Abstract
Non-selective cyclooxygenase (COX) inhibitors exert effects on lower urinary tract function in several species. The exact contributions of COX-1 and COX-2 isozymes have not been studied much. The present studies investigated the effects of non- and selective COX inhibitors on bladder irritation in the cat.
Chloralose-anaesthetised female cats were catheterised through the bladder dome for cystometric evaluation of bladder responses to intravesical infusion of saline or acetic acid. Bladder capacity, voiding efficiency, threshold pressure, and reflex-evoked bladder contraction amplitude and duration were measured. The cat COX selectivity of the doses of inhibitors examined was determined using an in vitro whole-blood assay and analysis of plasma levels.
Pretreatment with indomethacin or ketoprofen (non-selective COX inhibitors; 0.3 mg kg−1 i.v.) inhibited acetic acid-evoked irritation (characterised by a decrease in bladder capacity in vehicle pretreated animals). FR-122047 (selective COX-1 inhibitor), NS-398 and nimesulide (selective COX-2 inhibitors; 1 and 3 mg kg−1 i.v.) had no effects on bladder irritation. Analysis of plasma levels of the doses examined and determination of COX-1 and COX-2 inhibition in cat whole blood confirmed the reported selectivity of these compounds in this species.
The present studies suggest that dual COX inhibition is required to attenuate acetic acid-evoked bladder irritation in the cat.
Keywords: Urinary bladder, micturition, cat, cyclooxygenase, irritation
Introduction
Prostaglandins (PGs), produced from COX-induced metabolism of arachadonic acid, are known to play roles in the regulation of the micturition reflex. PGs are released from the bladder in response to a variety of stimuli, including distension, pelvic nerve stimulation (Khalaf et al., 1979) and chemical irritation (Morikawa et al., 1990). These locally produced PGs have been suggested to modulate bladder afferent nerve activity (Maggi, 1992; Wibberley, 2005). Two distinct isoforms of COX have been identified. COX-1 is constitutively expressed as a 'housekeeping' enzyme in nearly all tissues, and mediates physiological responses, whereas COX-2, expressed by cells involved in inflammation, has emerged as the isoform that is primarily responsible for the synthesis of prostanoids involved in acute and chronic inflammatory states (Simmons et al., 2004). Non-selective COX inhibitors have been shown to increase the threshold for reflex micturition, in both non- and irritation based bladder models (Khalaf et al., 1981; Morikawa et al., 1990). However, few studies have evaluated the exact contributions of COX-1 and COX-2 isozymes in the control of bladder function (Lecci et al., 2000; Wheeler et al., 2001; Hu et al., 2003). Furthermore, although the cat is increasingly being used as an animal model of human bladder function (Cheng et al., 1999; Thor et al., 2002; Roppolo et al., 2005), the effects of COX inhibitors on bladder function have not been evaluated in this species. Thus, the present studies evaluated the effects of non-selective COX inhibitors in acetic acid-evoked bladder instability in the cat. Moreover, selective COX-1 and COX-2 inhibitors were used to determine the exact contributions of each of these isozymes under these experimental conditions. The cat COX selectivity of the doses of inhibitors examined was determined using an in vitro whole-blood assay and analysis of plasma levels.
Methods
In vivo studies
All experiments were reviewed and approved by the Institutional Animal Care and Use Committee of GlaxoSmithKline. All procedures on animals were performed in compliance with ILAR Guide for the Care and Use of Laboratory Animals (National Research Council, 1966). After completion of experiments, animals were euthanised by an overdose of pentobarbitone sodium (120 mg kg−1 i.v.).
Experiments were performed on 41 female mixed breed adult cats (1.4–3.7 kg; Liberty Research, Waverly, NY, U.S.A.). They were maintained in a 12 h light – dark cycle and fasted overnight with free access to water. The methodologies used were similar to those described by previous investigators, including the use of acetic acid to evoke bladder irritation (Cheng et al., 1999; Thor et al., 2002; Roppolo et al., 2005). Cats were anaesthetised with isoflurane (5% in 100% oxygen for induction and 3–4% for maintenance). Depth of anaesthesia was assessed by the stability of blood pressure and heart rate, and by an absence of hind limb withdrawal in response to paw pinch. The trachea was intubated to maintain a patent airway. The left femoral vein was cannulated for anaesthetic and drug administration, and the left femoral artery was cannulated with a heparinised cannula (20 units ml−1 heparin in 0.9% w v−1 saline) for the measurement of mean arterial blood pressure (MAP) and for sampling arterial blood for blood gas and plasma analysis. Body temperature was maintained between 36° and 38°C using a blanket system (Gaymar TP-500). Blood pressure was measured using a pressure transducer (Gould Statham P23XL), and the heart rate (HR) derived electronically on-line from the blood pressure signal using AcqKnowledge version 3.7 software (Biopac Systems Inc., U.S.A.). The urinary bladder was exposed by a midline abdominal incision and the ureters leading from the kidneys to the bladder were exposed on either side of the descending colon and cut and drained with a gauze wick to prevent the bladder filling with urine during experiments. A 13G needle was inserted into the bladder through the bladder dome and a cannula (PE60) was inserted into the bladder through the needle. The needle was removed and the cannula secured with a purse-string suture. The cannula, via a 3-way tube connector (Small Parts Inc., Miami, Florida, U.S.A.), was connected to a pressure transducer (Gould Statham P23XL) to record intravesical bladder pressure, and to a syringe pump (Harvard Apparatus Infusion Pump, model 975) for the intravesical infusion of saline or acetic acid. Backflow through the cannula allowed the bladder to be emptied of saline to measure residual volume. At the end of all surgical procedures, isoflurane anaesthesia was discontinued and α-chloralose administered (50–70 mg kg−1, i.v.). Depth of anaesthesia was assessed by the stability of blood pressure and HR, and by an absence of hind limb withdrawal in response to paw pinch. Supplementary doses of α-chloralose (5–10 mg kg−1, i.v.) were given where necessary. Animals were artificially ventilated (rate 80 min−1, stroke volume 8 ml kg−1) with room air by use of a positive pressure pump (Large Animal Ventilator, Harvard Apparatus, MA, U.S.A.). Blood gases were monitored and maintained between 90–130 mmHg PO2, 20–35 mmHg PCO2 and pH 7.3–7.4 with a Corning pH/blood gas analyser (Model 248). Adjustments of the respiratory pump rate and volume were made as necessary to maintain blood gas and pH balance. Following surgery, animals were allowed to stabilize for 30 min during which blood gases were monitored and maintained. Warmed saline (0.9%) was then infused into the bladder (0.5 ml min−1) until voiding (the micturition reflex) occurred, characterized by a large amplitude bladder contraction with the concomitant release of fluid from the urethral opening. This fluid was measured and termed the void volume (ml). Fluid remaining in the bladder following voiding was also measured and this was termed the residual volume (ml). Bladder capacity (ml) was defined as the sum of void volume plus residual volume. Voiding efficiency (VE) was calculated by dividing voided volume by bladder capacity and multiplying by 100. Fluid was emptied from the bladder over a 10 min period (until the bladder was as empty as possible), following which the bladder was re-infused with saline to evoke a further micturition reflex. This was continued until five control voids had been evoked. Non-selective (indomethacin or (S(+)-ketoprofen), selective COX-1 (FR-122047) and COX-2 inhibitors (NS-398 or nimesulide) or vehicle (polyethylene glycol 400; PEG 400) were then administered i.v. followed by a 5 min period to allow stabilisation of any changes in baseline parameters. The saline infusion into the bladder was then replaced with 0.7% acetic acid in 0.9% saline (pH 3.0) and six acetic acid voids were sequentially evoked as outlined in the protocol above. Blood was removed for analysis of drug levels following the second, fourth and sixth acetic acid voids. Time-matched saline only controls were performed in a separate series of experiments. Arterial blood and bladder pressures were continuously displayed on a chart recorder (Grass Instruments) and captured (200 samples s−1) by a MP 100 WSW interface (Biopac Systems Inc., U.S.A.) to allow data to be acquired and analysed off-line using AcqKnowledge version 3.5.3 software (Biopac Systems Inc., U.S.A.). HR were derived electronically on-line from the blood pressure signal using this software (Biopac Systems Inc., U.S.A.). All baseline values were the mean values over a 30 s period and were measured 1 min before start of fluid infusion into the bladder. The amplitude (mmHg) and duration (s) of each reflex-evoked bladder contraction was measured. The micturition pressure thresholds were taken as the bladder pressure (mmHg) at which voiding was initiated. The mean control reflex values for these parameters were calculated from the last three control voids (infusing the bladder with saline). Mean baseline MAP and HRs were also calculated from the baseline measurements taken for the last three control voids. Changes in baseline and reflex-evoked values evoked by saline or acetic-acid bladder infusion and following drug or vehicle administration were compared using two-way Anova followed by post-hoc Bonferroni test. P<0.05 was considered statistically significant.
Plasma analysis
Analysis of cat plasma for indomethacin, ketoprofen, FR-122047, nimesulide and NS-398 was performed using chromatography/tandem mass spectrometric (LC/MS/MS) detection, employing discrete standard curves for the quantitation of each analyte. To correspond with the actual study samples, standards were prepared in 50 μl of female cat plasma, and were made fresh on the bioanalytical study day. Plasma samples were thawed, plasma proteins were precipitated with 200 μl of 95/5 acetonitrile/10 mM aqueous ammonium formate (pH 3.0), containing a proprietary mass spectral internal standard, and the resulting mixture was vortex-mixed for 2 min followed by centrifugation for 30 min at >2000 × g. A measure of 2 μl of the resulting supernatant was injected onto the LC/MS/MS system using an HTS PAL autosampler (CTC Analytics, Zwingen, Switzerland). The mobile phase consisted of a gradient that transitioned linearly from 95/5% of 0.1% aqueous formic acid/acetronitrile to 100% acetonitrile over 1.50 min (500 μl min−1 flow rate). A 2 × 50 mm, 4 μ, Synergi MAX-RP analytical column (Phenomenex, Torrance, CA, U.S.A.) was used. The eluent flowed into a Sciex API4000 triple-quadrupole mass spectrometer (Applied Biosystems, Foster City, CA, U.S.A.) using negative-ion electrospray multiple-reaction monitoring set at optimized collision energies for indomethacin, ketoprofen, NS-398 and nimesulide, while positive-ion electrospray multiple-reaction monitoring was used for the detection of FR-122047. Quantitative concentrations of compounds were determined by standard calibration curve analysis, using linear fitting of a 1/x-weighted plot of the analyte/internal standard peak area ratios vs analyte concentration for indomethacin, ketoprofen and FR-122047 while quadratic line equations were used for NS-398 and nimesulide.
In vitro cat whole-blood COX
Seven female mixed breed adult cats (Liberty Research, Waverly, NY, U.S.A.) were anaesthetised with isoflurane (5% in 100% oxygen). Animals were held in sternal recumbency and 20–30 ml of blood removed via cathertization of the jugular vein into heparinized collection tubes. Compounds were made to 30 mM stock solutions in DMSO and diluted with methanol. Ninety-six-well v-bottom plates were pre-loaded with 1 μl of vehicle or test compound at 100 × final concentration and the methanol allowed to evaporate. For COX-1 inhibition, 100 μl of heparinized whole blood was added to each well and the plate incubated at 37°C for 1 h in a cell culture incubator. A volume of 0.5 μl of calcium ionophore (final concentration of 50 μM) was added to each well and the plate incubated for an additional 30 min. For COX-2 inhibition, heparinised whole blood was incubated for 1 h at 37°C with aspirin (100 μM final) to inactivate COX-1. A measure of 100 μl of heparinised whole blood was added to each well followed by lipopolysaccharide (LPS: 100 μg ml−1, final) and the plate was incubated at 37°C for 24 h in a cell culture incubator. For both COX-1 and COX-2, the incubations were stopped by centrifuging the plate at 4°C for 5 min. Plasma was removed and stored at −80°C until assayed for thromboxane (TXB2; COX-1) or PGE2 (COX-2) by ELISA (Cayman Chemical Company, U.S.A.) following the kit instructions. Results were expressed as percentages of vehicle-evoked responses, COX-1 and COX-2 IC50 and IC80 values for each test compound used were determined and selectivity ratios (COX-1 IC50 or IC80 divided by COX-2 IC50 or IC80, respectively) calculated.
Drugs
Indomethacin, S(+)-Ketoprofen and nimesulide were obtained from Sigma-Aldrich (Saint Louis, MO, U.S.A.). FR-122047 (1-((4,5-bis(4-methoxyphenyl)-2-thiazolyl)carbonyl)-4-methylpiperazine hydrochloride) and NS-398 (N-(2-cyclohexyloxy-4-nitrophenylmethanesulfonamide) were obtained from Tocris Cookson (Ellisville, MO, U.S.A.).
Results
Control micturition reflexes
Infusion of saline into the bladder evoked the micturition reflex, characterised by the appearance of a large amplitude (27.5±2.43 mmHg) bladder contraction lasting 68.5±9.01 s, with the concomitant release of 4.64±0.57 ml of fluid from the urethral opening (void volume; n=41). The mean residual volume was 3.51±0.66 ml, with a calculated mean bladder capacity of 8.15±0.80 ml. Mean voiding efficiency was 59.8±4.22%. The mean bladder pressure threshold to evoke the micturition reflex was 11.2±0.90 mmHg (micturition pressure threshold). The mean control data for micturition reflexes and baseline parameters are shown in Table 1. A variation in control bladder capacities between some treatment groups was observed, in similarity to previously observed variation in this parameter in the cat (Cheng et al., 1999). This is likely a consequence of biological variability in the micturition reflex, and this did not affect the degree of acetic acid irritation observed (Table 2).
Table 1.
Control values for all treatment groups
| Treatment | Dose (mg kg−1) | n | Bladder capacity (ml) | Voiding efficiency (%) | Micturition threshold (mmHg) | Bladder contraction amplitude (mmHg) | Bladder contraction duration (s) | MAP (mmHg) | HR (beats min−1) |
|---|---|---|---|---|---|---|---|---|---|
| Salinea |
— |
3 |
5.12±1.56 |
60.2±20.5 |
7.86±2.03 |
25.3±5.68 |
96.3±41.2 |
105±20.3 |
201±24.5 |
| Vehicle |
— |
5 |
4.78±0.87 |
55.9±14.4 |
12.7±1.44 |
22.7±3.67 |
23.7±2.14 |
94.4±11.1 |
163±8.17 |
| Ketoprofen |
0.3 |
4 |
5.96±1.39 |
74.6±15.1 |
9.22±3.96 |
40.4±14.4 |
88.0±18.0 |
117±7.79 |
195±17.0 |
| Indomethacin |
0.3 |
4 |
5.26±1.29 |
43.8±9.41 |
10.2±2.46 |
31.1±9.39 |
41.9±14.0 |
140±10.8 |
185±20.7 |
| FR122047 |
1 |
3 |
12.3±7.50 |
51.6±26.7 |
10.5±2.27 |
15.7±6.63 |
56.4±13.1 |
125±9.53 |
210±28.9 |
| |
3 |
5 |
10.3±2.15 |
53.5±13.7 |
11.7±3.20 |
26.5±5.70 |
60.9±15.9 |
129±12.1 |
185±20.3 |
| NS398 |
1 |
3 |
11.9±2.71 |
76.6±13.2 |
8.78±2.04 |
22.0±7.91 |
105±28.4 |
119±15.4 |
204±32.9 |
| |
3 |
5 |
12.3±2.12 |
58.6±12.2 |
8.45±1.71 |
24.7±4.12 |
88.5±32.9 |
120±8.77 |
199±9.07 |
| Nimesulide |
1 |
3 |
11.9±2.71 |
76.6±13.2 |
8.78±1.96 |
22.0±7.91 |
105±28.4 |
119±15.4 |
204±32.9 |
| 3 | 6 | 6.49±1.28 | 63.2±11.7 | 14.7±2.93 | 34.4±7.82 | 112±37.1 | 116±10.5 | 194±12.9 |
Experiments during which saline alone was infused into the bladder for comparison with the effects of intravesical acetic acid.
All values are mean±s.e.m.
Table 2.
Maximum change in parameter measured during acetic acid-evoked bladder irritation following pre-treatment with test substance
| |
|
Max % change in parameter measured |
|||||
|---|---|---|---|---|---|---|---|
| Drug | Dose (mg kg−1 i.v.) | Voiding efficiency | Bladder contraction amplitude (mmHg) | Bladder contraction duration (s) | Micturition pressure threshold (mmHg) | Mean arterial pressure (mmHg) | Heart rate (beats min−1) |
| Salinea |
— |
−19.8±4.80 |
28.7±55.6 |
21.6±16.3 |
10.1±15.4 |
8.71±12.6 |
−15.6±5.12 |
| Vehicle |
— |
48.2±22.4 |
76.7±54.1 |
58.4±34.0 |
22.5±20.4 |
16.7±12.1 |
−9.59±3.99 |
| Ketoprofen |
0.3 |
−30.7±17.7 |
21.8±16.1 |
−25.8±7.45 |
17.6±25.2 |
13.5±10.8 |
15.4±4.90 |
| Indomethacin |
0.3 |
37.9±44.9 |
−10.8±8.15 |
161±127 |
17.6±7.74 |
−9.24±4.38 |
2.42±1.20 |
| FR122047 |
1 |
13.3±9.74 |
73.1±59.9 |
−18.0±20.4 |
−38.6±24.7 |
−13.0±8.26 |
−2.84±0.77 |
| |
3 |
39.3±32.5 |
24.9±25.9 |
153±163 |
−38.9±14.0 |
−10.0±9.91 |
−5.57±3.21 |
| NS398 |
1 |
25.5±17.1 |
42.1±42.1 |
149±102 |
56.9±40.2 |
18.4±15.1 |
−12.2±3.22 |
| |
3 |
26.9±31.7 |
−13.7±4.77 |
−44.6±8.45 |
87.8±73.1 |
−11.1±5.20 |
16.3±4.04 |
| Nimesulide |
1 |
37.8±23.7 |
34.6±16.4 |
−43.6±28.4 |
−26.7±7.49 |
11.4±6.08 |
−19.7±14.2 |
| 3 | 14.0±25.0 | 16.8±16.0 | 36.8±37.5 | −11.4±10.1 | 18.7±7.86 | −4.87±2.74 | |
Experiments during which saline alone was infused into the bladder.
All values are mean±s.e.m.
Effects of intravesical administration of saline or acetic acid following vehicle pretreatment
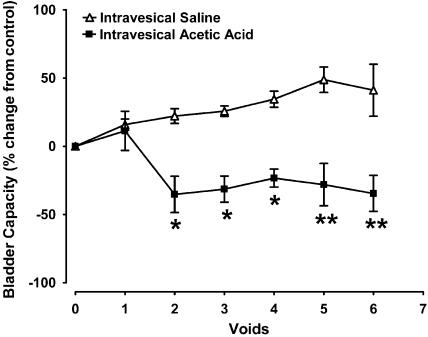
Intravesical infusion of a dilute solution of acetic acid (0.7%; pH 3.0) in vehicle-pretreated animals evoked a significant decrease in bladder capacity compared to vehicle controls with continued intravesical saline infusion over a similar time period (n=7; Figure 1). This decrease was evident in all animals after two acetic acid voids, reaching a decrease in capacity of 34.5±13.2% after six acetic acid voids (n=5; Figure 1). The voiding efficiency, amplitude and duration of reflex-evoked bladder contractions and micturition pressure thresholds were unchanged following intravesical acetic acid infusion. Baseline MAP and HR were also unchanged in all animals (Table 2).
Figure 1.
Effects of intravesical infusion of saline (n=3) or acetic acid (n=5) on bladder capacity in the anaesthetised female cat. *P<0.05, **P<0.01 compared to intravesical saline infusion.
Effects of the non-selective COX inhibitors, indomethacin and ketoprofen on acetic acid-evoked bladder irritation
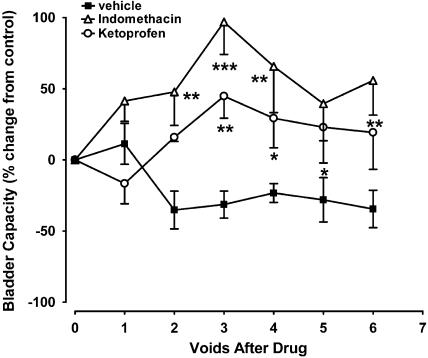
Indomethacin and ketoprofen pretreatment (0.3 mg kg−1 i.v.) significantly attenuated acetic acid-evoked bladder instability, resulting in maximal increases in bladder capacity of 96.9±22.8% (n=4) and 44.8±15.5% (n=4), respectively, after the third acetic acid void (Figure 2). Increases in bladder capacity evoked by both indomethacin and ketoprofen were sustained over the course of the experiment (Figure 2). Both non-selective COX inhibitors had no effects on all other parameters that were measured compared to vehicle controls, including voiding efficiency, bladder contraction amplitude and duration, micturition pressure threshold and baseline MAP or HR (Table 2).
Figure 2.
Effects of non-selective COX inhibitors, indomethacin and ketoprofen (0.3 mg kg−1, i.v.; n=4 for each compound) on bladder capacity in acetic acid-evoked bladder irritation in the anaesthetised female cat. *P<0.05, **P<0.01 and ***P<0.001 compared to vehicle controls.
Effects of COX-1 selective (FR-122047) and COX-2 selective (NS-398 and nimesulide) inhibitors, on acetic acid-evoked bladder irritation
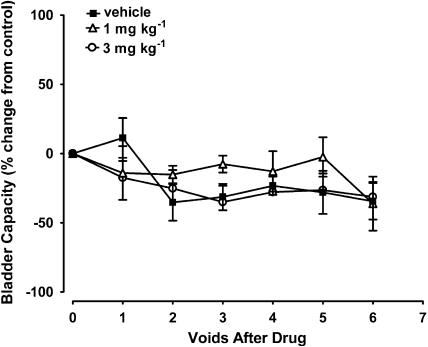
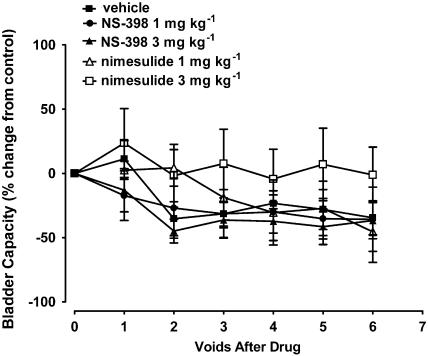
FR-122047, NS-398 nor nimesulide (1 and 3 mg kg−1 i.v.) had no significant effects on acetic acid-evoked decreases in bladder capacity (Figures 3 and 4). Nimesulide tended to attenuate this parameter, but this did not reach statistical significance. Neither compounds tested had effects on voiding efficiency, bladder contraction amplitude and duration, micturition pressure threshold and baseline MAP or HR (Table 2).
Figure 3.
Effects of the selective COX-1 inhibitor FR-122047 on bladder capacity in acetic acid-evoked bladder irritation in the anaesthetised female cat (n=8).
Figure 4.
Effects of the selective COX-2 inhibitors, NS-398 (n=8) and nimesulide (n=9), on bladder capacity in acetic acid-evoked bladder irritation in the anaesthetised female cat.
Cat whole-blood COX inhibition and plasma drug levels of indomethacin, ketoprofen, FR-122047, NS-398 and nimesulide
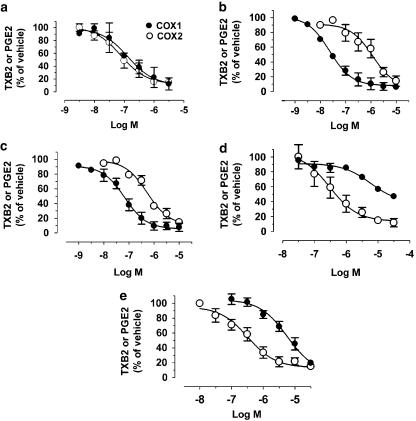
The plasma levels of the test compounds used in the present study following acetic-acid voids 2, 4 and 6 and the whole-blood COX inhibitory activities (IC50 and IC80) are shown in Table 3. Figure 5 shows the concentration-response curves of the compounds on the activity of COX-1 and COX-2 in cat whole blood. The whole-blood COX-1 and COX-2 selectivity ratios (calculated using both IC50 and IC80 values) for the non- and selective COX inhibitors used in the present study showed that ketoprofen and FR-122047 were COX-1 preferring, NS-398 and nimesulide COX-2 selective and indomethacin non-selective in the cat. Plasma levels of indomethacin and ketoprofen following administration were consistent with an estimated 100% inhibition of both COX-1 and COX-2 from the whole-blood COX IC80s after acetic acid voids 2 and 4. The plasma levels and whole-blood IC80 values of FR-122047 showed an approximate 50–80% inhibition of COX-1 and 5–10% inhibition of COX-2 at the lowest dose tested (1 mg kg−1 i.v.) and 100% inhibition of COX-1 and 10–20% inhibition of COX-2 at the highest dose (3 mg kg−1 i.v.), consistent with the COX-1 preferring profile of this compound. Conversely, NS-398 was COX-2 preferring with the limitation that IC80 values for COX-1 could not be obtained due to the lack of complete inhibition of this enzyme by NS-398. From the IC50 values, at the low dose tested (1 mg kg−1 i.v.), NS-398 would produce approximately 100% inhibition of COX-2 and 15–20% of COX-1. At the highest dose, approximately 50–80% inhibition of COX-1 and 100% of COX-2 was calculated. Nimesulide evoked approximately 50 and 100% inhibition of COX-1 and COX-2, respectively, at the low dose (1 mg kg−1 i.v.), however, at the higher dose (3 mg kg−1 i.v.) 100% inhibition of both enzymes would be expected.
Table 3.
Cat whole blood COX-1 and COX-2 inhibitory activities and plasma levels attained following the second, fourth and sixth acetic acid voids for non- and selective COX inhibitors examined
| |
|
|
|
|
|
|
|
Plasma level of drug at acetic acid void (μM) |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Dose (mg kg−1 i.v.) | COX-1 IC50 (μM) | COX-2 IC50 (μM) | COX-1 IC80 (μM) | COX-2 IC80 (μM) | IC50 ratio | IC80 ratio | 2 | 4 | 6 |
| Ketoprofen |
0.3 |
0.023±3 × 10−4 |
1.18±0.04 |
0.12±0.03 |
3.7±2.4 |
0.019 |
0.03 |
7.81±1.52 |
3.52±1.40 |
1.86±0.78 |
| Indomethacin |
0.3 |
0.13±0.01 |
0.08±2 × 10−3 |
0.41±0.07 |
0.93±0.39 |
1.63 |
0.44 |
1.87±0.46 |
0.99±0.31 |
0.51±0.17 |
| FR-122047 |
1 |
0.07±1 × 10−2 |
0.53±0.01 |
0.37±0.2 |
2.78±1.65 |
0.13 |
0.13 |
0.29±0.07 |
0.18±0.05 |
0.14±0.02 |
| |
3 |
|
|
|
|
|
|
0.32±0.08 |
0.53±0.07 |
0.77±0.02 |
| NS-398 |
1 |
24.0±0.75 |
0.30±8 × 10−3 |
—a |
3.34±2.77 |
80 |
—a |
5.15±1.53 |
3.68±0.75 |
2.62±0.75 |
| |
3 |
|
|
|
|
|
|
19.0±5.46 |
11.4±2.46 |
8.83±1.17 |
| Nimesulide |
1 |
5.69±0.13 |
0.32±8 × 10−3 |
46.0±17.7 |
5.33±2.72 |
18 |
8.6 |
24.9±2.45 |
23.6±1.42 |
19.6±1.14 |
| 3 | 49.9±3.38 | 38.6±1.12 | 56.8±3.43 | |||||||
COX-1 IC80 value and ratio for NS-398 could not be determined due to lack of complete inhibition of COX-1.
All values are mean±s.e.m.
Figure 5.
The effects of indomethacin (a), ketoprofen (b), FR-122047 (c), NS-398 (d) and nimesulide (e) on the activity of COX-1 and COX-2 in cat whole blood. All data points are mean±s.e.m. of n=7.
Discussion
The results of the present study suggest that dual COX-1 and COX-2 inhibition is required to inhibit acetic-acid-evoked bladder irritation in the anaesthetised female cat. This is supported by the observation that the non-selective COX inhibitors, indomethacin and ketoprofen demonstrated a similar ability to inhibit bladder irritation, characterized by a decrease in bladder capacity, in this species. This is likely due, in part, to the ability of non-selective COX inhibitors to increase baseline bladder capacity alone independently of the effects of acetic acid, suggesting physiological antagonism. However, non-selective COX inhibitors will also likely exert antagonistic effects on changes in bladder function in the irritated state. These findings are in agreement with the ability of dual COX inhibitors to increase bladder capacity in non- and irritation-based bladder assays in other species (Khalaf et al., 1981; Morikawa et al., 1989; 1990; Maggi, 1992; Takagi-Matsumoto et al., 2004). The role of PGs in the regulation of the micturition reflex in man is controversial and is complicated by gastrointestinal tolerability issues with non-selective COX inhibitors which confer dosage and treatment duration limitations. However, available data suggests that dual COX inhibitors also modulate bladder function in man (Wibberley, 2005).
