholar]