Abstract
Mast cells are involved in allergic reactions, but also in innate immunity and inflammation. Crosslinkage of mast cell Fc immunoglobulin E receptors (FcɛRI) by multivalent antigen triggers secretion of granule-stored mediators, as well as de novo synthesis of cytokines, including interleukin (IL)-6.
We showed recently that the proinflammatory cytokine IL-1 stimulates human leukemic mast cells (HMC-1) and human umbilical cord blood-derived cultured mast cells (hCBMCs) to release newly synthesized IL-6 without tryptase in the absence of degranulation.
Here, we investigated several signal-transduction pathways activated by IL-1 leading to IL-6 production by HMC-1 and hCBMCs.
We also investigated the effect of the flavonol quercetin that was recently shown to strongly inhibit IL-6 secretion in response to allergic stimulation from hCBMCs.
IL-1 stimulated p38, but did not activate extracellular signal-regulated kinase (ERK) or c-jun N-terminal kinase (JNK); it also did not activate protein kinase C (PKC) isozymes α, β, μ and ζ, except for PKC-θ, which was phosphorylated.
The p38 inhibitor SB203580 and the PKC inhibitors Calphostin C and Gö6976 completely inhibited IL-1-induced IL-6 production.
Quercetin 1–100 μM inhibited IL-1-induced IL-6 secretion, p38 and PKC-θ phosphorylation in a dose-dependent manner.
These results indicate that IL-1-stimulated IL-6 production from human mast cells is regulated by biochemical pathways distinct from IgE-induced degranulation and that quercetin can block both IL-6 secretion and two key signal transduction steps involved.
Keywords: Inflammation, interleukin-1, interleukin-6, mast cells, protein kinase c, quercetin
Introduction
Mast cells are necessary for the development of allergic reactions, but have been increasingly implicated in innate and acquired (Galli et al., 2005) immunity, as well as in inflammatory diseases (Theoharides & Cochrane, 2004). Crosslinkage of IgE receptors (Fc immunoglobulin E receptors, FcɛRI) by multivalent antigen in mast cells results in secretion of granule-associated mediators and release of de novo synthesized cytokines, including interleukin (IL)-6 (Wedemeyer et al., 2000; Marone et al., 2002). FcɛRI crosslinkage leads to a number of distinct signaling steps (Metzger et al., 2002) that involve calcium influx, as well as activation of mitogen-activated protein (MAP) kinases, in a serine/threonine protein kinase C (PKC)-dependent or -independent manner (Zhang et al., 1997; Kawakami et al., 1998; Siraganian, 2003). All isozymes of PKC have been implicated in IgE-induced release of preformed mediators (Ozawa et al., 1993a; Chang et al., 1997; Kimata et al., 1999), as well as in cytokine production (Razin et al., 1994; Chang et al., 1997); these include the classical calcium-sensitive isozymes α, β; the calcium-insensitive δ, ɛ, θ, as well as the atypical ζ isozyme (White & Metzger, 1988; Ozawa et al., 1993b; Liu et al., 2001). The calcium and phorbol ester-independent PKC-θ was shown to augment antigen-induced degranulation of rat basophil leukemia (RBL) cells (Liu et al., 2001). All three MAP kinase pathways p38, ERKs and JNKs have been implicated in regulation of IL-6 mRNA or protein production in response to FcɛRI aggregation (Offermanns et al., 1994).
Activation of MAP kinases and PKC are typical signaling events induced by the proinflammatory cytokine interleukin-1 (IL-1) in several cell types (Dinarello, 1994). With few exceptions, IL-1 induces formation of diacylglycerol (DAG), ceramide or phosphatidic acid in the absence of phosphatidylinositol hydrolysis and, therefore, does not raise cytoplasmic calcium (Dinarello, 1994). The atypical PKC isozyme ζ is, therefore, most consistently activated by IL-1 in different cell types (Ganz et al., 1996; Rzymkiewicz et al., 1996; Esteve et al., 2002) and has been implicated in activation of ERKs (Berra et al., 1995).
We recently showed that IL-1 stimulates selective release of newly synthesized IL-6 through a calcium-independent process that does not involve degranulation (Kandere-Grzybowska et al., 2003). We also recently showed that certain flavonols, especially quercetin, could inhibit FcɛRI-induced cytokine release from human umbilical cord blood-derived mast cells (hCBMCs) and phosphorylation of PKC-θ (Kempuraj et al., 2005). Here, we investigated the effect of IL-1 on phosphorylation of p38, ERK and JNK, as well as activation of PKC isozymes in human leukemic mast cells (HMC-1) and in hCBMCs. We also showed that pretreatment with quercetin inhibits IL-1-induced IL-6 release, as well as PKC-θ and p38 activation. We are not aware of any previous studies on activation of intracellular signaling pathways in human mast cells by IL-1 or its inhibition.
Methods
Human recombinant IL-1α, IL-4, IL-6 and TNF-α were purchased from Chemicon Inc. (Temecula, CA, U.S.A.). Recombinant human stem cell factor (rhSCF) was a gift from Amgen, Inc. (Thousand Oaks, CA, U.S.A.). Monoclonal mouse antibody to PKC-α was from Upstate Cell Signaling Solutions (Lake Placid, NY, U.S.A.) and to PKC-β from BD Transduction Laboratories (Lexington, KY, U.S.A.). Polyclonal rabbit antibodies to PKC-ζ and actin were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, U.S.A.) and phosphorylated (p) PKC isozymes p-PKC-μ, p-PKC-ζ and p-PKC-θ were from Cell Signaling Technologies (Beverly, MA, U.S.A.). Stock solutions of SB203580, an inhibitor of the p38 MAP kinase, and of the PKC inhibitors Calphostin C and Gö6976 (Table 1, all from Calbiochem, EMD Bioscience Inc., La Jolla, CA, U.S.A.) were prepared in DMSO and diluted so that the final DMSO concentration was <0.1%. Goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (HRP) was from Cell Signaling and goat anti-mouse HRP-conjugated antibody was from Upstate Signaling Solutions. Quercetin was obtained from Sigma (St Louis, MO, U.S.A.) and was diluted in DMSO prior to final dilutions in culture medium; the final DMSO concentration was <1%.
Table 1.
Signal transduction pathways involved in IL-6 production from mast cellsa
|
Target |
Inhibitorb |
HMC-1 (IL-1, 10 ng ml−1) |
hCBMCs (IL-1, 50 ng ml−1) |
MCc (Anti-IgE) (%) |
|---|---|---|---|---|
| Inhibition (% total) | ||||
| p38 MAP kinase |
SB203580 (10 μM) |
56.7±2.1 |
52.8±10.0 |
0d |
| PKC |
Calphostin C (0.5 μM) |
80.7±6.5 |
95.8±1.7 |
|
| PKC α, β, μ | Gö6976 (0.5 μM) | 78.3±4.5 | 77.1±11.2 | 100e |
Percent inhibition of IL-6 release during 6 h stimulation in hCBMCs or 18 h stimulation in HMC-1 cells.
SB203580, Calphostin C, Gö6976 were added 30 min before IL-1.
MC=mast cells; data from various sources indicated with references.
hCBMCs (this study).
rat peritoneal MCs (Leal-Berumen et al., 1994).
Mast cell culture
Human cord blood was obtained from normal deliveries in accordance with established institutional guidelines. hCBMCs were derived by the culture of CD34+ progenitor cells as previously described (Kempuraj et al., 1999) with minor modifications. Briefly, mononuclear cells were isolated by pipetting heparin-treated cord blood onto Lymphocyte Separation Medium (INC Biomedical, Aurora, OH, U.S.A.). CD34+ progenitor cells were isolated from mononuclear cells by selection of cells positive for the AC133 antigen (CD133+/CD34+) by magnetic cell sorting (Miltenyi Biotec, Auburn, CA, U.S.A.). For the first 4 weeks, CD34+ cells were cultured in IMDM (GIBCO BRL, Long Island, NY, U.S.A.) supplemented with 0.55 μM 2-mercaptoethanol, 100 mg l−1 Insulin-Transferrin-Selenium supplement (ITS) from GIBCO, 0.1% bovine serum albumin (BSA; Sigma), 1% penicillin/streptomycin (GIBCO), 100 ng ml−1 SCF (Amgen) and 50 ng ml−1 IL-6 (Chemicon) at 37°C in 5% CO2-balanced air. After 4 weeks of culture, BSA and ITS in the culture medium were substituted with 10% fetal bovine serum (FBS) (GIBCO). By 8 weeks, 95% of the cells in the culture were identified as mast cells by immunostaining for tryptase. HMC-1 cells were obtained from Dr J.H. Butterfield (Mayo Clinic, St Paul, MN, U.S.A.) and were cultured in IMDM supplemented with 10% FBS (Hyclone, Logan, UT, U.S.A.), penicillin/streptomycin and 2 μM α-thioglycerol (GIBCO) (Butterfield et al., 1988).
Mediator release experiments
For stimulation of IL-6 production, 8–14-week-old hCBMCs were washed once in each Dulbecco's phosphate-buffered saline (DPBS) and human tyrode buffer and resuspended in fresh medium containing recombinant human stem cell factor (rhSCF) (100 ng ml−1) alone, which was included in the stimulation medium in all experiments for optimal mast cell viability. In anti-IgE stimulation experiments, hCBMCs were resuspended (106 cells ml−1) and passively sensitized by incubation with human myeloma IgE (2 μg ml−1 106 cells; Chemicon) for 48–72 h in IL-6-free medium at 37°C. Cells were then washed, resuspended in medium containing rhSCF alone and distributed to 96-well ‘U bottom' microtiter assay plates (1 × 105 cells 200 μl−1) in duplicate or triplicate for stimulation with IL-1 (50 ng ml−1, Chemicon) or anti-IgE (5–10 μg ml−1; DAKO, Carpinteria, CA, U.S.A.) at 37°C in 5% CO2 for 6 h. HMC-1 were also distributed to 96-well microtiter assay plates (2 × 105 cells 200 μl−1) in duplicate or triplicate and stimulated in complete culture medium. Optimal concentrations were determined from previous dose–response and time-course experiments (Kandere-Grzybowska et al., 2003). IL-6 was determined in cell-free supernatants with commercial ELISA kit (R&D Systems, Minneapolis, MN, U.S.A.) according to the manufacturer's directions (the sensitivity of the assay was 3 pg ml−1).
For the inhibitor studies, HMC-1 or hCBMCs were stimulated in 96-well plates (2 × 105 cells 200 μl−1 well−1) in medium containing 0.5% FBS with IL-1 (10 ng ml−1) for 18 h. In order to avoid loss of cell viability, hCBMCs (1 × 105 cells 200 μl−1 well−1) were stimulated with IL-1 (50 ng ml−1) or anti-IgE (10 μg ml−1) for 6 h in culture medium containing 10% FBS and SCF (but not IL-6). Inhibitors (SB203580, Calphostin C or quercetin) were added 30 min prior to stimulation; these conditions and the concentrations of the inhibitors were selected from published studies in other cell types and were tested and found to be optimal for the present studies.
Cell lysates and ELISA for phosphorylated MAP kinases
For measurements of MAP kinase phosphorylation, HMC-1 were washed two times in DPBS, resuspended in medium containing 0.5% FBS, distributed to 48-well plates (2 × 105 cells 200 μl−1) in each well, and preincubated for 3 h (37°C, 5% CO2) to reduce background induction. In order to maintain optimal cell viability, hCBMCs (2 × 105 cells 200 μl−1) were stimulated in culture medium containing 10% FBS and rhSCF, but not IL-6. In preliminary studies, no difference in activation of p38 was observed in the presence or absence of either FBS or SCF (data not shown). Cells were stimulated for the indicated times with IL-1 (10 ng ml−1 for HMC-1 and 50 ng ml−1 for hCBMCs) or anti-IgE (only for hCBMCs; 10 μg ml−1). Stimulation was terminated by the addition of ice cold DPBS, and cells were washed two times in DPBS. Cells were then lysed in 50 μl of cell extraction buffer (10 mM Tris pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, 1 mM PMSF and protease inhibitor cocktail from Sigma (cat # P-2714) for 30 min on ice.
Activation of MAP kinases was determined by quantifying the amounts of phosphorylated and total kinase by ELISA (Biosource International, Camarillo, CA, U.S.A.). Data in each sample was normalized against total amount of kinase and expressed as fold induction. Samples were diluted appropriately (1 : 10 or 1 : 20) to allow them to fall on the straight part of the standard curve. ELISA kits were specific for dually phosphorylated p38 MAP kinase at residues threonine 180 and tyrosine 182; ERK1 at threonine 202 and tyrosine 204 and ERK2 at threonine 185 and tyrosine 187; JNK1 and JNK2 at threonine 183 and tyrosine 185.
Immunoblotting for PKC isozymes
HMC-1 were serum starved (0.5% FBS) overnight, washed two times with PBS, and placed in six-well plates (2 × 107 cells 4 ml−1 well−1) in fresh medium containing 0.5% FBS. IL-1 (10 ng ml−1) and PMA (25 ng ml−1) were added for the indicated times and stimulation was terminated by addition of ice-cold PBS. The contents of two wells were pooled (4 × 107 cells sample−1) and were disrupted (4 × 10 s) with a Polytron homogenizer (Kinematica Inc., Cincinnati, OH, U.S.A.) in 500 μl of an extraction buffer: 50 mM Tris at pH 7.4, 2 mM EGTA, 2 mM DTT, 0.5 mM PMSF, 1 μg ml−1 aprotinin, 1 μg ml−1 leupeptin. Cell extracts were centrifuged (700 × g, 15 min) to remove nuclei, followed by separation of cytosolic and membrane fractions by centrifugation (43,000 × g, 1 h). The pellet (now the membrane fraction) was washed once more with extraction buffer followed by centrifugation (43,000 × g, 1 h). The membrane fraction was further homogenized in 500 μl extraction buffer, containing in addition 1% Triton-X. The protein concentration was determined in all samples with the BioRad protein assay. Cytosolic and membrane fractions were aliquoted and stored at −80°C. For the detection of phosphorylated forms of PKC isozymes (p-PKC), overnight serum starved (0.5% FBS) HMC-1 (4 × 106 cells 4 ml−1) were stimulated with IL-1 (10 ng ml−1) or PMA (25 ng ml−1) for the indicated times. Cells were lysed in 200 μl SDS lysis buffer (62.5 mM Tris-HCl of pH 6.8, 2% SDS, 19% glycerol, 50 mM DTT, 0.01% bromphenol blue). Proteins (20 μg for the two fractions from 20 μl of cell lysates ∼equivalent to 4 × 105 cells) were loaded on 10% Tris-HCl precast mini gels (Bio-Rad Laboratories, Hercules, CA, U.S.A.). The separated proteins were then transferred to polyvinylidene diflouride membrane (Bio-Rad Laboratories). Immunoreactive proteins were detected by isozyme-specific antibodies at the following dilutions: PKC-α at 1 : 2000; p-PKC-μ, p-PKC-ζ, p-PKC-θ and actin at 1 : 1000; PKC-β at 1 : 250. Secondary antibody, either goat anti-mouse IgG or goat anti-rabbit IgG, was used at 1 : 2000 dilution. Blots were developed with the chemiluminescence detection system and imaged with Kodak Digital Science 1D Image Station (Eastman Kodak Company, Rochester, NY, U.S.A.).
Expression and analysis of results
The results are presented as mean±s.e.m. of 3–7 experiments performed in duplicate or triplicate. Results were compared to control using the nonparametric Mann–Whitney U-test. Comparisons, among different conditions where appropriate, were made using ANOVA. Significance was set at P<0.05.
Results
Selective activation of p38 MAP kinase by IL-1
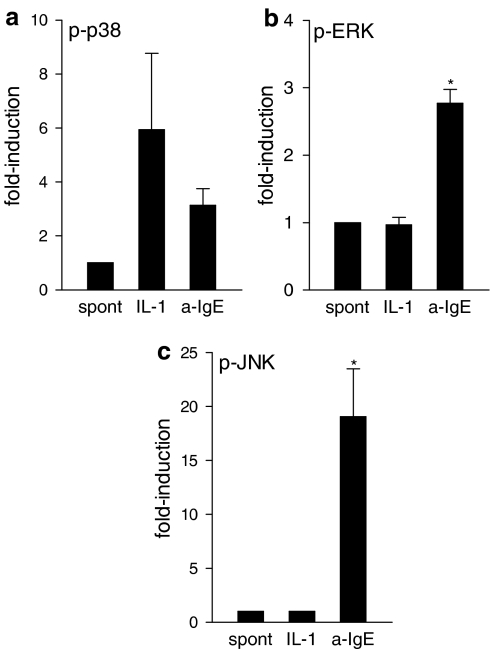
In view of potential cell type specificity in IL-1 signaling, we determined the amount of total and phosphorylated forms of p38, ERK1/2 and JNK1/2 at the indicated time intervals, selected by preliminary studies based on the best time points shown for FcɛRI (Kimata et al., 2000) after stimulation with IL-1 or anti-IgE of hCBMCs. Phosphorylation only of p38 was enhanced about three-fold above baseline (n=5) by stimulation with either IL-1 or anti-IgE (Figure 1a). In contrast, anti-IgE, but not IL-1, stimulated (n=8) the phosphorylation of ERK1/2 about two-fold and JNK1/2 about 15-fold in hCBMCs (Figure 1b and c). Phosphorylation of ERK1/2 was not increased above baseline (n=4) by addition of IL-1, while phosphorylation of JNK1/2 was under the detection limit in the presence or absence of IL-1. The fact that there was phosphorylation of p38 and ERK1/2 in the absence of any stimulation suggests constitutive activation of these protein kinases in normal human mast cells. Similar results were obtained in HMC-1 cells (results not shown).
Figure 1.
IL-1 selectively stimulates phosphorylation of p38 MAP kinase in hCBMCs. The phosphorylation status of MAP kinases was determined in unstimulated and IL-1α (50 ng ml−1)- or anti-IgE (10 μg ml−1)-treated hCBMCs by using ELISA. Amounts of phosphorylated and total MAPKs were determined; data were normalized and expressed as fold-induction. Cells were stimulated for 3 min for p38 (a; n=5), 5 min for ERK (b; n=8) and 15 min for JNK (c; n=4) assay, conditions for optimal anti-IgE activation of MAPKs. Data is mean±s.e.m. of the number of experiments shown in the parentheses above, *P<0.05 for each experimental condition compared to control; spont=spontaneous; a-IgE=anti-IgE.
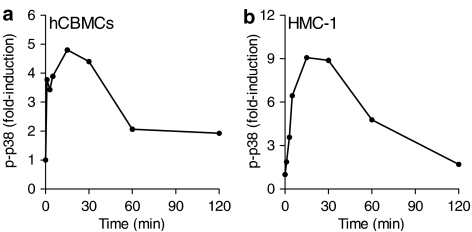
In view of the unique stimulation of p38 by IL-1, we further investigated the time course of p38 phosphorylation. Phosphorylation of p38 in hCBMCs started immediately following addition of IL-1 and peaked (about five-fold) at 15–30 min and declined close to basal levels by 2 h (Figure 2a); stimulation by anti-IgE gave a similar time course, but with peak induction that varied 3–5-fold (results not shown). The kinetics of p38 phosphorylation in response to IL-1 in hCBMCs were similar to HMC-1 cells with a peak of nine-fold at 15 min (Figure 2b). Lack of ERK and JNK phosphorylation in response to IL-1 was verified at 3, 15, 30 and 60 min in both cell types (data not shown). From these data, we conclude that p38 is the only MAP kinase activated selectively in human mast cells in response to IL-1.
Figure 2.
Kinetics of p38 phosphorylation stimulated by IL-1. The phosphorylation of p38 in IL-1-treated (a) hCBMCs (50 ng ml−1) or (b) HMC-1 cells (10 ng ml−1) determined by ELISA. p-p38, phosphorylated p38. Representative experiment from four experiments in duplicate is shown; data are normalized against total p38 expression.
Inhibition of IL-1-stimulated IL-6 production by the p38 inhibitor SB203580
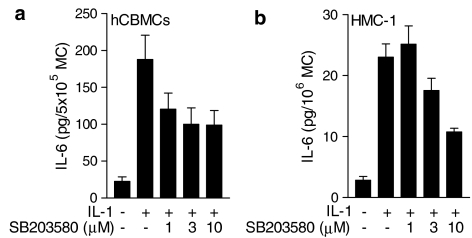
The specific inhibitor for p38 MAP kinase, SB203580 (Cuenda et al., 1995), was used (1–10 μM) to determine the role of p38 in IL-1-induced IL-6 production in human mast cells. SB203580 (3 μM) inhibited IL-1-stimulated IL-6 production in hCBMCs by 52.8±10.0% (n=4), but there was no statistical difference between 1 and 10 μM of the inhibitor (Figure 3a). In contrast, anti-IgE-stimulated IL-6 production was not affected by SB203580 (n=3; Table 1). Similarly, IL-1-stimulated IL-6 in HMC-1 cells was reduced (n=5) by 56.7±2.1% at 10 μM SB203580 (Figure 3b). These data demonstrate that the p38 MAP kinase contributes to IL-1-stimulated IL-6 production in human mast cells.
Figure 3.
Inhibition of IL-1-stimulated IL-6 production by the p38 inhibitor SB203580. Cells were pretreated with SB203580 for 30 min prior to stimulation. (a) hCBMCs were stimulated for 6 h with 50 ng ml−1 of IL-1 (n=5) and (b) HMC-1 cells were stimulated for 18 h with 10 ng ml−1 of IL-1 (n=5). Data=mean±s.e.m., P<0.05 for all concentrations, except for 1 μM SB253080, compared to IL-1 alone. Please note IL-6 units differ as they are listed as pg per 5 × 105 for hCBMCs, but as pg per 106 for HMC-1 cells due to the abundance of the latter. MC=mast cells.
Inhibition of IL-1-stimulated IL-6 production by PKC inhibitors
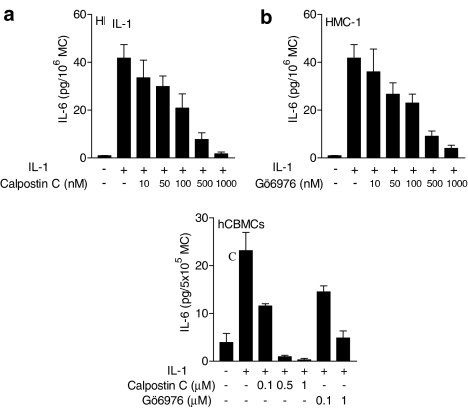
To determine whether PKC is involved in IL-1-induced IL-6 production in mast cells, we used the nonspecific PKC inhibitor Calphostin C (IC50=0.05 μM). Pretreatment of HMC-1 cells for 30 min with Calphostin C (0.01–1 μM) prior to stimulation with IL-1 (10 ng ml−1) inhibited (n=4) IL-6 production in a concentration-dependent manner (Figure 4a and b). At 0.5 μM, Calphostin C and Gö6976 inhibited IL-1-induced IL-6 production in HMC-1 cells by 80.7±6.5 and 78.3±4.5%, respectively. At 0.5 μM, Calphostin C also inhibited IL-6 production induced by IL-1 (50 ng ml−1) in hCBMCs by 95.8±1.7% (Figure 4b).
Figure 4.
Inhibition of IL-1-stimulated IL-6 production by PKC inhibitors. Cells were pretreated with different concentrations of Calphostin C (a, b) for 30 min prior to stimulation with IL-1 (50 ng ml−1) for 6 h for hCBMCs or 18 h for HMC-1 cells (10 ng ml−1). Results are expressed as mean±s.e.m., n=4, P<0.05 for all concentrations. MC=mast cells.
Effect of IL-1 on activation of PKC isozymes
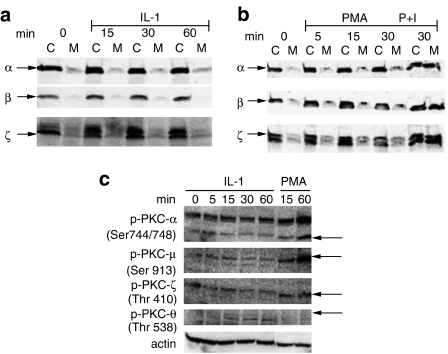
Since Calphostin inhibited IL-1-induced IL-6 release, which had previously been shown to be calcium independent, we investigated the activation of PKC isozymes in response to IL-1 and phorbol ester (PMA) with or without the calcium ionophore A23187 (0.5 μg ml−1) used as a positive control in HMC-1. These studies were performed using serum-starved (0.5% FCS) cells in order to reduce the background and increase the sensitivity of the assay. We investigated the calcium-sensitive PKC-α and β, the calcium-insensitive/PMA-sensitive PKC-ζ (the PKC isozyme most often activated by IL-1 in other cell types), as well as the calcium and PMA-insensitive PKC-θ. We investigated the translocation of PKC α, β and ζ from the cytoplasm to the membrane in HMC-1 cells by Western blotting using isozyme-specific antibodies (Figure 5). IL-1 did not stimulate translocation of PKC isozymes α, β or ζ to the plasma membrane during the observed time (0–60 min). Treatment with PMA (25 ng ml−1) by itself did not have any effect but PMA (25 ng ml−1) together with the calcium ionophore A23187 (0.5 μg ml−1) at 30 min increased translocation of all three isozymes (Figure 5b). In contrast, PKC-θ translocated to the plasma membrane, but was not affected by PMA (not shown).
Figure 5.
Effect of IL-1 on activation of PKC isozymes in HMC-1 cells. (a, b) HMC-1 cells were stimulated with IL-1 (10 ng ml−1), PMA (25 ng ml−1) or PMA and calcium ionophore A23187 (0.5 μg ml−1); the cytosolic (C) and membrane (M) fractions were separated and immunoblotted by antibodies to PKC-α, PKC-β or PKC-ζ. (c) HMC-1 cells were stimulated with IL-1 (10 ng ml−1) or PMA (25 ng ml−1) for 60 min. Lysates were immunoblotted with antibodies to p-PKC-α, p-PKC-μ, p-PKC-ζ, p-PKC-θ or actin; P=phospho; P+I=PMA and ionophores. The arrows indicate the respective phospho proteins. There was only one loading control for both cytosolic and membrane fractions for all blots. Each gel is representative of three similar ones.
Activation of PKC isozymes was also examined by immunoblotting with antibodies specific for PKC phosphorylated isoforms. There was some basal phosphorylation of PKC-α and PKC-μ that was not further increased by IL-1, during 5–60 min stimulation; instead, PMA (25 ng ml−1) stimulated its phosphorylation during the observed times (5–60 min) (Figure 5c). Activation of PKC-ζ was also evaluated by immunoblotting cell lysates with antibody to PKC-ζ phosphorylated at Thr410, which has been correlated with PKC-ζ activation. There was some baseline PKC-ζ phosphorylation in response to IL-1 while PMA stimulated its phosphorylation (Figure 5c).
IL-1 did stimulate phosphorylation of the novel PKC-θ at 15–60 min, with decrease at 120 min, as determined with immunoblotting using antibody to PKC-θ, phosphorylated at Thr538 (Figure 5c); PMA 25 ng ml−1 also phosphorylated this PKC isozyme at 5 min, but this effect was absent at 15 and 60 min. Equal loading was verified by immunoblotting the same membranes with antibodies to actin (Figure 5c). Pretreatment of HMC-1 with Calphostin C (1 μM) for 30 min prior to IL-1 stimulation inhibited translocation of PKC-θ (n=2, results not shown).
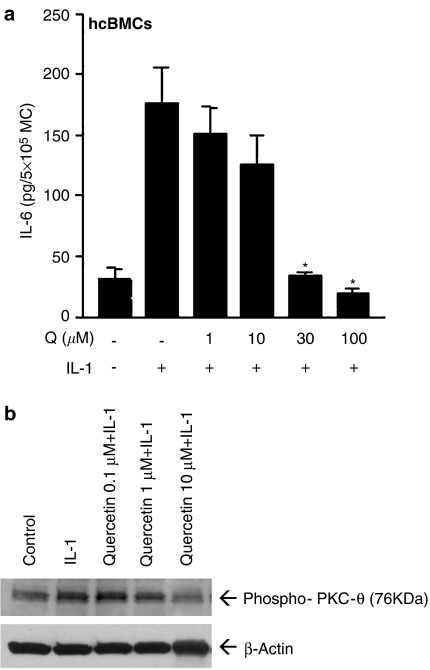
Effect of quercetin on IL-1-induced IL-6 release and phosphorylation of PKC-θ
Pretreatment of hCBMCs for 10 min with various amounts of quercetin prior to stimulation with IL-1 (10 ng ml−1) for 30 min resulted in dose-dependent inhibition of IL-6 release that reached 79% at 100 μM (Figure 6a). Incubation of HMC-1 cells with IL-1 (10 ng ml−1) for 30 min stimulated phosphorylation of PKC-θ (Thr538). However, preincubation of HMC-1 cells with quercetin (1 or 10 μM) for 15 min prior to stimulation with IL-1 (10 ng ml−1) for 30 min inhibited PKC-θ phosphorylation. Equal loading was verified by immunoblotting the same membrane with antibody to actin (Figure 6b).
Figure 6.
Effect of quercetin on IL-1-induced (a) IL-6 release (n=4), (b) PKC-θ phosphorylation. Incubation of HMC-1 cells with IL-1 (10 ng ml−1) stimulated phosphorylation of PKC-θ (Thr538). HMC-1 cells were preincubated with quercetin (1 or 10 μM) for 15 min prior to incubation with IL-1 (10 ng ml−1) for 30 min. Equal loading was verified by immunoblotting the same membrane with antibody to actin (representative gel of three similar ones). Q=quercetin.
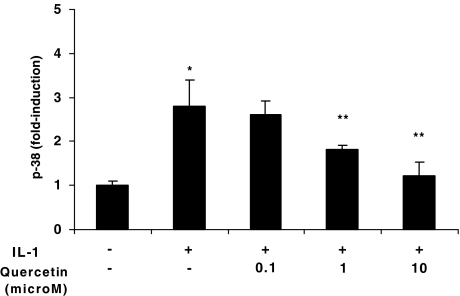
Effect of quercetin on IL-1-induced p38 phosphorylation
Pretreatment of HMC-1 cells with quercetin (0.1, 1.0 or 10 μM) for 15 min prior to stimulation with IL-1 (10 ng ml−1) for 30 min inhibited p38 phosphorylation in a dose-dependent manner (Figure 7).
Figure 7.
Effect of quercetin on IL-1-induced p38 phosphorylation. HMC-1 cells were preincubated with quercetin (0.1, 1 or 10 μM) for 15 min prior to stimulation with IL-1 (10 ng ml−1) in the presence or absence of quercetin (0.1, 1 or 10 μM) for another 3 min. Cell lysates were then prepared and the phosphorylation status of p38 MAPK was determined by ELISA. Amounts of phosphorylated and total p38 was determined; data were normalized and expressed as fold-induction. Q=quercetin. Data represented as mean±s.e.m. (n=3). *P=0.05 versus control, **P<0.05 versus IL-1.
Discussion
We have reported that IL-1 stimulates de novo synthesis and selective release of IL-6 without tryptase and without degranulation from human mast cells in a calcium-independent manner (Kandere-Grzybowska et al., 2003). In contrast to FcɛRI-dependent aggregation by anti-IgE, which activates all three MAP kinases, IL-1 selectively activated only the p38 MAP kinase in HMC-1 and hCBMCs. Similar to our results, IL-1 stimulated IL-6 production in lung and synovial fibroblasts, as well as in rat cardiac myocytes, only through the p38 MAP kinase (Ridley et al., 1997; Miyazawa et al., 1998). Moreover, only the p38 MAP kinase, but not ERK or JNK, was activated by IL-1 in human neutrophils, whereas all three MAP kinases are activated by IL-1 in human monocytes, human vascular endothelial cells, human fibroblasts and human hepatoma cells (Ridley et al., 1997; Kumar et al., 1998; Suzuki et al., 2001). IL-1RI signaling involves activation of several tyrosine and serine/threonine protein kinases, including MAP kinases (Dinarello, 1994). To date, at least three different MAP kinases, p38, ERK1/2 and JNKs1/2 have been implicated in IL-1RI signaling in a cell type-specific manner (O'Neill, 2002). The MAP kinases comprise parallel signaling cascades that are known to regulate production of several cytokines by activating transcription of their target genes (Vanden Berghe et al., 1998).
The specific inhibitor of p38, SB203580 (Cuenda et al., 1995), substantially (∼50%) reduced IL-1, but not anti-IgE-stimulated IL-6 production in hCBMCs, without a classic dose–response curve. These results suggest that in human mast cells, IL-1 induces some additional signals that allow mRNA induction and protein synthesis in the absence of other costimulatory signals, or the presence of some resistant p38 isoform. One possibility is that p38 stabilizes IL-6 mRNA through its AU-repeat-rich 3′-untranslated region (3′-UTR). For instance, in mouse mast cells, IL-1 did not induce transcription of IL-6 mRNA, but acted to stabilize IL-6 mRNA induced by costimulation with IL-10 and SCF (Lu-Kuo et al., 1996). Many cytokines, including IL-6, have AU-repeats at their 3′-UTR and p38 MAP kinase mediates the stabilization of these mRNAs in AU-rich-region-dependent manner (Miyazawa et al., 1998; Winzen et al., 1999).
The potent inhibition of IL-1-stimulated IL-6 by the PKC inhibitor Calphostin C strongly suggests the involvement of PKC. However, we did not find any evidence of activation of PKC isozymes α, β, ζ or μ in response to IL-1 in HMC-1 cells. Instead, the novel isozyme PKC-θ was uniquely activated in response to IL-1. Both classical and novel PKC isozymes have been implicated in IL-6 production due to FcɛRI crosslinkage (Metzger et al., 2002; Blank & Rivera, 2004). PKC-β, in particular, has been linked to IgE-dependent IL-6 production in mast cells by showing that overexpression of PKC-β (but not α, ɛ, δ, or η) in RBL-2H3 cells resulted in increased IL-6 mRNA levels (Chang et al., 1997). Moreover, in vivo studies implicated both PKC-β and δ isozymes since mast cells from PKC-β−/− and PKC-δ−/− knockout mice displayed impaired FcɛRI-mediated IL-6 production (Nechushtan et al., 2000). Long-term treatment (24 h) of rat peritoneal mast cells with PMA resulted in complete inhibition of IgE-dependent IL-6 production, implicating calcium-dependent PKC isozymes (Leal-Berumen et al., 1994). The atypical PKC-ζ and the novel calcium-insensitive PKC-θ have received attention due to their role in immune responses in the context of antigen receptor signaling (Isakov & Altman, 2002). PKC-ζ−/− mice have alterations in the development of secondary lymphoid organs, and induction of IL-6 mRNA is reduced in B cells (Leitges et al., 2001). Similar to our results, PKC-ζ did not translocate to the membrane in response to IgE and specific antigen stimulation (Ozawa et al., 1993a). PKC-θ was phosphorylated in response to IL-1 and by PMA, even though the timing was different. PKC-θ also translocated and was involved in activation of ERKs in response to FcɛRI signaling (Liu et al., 2001). Moreover, PKC-θ was also shown to translocate in human T lymphocytes (Freeley et al., 2005) and in bone marrow-derived mouse mast cells (BMDMs) (Peng & Beaven, 2005) in response to PMA. Such apparent differences may be cell type or stimulation condition specific.