The requirement for dual COX inhibition to affect bladder irritation in the present studies is also supported by the lack of effect of FR-122047, a selective COX-1 inhibitor (Ochi et al., 2000; Ochi & Goto, 2002) and NS-398 and nimesulide, reported selective COX-2 inhibitors (Futaki et al., 1993; Singla et al., 2000).
The whole-blood assay was used in the present studies to determine the COX-1 and COX-2 inhibitory activities of the non- and selective COX inhibitors tested in the cat. To compare the relative potencies of COX inhibitors against COX-1 and COX-2, IC50 values are commonly used. However, as evident in the present studies, the COX-1 and COX-2 inhibitor curves are often not parallel, suggesting that as the concentration of a COX inhibitor increases so does the relative potency. Furthermore, therapeutic doses of commonly used COX inhibitors produce greater than 50% COX inhibition (Pairet & Van Ryn, 1998; Warner et al., 1999). Thus, comparison of the potencies of COX inhibitors against COX-1 and COX-2 at the IC80 value appears most appropriate and as such was used in the present studies. The selectivity ratios of the IC80 values for COX-1 and COX-2 (the higher the ratio, the greater the COX-2 selectivity) for indomethacin, ketoprofen, FR-122047, NS-398 (IC50 had to be used due to incomplete inhibition of COX-1 by NS-398) and nimesulide in cat whole blood are consistent with their reported selectivity in other animal and human whole-blood assays (Pairet & Van Ryn, 1998; Warner et al., 1999; Brideau et al., 2001). Furthermore, with the exception of nimesulide, calculation of the approximate level of inhibition of COX-1 and COX-2 based on plasma exposures during the experiment supports the finding that dual COX inhibition with indomethacin and ketoprofen attenuates acetic acid-evoked bladder irritation, while selective COX-1 (FR-122047) or COX-2 (NS-398) does not. The reason for the lack of effect of nimesulide, despite apparent 100% in vitro inhibition of both COX enzymes remains unclear. Nimesulide tended to attenuate acetic acid-evoked decreases in bladder capacity but this did not reach statistical significance. A general property of COX inhibitors is high protein plasma binding (>95%) and a low volume of distribution. The data available for nimesulide suggests extensive binding to serum (>99%) and volumes of distribution less than 0.4 l kg−1 in human and 0.18 l kg−1 in the dog (Bree et al., 1993; Pairet & Van Ryn, 1998; Toutain et al., 2001). Moreover, comparisons of the properties of dual COX inhibitors between various animal species and man suggest that considerable species differences may exist in the degree of plasma protein binding and distribution volumes (Davies & Skjodt, 2000; Kim et al., 2001; Lees et al., 2004a, 2004b). Protein binding and distribution volume data for the compounds used in the present studies specifically in the cat have not been reported. Indeed, although the protein plasma binding of compounds should be factored in the whole-blood assay, differences in the on–off rates to plasma proteins, distribution volume and tissue site (i.e. bladder drug levels) in vivo may account for the lack of effect of nimesulide on micturition in the cat despite apparent complete dual COX inhibition in vitro. An ex vivo (animals that have been treated previously with compound) determination of the degree of COX inhibition of nimesulide would allow a more direct comparison of the in vivo relevance of these in vitro findings. Despite this anomaly, the data with the highly selective COX-1 and COX-2 inhibitors, FR-122047 and NS-398, respectively, and a COX-2 preferring low dose of nimesulide, suggests dual COX inhibition is required to inhibit acetic acid-evoked bladder irritation in the cat. However, preliminary studies with combinations of FR-122047 and NS-398 in this assay revealed a lack of additive effects (unpublished observations). Interactions of selective COX-2 inhibitors with COX-1 inhibitors have been reported. Concentrations of NS-398 that did not directly inhibit COX-1 activity attenuated the inhibition of COX-1 by aspirin or indomethacin in various in vitro assays. The same phenomena have also been shown to occur with both nimesulide and DuP697, another selective COX-2 inhibitor (Rosenstock et al., 1999; Ouellet et al., 2001). The molecular mechanisms underlying these interactions remain unknown and although data does not exist for a similar relationship with selective COX-2 inhibitors and the affinity of FR-122047 for COX-1, these findings may be a confounding factor in the design of combination studies. Further, tissue distributions and/or pharmacokinetic differences may preclude the ability to mimic dual acting compounds by combinations of singly acting tools.
The mechanism underlying the requirement for dual COX inhibition to inhibit acetic acid-evoked bladder irritation remains unclear. This phenomenon clearly differs from other nociceptive processes, such as inflammatory pain, in which both non- and selective COX inhibitors are efficacious (Futaki et al., 1993; Ochi et al., 2000; Ochi & Goto, 2002). A variety of prostanoids are released from the bladder in response to several physiological and noxious stimuli, including innocuous bladder distension and chemical irritation, and it is possible that owing to the prominent and complex role of prostaglandins in the bladder, inhibition of only a portion of these inflammatory mediators with either selective COX-1 or COX-2 inhibition may not be sufficient to result in bladder efficacy (Wibberley, 2005). Additional antinociceptive effects mediating the bladder efficacy of the dual COX inhibitors indomethacin and ketoprofen cannot be ruled out (Lees et al., 2004a).
Few studies have investigated the exact contributions of COX-1 and COX-2 in the control of bladder function in preclinical assays (Lecci et al., 2000, Wheeler et al., 2001; Hu et al., 2003). NS-398 had no effect on bladder function when administered 15 min following surgical implantation of a bladder catheter in ‘normal' rats, but reduced micturition frequency 3 h following surgery (Lecci et al., 2000). NS-398 had no effects in the cat when administered approximately 2–3 h following surgical placement of a bladder catheter, suggesting potential species differences in the role of COX-2 under these experimental conditions. Differences in surgical technique, anaesthetic or sex differences (male Wistar rats were utilised by Lecci et al.) may explain these discrepancies. Supporting data exists for a role for COX-2 in mediating chemically-evoked inflammatory bladder responses in the rat. NS-398 pretreatment prevented lipopolysaccharide (LPS)-evoked bladder irritation and reversed cyclophosphamide (CYP)-evoked bladder irritation (Lecci et al., 2000). Thus, species differences in the role of COX-2 in bladder inflammation between the rat and cat may exist. Further, differences in COX responses to chemical irritants (e.g. CYP or LPS vs acetic acid) or differences in the degree or time course of COX-2 up-regulation under varying experimental conditions are potential explanations of these differing results with NS-398 between the present, and previous, studies. In this respect, both LPS and CYP have been shown to increase COX-2 levels in rat bladder 4 h following administration (Wheeler et al., 2001; Hu et al., 2003). Furthermore, Park et al. (1999) have shown an increase in COX-2 expression following bladder outlet obstruction in the female mouse. Thus, COX-2 may play a role in bladder inflammatory responses in the cat, but the experimental conditions used here were not sufficient in type of irritant or time course to induce COX-2. Under the current experimental conditions, selective COX-1 or COX-2 inhibitors did not demonstrate an ability to inhibit acetic acid-evoked bladder irritation in the female cat, while dual COX inhibitors were effective.
In conclusion, the present results suggest that dual COX inhibition is required to inhibit acetic acid-evoked bladder irritation in the female cat. This is supported by the effects of dual, and not selective COX-1 or COX-2 inhibitors, at doses that would, for the most part, maintain respective selectivity, under the current acute experimental conditions. The exact role of COX-1 and COX-2 in chronic inflammatory bladder conditions in the cat remains to be determined.
Abbreviations
- COX
cyclooxygenase
- MAP
mean arterial pressure
- PG
prostaglandin
References
- BREE F., NGUYEN P., URIEN S., ALBENGRES E., MACCIOCCHI A., TILLEMENT J.P. Nimesulide binding to components within blood. Drugs. 1993;46:83–90. doi: 10.2165/00003495-199300461-00016. [DOI] [PubMed] [Google Scholar]
- BRIDEAU C., VAN STADEN C., CHAN C.C. In vitro effects of cyclooxygenase inhibitors in whole blood of horses, dogs, and cats. Am. J. Vet. Res. 2001;62:1755–1760. doi: 10.2460/ajvr.2001.62.1755. [DOI] [PubMed] [Google Scholar]
- CHENG C.L., LIU J.C., CHANG S.Y., MA C.P., DE GROAT W.C. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am. J. Physiol. 1999;277:R786–R794. doi: 10.1152/ajpregu.1999.277.3.R786. [DOI] [PubMed] [Google Scholar]
- DAVIES N.M., SKJODT N.M. Choosing the right nonsteroidal anti-inflammatory drug for the right patient: a pharmacokinetic approach. Clin. Pharmacokinet. 2000;38:377–392. doi: 10.2165/00003088-200038050-00001. [DOI] [PubMed] [Google Scholar]
- FUTAKI N., YOSHIKAWA K., HAMASAKA Y., ARAI I., HIGUCHI S., IIZUKA H., OTOMO S. NS-398, a novel non-steroidal anti-inflammatory drug with potent analgesic and antipyretic effects, which causes minimal stomach lesions. Gen. Pharmacol. 1993;24:105–110. doi: 10.1016/0306-3623(93)90018-s. [DOI] [PubMed] [Google Scholar]
- HU V.Y., MALLEY S., DATTILIO A., FOLSOM J.B., ZVARA P., VIZZARD M.A. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am. J. Physiol. 2003;284:574–585. doi: 10.1152/ajpregu.00465.2002. [DOI] [PubMed] [Google Scholar]
- KHALAF I.M., GHONEIM M.A., ELHILALI M.M. The effect of exogenous prostaglandins F2 alpha and E2 and indomethacin on micturition. Br. J. Urol. 1981;53:21–28. doi: 10.1111/j.1464-410x.1981.tb03123.x. [DOI] [PubMed] [Google Scholar]
- KHALAF I.M., LEHOUX J.G., ELSHAWARBY L.A., ELHILALI M.M. Release of prostaglandins into the pelvic venous blood of dogs in response to vesical distention and pelvic nerve stimulation. Invest. Urol. 1979;17:244–247. [PubMed] [Google Scholar]
- KIM S.Y., LEE Y.M., SHIN H.J., KANG J.S. Indomethacin-loaded methoxy poly(ethylene glycol)/poly(ɛ-caprolactone) diblock copolymeric nanosphere: pharmacokinetic characteristics of indomethacin in the normal Sprague–Dawley rats. Biomaterials. 2001;22:2049–2056. doi: 10.1016/s0142-9612(00)00393-8. [DOI] [PubMed] [Google Scholar]