The flavonoid quercetin inhibited IL-6 secretion, as well as p38 and PKC-θ phosphorylation induced by IL-1. These results indicate that quercetin is uniquely able to inhibit this selective mast cell activation. We recently showed that quercetin could also inhibit FcɛRI-induced cytokine release, as well as calcium ion elevations and PKC-θ phosphorylation (Kempuraj et al., 2005) in hCBMCs. Flavonoids are naturally occurring polyphenolic compounds that can inhibit a variety of enzymes (Middleton et al., 2000). Quercetin, the flavonoid component in Ginkgo biloba, inhibited LPS-induced TNF-α transcription by inhibiting ERK1/2 and p38 (Wadsworth et al., 2001). Quercetin also inhibited LPS-induced expression of TNF-α, IL-1β and IL-6 by suppressing activation of ERK and p38 MAP kinases in macrophages (Cho et al., 2003). Another related flavonoid, luteolin, also inhibited LPS-induced inflammatory gene expression in macrophages by reducing activation of ERK and p38 (Xagorari et al., 2002). Certain O-methylated catechins were also shown to inhibit FcɛRI-induced histamine, cytokine and leukotriene release, as multiple protein kinases in mast cells (Maeda-Yamamoto et al., 2004). The ability of quercetin to inhibit allergic and nonallergic human mast cell release of IL-6 suggests that it may be useful in inflammatory conditions involving IL-6 and mast cells such as multiple sclerosis (Theoharides & Cochrane, 2004), migraines (Theoharides et al., 2005), inflammatory arthritis (Theoharides, 2005) and interstitial cystitis (Theoharides & Sant, 2005).
Acknowledgments
We thank Amgen, Inc. (Thousand Oaks, CA, U.S.A.) for the recombinant human SCF, Dr M.J. May (Howard Hughes Medical Institute, New Haven, CT, U.S.A.) for the NBD peptides and Dr J.H. Butterfield (Mayo Clinic, St Paul, MN, U.S.A.) for the HMC-1 cells. We are grateful to Dr Achilles Athanassiou and all the nurses of the Labor and Delivery Division of Tufts – New England Medical Center (Boston, MA, U.S.A.) for the umbilical cord blood collection. This work was supported in part by NIH Grant No. AR47652 to T.C. Theoharides and Theta Biomedical Consulting and Development Co., Inc. (Brookline, MA, U.S.A.) to T.C.T. Thanks are also due to Ms Jessica Christian for her patience and word processing skills.
Abbreviations
- FcɛRI
Fc immunoglobulin E receptors
- hCBMCs
human umbilical cord blood-derived cultured mast cells
- HMC-1
human leukemic mast cell line
- IC50
inhibitory concentration for 50%
- IL
interleukin
- MC
mast cell
- NF-κB
nuclear factor kappa B
- PKC
protein kinase C
- SCF
stem cell factor
References
- BERRA E., DIAZ-MECO M.T., LOZANO J., FRUTOS S., MUNICIO M.M., SANCHEZ P., SANZ L., MOSCAT J. Evidence for a role of MEK and MAPK during signal transduction by protein kinase C zeta. EMBO J. 1995;14:6157–6163. doi: 10.1002/j.1460-2075.1995.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLANK U., RIVERA J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004;25:266–273. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- BUTTERFIELD J.H., WEILER D., DEWALD G., GLEICH G.J. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk. Res. 1988;12:345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- CHANG E.Y., SZALLASI Z., ACS P., RAIZADA V., WOLFE P.C., FEWTRELL C., BLUMBERG P.M., RIVERA J. Functional effects of overexpression of protein kinase C-alpha, -beta, -delta, -epsilon, and -eta in the mast cell line RBL-2H3. J. Immunol. 1997;159:2624–2632. [PubMed] [Google Scholar]
- CHO S.Y., PARK S.J., KWON M.J., JEONG T.S., BOK S.H., CHOI W.Y., JEONG W.I., RYU S.Y., DO S.H., LEE C.S., SONG J.C., JEONG K.S. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell. Biochem. 2003;243:153–160. doi: 10.1023/a:1021624520740. [DOI] [PubMed] [Google Scholar]
- CUENDA A., ROUSE J., DOZA Y.N., MEIER R., COHEN P., GALLAGHER T.F., YOUNG P.R., LEE J.C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- DINARELLO C.A. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8:1314–1325. [PubMed] [Google Scholar]
- ESTEVE P.O., CHICOINE E., ROBLEDO O., AOUDJIT F., DESCOTEAUX A., POTWOROWSKI E.F., ST-PIERRE Y. Protein kinase C-zeta regulates transcription of the matrix metalloproteinase-9 gene induced by IL-1 and TNF-alpha in glioma cells via NF-kappa B. J. Biol. Chem. 2002;277:35150–35155. doi: 10.1074/jbc.M108600200. [DOI] [PubMed] [Google Scholar]
- FREELEY M., VOLKOV Y., KELLEHER D., LONG A. Stimulus-induced phosphorylation of PKC theta at the C-terminal hydrophobic-motif in human T lymphocytes. Biochem. Biophys. Res. Commun. 2005;334:619–630. doi: 10.1016/j.bbrc.2005.06.136. [DOI] [PubMed] [Google Scholar]
- GALLI S.J., NAKAE S., TSAI M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- GANZ M.B., SAKSA B., SAXENA R., HAWKINS K., SEDOR J.R. PDGF and IL-1 induce and activate specific protein kinase C isoforms in mesangial cells. Am. J. Physiol. 1996;271:F108–F113. doi: 10.1152/ajprenal.1996.271.1.F108. [DOI] [PubMed] [Google Scholar]
- ISAKOV N., ALTMAN A. Protein kinase C(theta) in T cell activation. Annu. Rev. Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- KANDERE-GRZYBOWSKA K., LETOURNEAU R., BOUCHER W., BERY J., KEMPURAJ D., POPLAWSKI S., ATHANASSIOU A., THEOHARIDES T.C. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J. Immunol. 2003;171:4830–4836. doi: 10.4049/jimmunol.171.9.4830. [DOI] [PubMed] [Google Scholar]