- LECCI A., BIRDER L.A., MEINI S., CATALIOTO R.M., TRAMONTANA M., GIULIANI S., CRISCUOLI M., MAGGI C.A. Pharmacological evaluation of the role of cyclooxygenase isoenzymes on the micturition reflex following experimental cystitis in rats. Br. J. Pharm. 2000;130:331–338. doi: 10.1038/sj.bjp.0703309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEES P., GIRAUDEL J., LANDONI M.F., TOUTAIN P.L. PK-PD integration and PK-PD modelling of nonsteroidal anti-inflammatory drugs: principles and applications in veterinary pharmacology. J. Vet. Pharmacol. Ther. 2004a;27:491–502. doi: 10.1111/j.1365-2885.2004.00618.x. [DOI] [PubMed] [Google Scholar]
- LEES P., LANDONI M.F., GIRAUDEL J., TOUTAIN P.L. Pharmacodynamics and pharmacokinetics of nonsteroidal anti-inflammatory drugs in species of veterinary interest. J. Vet. Pharmacol. Ther. 2004b;27:479–490. doi: 10.1111/j.1365-2885.2004.00617.x. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. Prostanoids as local modulators of reflex micturition. Pharmacol. Res. 1992;25:13–20. doi: 10.1016/s1043-6618(05)80059-3. [DOI] [PubMed] [Google Scholar]
- MORIKAWA K., FUKUOKA M., KAKIUCHI M., KATO H., ITO Y., GOMI Y. Detrusor hyperreflexia induced by intravesical instillation of xylene in conscious rats. Jap. J. Pharm. 1990;52:587–595. doi: 10.1254/jjp.52.587. [DOI] [PubMed] [Google Scholar]
- MORIKAWA K., ICHIHASHI M., KAKIUCHI M., YAMAUCHI T., KATO H., ITO Y., GOMI Y. Effects of various drugs on bladder function in conscious rats. Jap. J. Pharm. 1989;50:369–376. doi: 10.1254/jjp.50.369. [DOI] [PubMed] [Google Scholar]
- NATIONAL RESEARCH COUNCIL, COMMISSION ON LIFE SCIENCES, INSTITUTE OF LABORATORY ANIMAL RESOURCES . Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1966. [Google Scholar]
- OCHI T., GOTO T. Differential effect of FR122047, a selective cyclooxygenase-1 inhibitor, in rat chronic models of arthritis. Br. J. Pharmacol. 2002;135:782–788. doi: 10.1038/sj.bjp.0704511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OCHI T., MOTOYAMA Y., GOTO T. The analgesic effect profile of FR122047, a selective cyclooxygenase-1 inhibitor, in chemical nociceptive models. Eur. J. Pharmacol. 2000;391:49–54. doi: 10.1016/s0014-2999(00)00051-0. [DOI] [PubMed] [Google Scholar]
- OUELLET M., RIENDEAU D., PERCIVAL M.D. A high level of cyclooxygenase-2 inhibitor selectivity is associated with a reduced interference of platelet cyclooxygenase-1 inactivation by aspirin. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14583–14588. doi: 10.1073/pnas.251543298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAIRET M., VAN RYN J. Experimental models used to investigate the differential inhibition of cyclooxygenase-1 and cyclooxygenase-2 by non-steroidal anti-inflammatory drugs. Inflamm. Res. 1998;47:S93–S101. doi: 10.1007/s000110050289. [DOI] [PubMed] [Google Scholar]
- PARK J.M., YANG T., AREND L.J., SCHNERMANN J.B., PETERS C.A., FREEMAN M.R., BRIGGS J.P. Obstruction stimulates COX-2 expression in bladder smooth muscle cells via increased mechanical stretch. Am. J. Physiol. 1999;276:129–136. doi: 10.1152/ajprenal.1999.276.1.F129. [DOI] [PubMed] [Google Scholar]
- ROPPOLO J.R., TAI C., BOOTH A.M., BUFFINGTON C.A., DE GROAT W.C., BIRDER L.A. Bladder Adelta afferent nerve activity in normal cats and cats with feline interstitial cystitis. J. Urol. 2005;173:1011–1015. doi: 10.1097/01.ju.0000145591.35569.9e. [DOI] [PubMed] [Google Scholar]
- ROSENSTOCK M., DANON A., RIMON G. PGHS-2 inhibitors, NS-398 and DuP-697, attenuate the inhibition of PGHS-1 by aspirin and indomethacin without altering its activity. Biochim. Biophys. Acta. 1999;1440:127–137. doi: 10.1016/s1388-1981(99)00105-5. [DOI] [PubMed] [Google Scholar]
- SINGLA A.K., CHAWLA M., SINGH A. Nimesulide: some pharmaceutical and pharmacological aspects – an update. J. Pharm. Pharmacol. 2000;52:467–486. doi: 10.1211/0022357001774255. [DOI] [PubMed] [Google Scholar]
- SIMMONS D.L., BOTTING R.M., HLA T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- TAKAGI-MATSUMOTO H., NG B., TSUKIMI Y., TAJIMI M. Effects of NSAIDs on bladder function in normal and cystitis rats: a comparison study of aspirin, indomethacin, and ketoprofen. J. Pharmacol. Sci. 2004;95:458–465. doi: 10.1254/jphs.fp0040098. [DOI] [PubMed] [Google Scholar]
- THOR K.B., KATOFIASC M.A., DANUSER H., SPRINGER J., SCHAUS J.M. The role of 5-HT(1A) receptors in control of lower urinary tract function in cats. Brain Res. 2002;946:290–297. doi: 10.1016/s0006-8993(02)02897-4. [DOI] [PubMed] [Google Scholar]
- TOUTAIN P.L., CESTER C.C., HAAK T., METGE S. Pharmacokinetic profile and in vitro selective cyclooxygenase-2 inhibition by nimesulide in the dog. J. Vet. Pharmacol. Ther. 2001;24:35–42. doi: 10.1046/j.1365-2885.2001.00303.x. [DOI] [PubMed] [Google Scholar]
- WARNER T.D., GIULIANO F., VOJNOVIC I., BUKASA A., MITCHELL J.A., VANE J.R. Nonsteroid drug selectivities for cyclooxygenase-1 rather than cyclooxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc. Natl. Acad. Sci. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHEELER M.A., YOON J.H., OLSSON L.E., WEISS R.M. Cyclooxygenase-2 protein and prostaglandin E(2) production are up-regulated in a rat bladder inflammation model. Eur. J. Pharmacol. 2001;417:239–248. doi: 10.1016/s0014-2999(01)00911-6. [DOI] [PubMed] [Google Scholar]
- WIBBERLEY A. Overactive bladder: targeting prostaglandins in sensory pathways. Drug Discovery Today: Therapeutic Strategies. 2005;2:7–13. [Google Scholar]