- KAWAKAMI Y., HARTMAN S.E., HOLLAND P.M., COOPER J.A., KAWAKAMI T. Multiple signaling pathways for the activation of JNK in mast cells: involvement of Bruton's tyrosine kinase, protein kinase C, and JNK kinases, SEK1 and MKK7. J. Immunol. 1998;161:1795–1802. [PubMed] [Google Scholar]
- KEMPURAJ D., MADHAPPAN B., CHRISTODOULOU S., BOUCHER W., CAO J., PAPADOPOULOU N., CETRULO C.L., THEOHARIDES T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005;145:934–944. doi: 10.1038/sj.bjp.0706246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMPURAJ D., SAITO H., KANEKO A., FUKAGAWA K., NAKAYAMA M., TORU H., TOMIKAWA M., TACHIMOTO H., EBISAWA M., AKASAWA A., MIYAGI T., KIMURA H., NAKAJIMA T., TSUJI K., NAKAHATA T. Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood. 1999;93:3338–3346. [PubMed] [Google Scholar]
- KIMATA M., INAGAKI N., KATO T., MIURA T., SERIZAWA I., NAGAI H. Roles of mitogen-activated protein kinase pathways for mediator release from human cultured mast cells. Biochem. Pharmacol. 2000;60:589–594. doi: 10.1016/s0006-2952(00)00354-3. [DOI] [PubMed] [Google Scholar]
- KIMATA M., SHICHIJO M., MIURA T., SERIZAWA I., INAGAKI N., NAGAI H. Ca2+ and protein kinase C signaling for histamine and sulfidoleukotrienes release from human cultured mast cells. Biochem. Biophys. Res. Commun. 1999;257:895–900. doi: 10.1006/bbrc.1999.0557. [DOI] [PubMed] [Google Scholar]
- KUMAR A., MIDDLETON A., CHAMBERS T.C., MEHTA K.D. Differential roles of extracellular signal-regulated kinase-1/2 and p38(MAPK) in interleukin-1beta- and tumor necrosis factor-alpha-induced low density lipoprotein receptor expression in HepG2 cells. J. Biol. Chem. 1998;273:15742–15748. doi: 10.1074/jbc.273.25.15742. [DOI] [PubMed] [Google Scholar]
- LEAL-BERUMEN I., CONLON P., MARSHALL J.S. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J. Immunol. 1994;152:5468–5476. [PubMed] [Google Scholar]
- LEITGES M., SANZ L., MARTIN P., DURAN A., BRAUN U., GARCIA J.F., CAMACHO F., DIAZ-MECO M.T., RENNERT P.D., MOSCAT J. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol. Cell. 2001;8:771–780. doi: 10.1016/s1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- LIU Y., GRAHAM C., PARRAVICINI V., BROWN M.J., RIVERA J., SHAW S. Protein kinase C theta is expressed in mast cells and is functionally involved in Fcepsilon receptor I signaling. J. Leukoc. Biol. 2001;69:831–840. [PubMed] [Google Scholar]
- LU-KUO J.M., AUSTEN K.F., KATZ H.R. Post-transcriptional stabilization by interleukin-1beta of interleukin-6 mRNA induced by c-kit ligand and interleukin-10 in mouse one marrow-derived mast cells. J. Biol. Chem. 1996;271:22169–22174. doi: 10.1074/jbc.271.36.22169. [DOI] [PubMed] [Google Scholar]
- MAEDA-YAMAMOTO M., INAGAKI N., KITAURA J., CHIKUMOTO T., KAWAHARA H., KAWAKAMI Y., SANO M., MIYASE T., TACHIBANA H., NAGAI H., KAWAKAMI T. O-methylated catechins from tea leaves inhibit multiple protein kinases in mast cells. J. Immunol. 2004;172:4486–4492. doi: 10.4049/jimmunol.172.7.4486. [DOI] [PubMed] [Google Scholar]
- MARONE G., GALLI S.J., KITAMURA Y. Probing the roles of mast cells and basophils in natural and acquired immunity, physiology and disease. Trends Immunol. 2002;23:425–427. doi: 10.1016/s1471-4906(02)02274-3. [DOI] [PubMed] [Google Scholar]
- METZGER H., EGLITE S., HALEEM-SMITH H., REISCHL I., TORIGOE C. Quantitative aspects of signal transduction by the receptor with high affinity for IgE. Mol. Immunol. 2002;38:1207–1211. doi: 10.1016/s0161-5890(02)00065-2. [DOI] [PubMed] [Google Scholar]
- MIDDLETON E., JR, KANDASWAMI C., THEOHARIDES T.C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- MIYAZAWA K., MORI A., MIYATA H., AKAHANE M., AJISAWA Y., OKUDAIRA H. Regulation of interleukin-1beta-induced interleukin-6 gene expression in human fibroblast-like synoviocytes by p38 mitogen-activated protein kinase. J. Biol. Chem. 1998;273:24832–24838. doi: 10.1074/jbc.273.38.24832. [DOI] [PubMed] [Google Scholar]
- NECHUSHTAN H., LEITGES M., COHEN C., KAY G., RAZIN E. Inhibition of degranulation and interleukin-6 production in mast cells derived from mice deficient in protein kinase Cbeta. Blood. 2000;95:1752–1757. [PubMed] [Google Scholar]
- OFFERMANNS S., JONES S.V., BOMBIEN E., SCHULTZ G. Stimulation of mitogen-activated protein kinase activity by different secretory stimuli in rat basophilic leukemia cells. J. Immunol. 1994;152:250–261. [PubMed] [Google Scholar]
- O'NEILL L.A. Signal transduction pathways activated by the IL-1 receptor/toll-like receptor superfamily. Curr. Top. Microbiol. Immunol. 2002;270:47–61. [PubMed] [Google Scholar]
- OZAWA K., SZALLASI Z., KAZANIETZ M.G., BLUMBERG P.M., MISCHAK H., MUSHINSKI J.F., BEAVEN M.A. Ca(2+)-dependent and Ca(2+)-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J. Biol. Chem. 1993a;268:1749–1756. [PubMed] [Google Scholar]
- OZAWA K., YAMADA K., KAZANIETZ M.G., BLUMBERG P.M., BEAVEN M.A. Different isozymes of protein kinase C mediate feedback inhibition of phospholipase C and stimulatory signals for exocytosis in rat RBL-2H3 cells. J. Biol. Chem. 1993b;268:2280–2283. [PubMed] [Google Scholar]
- PENG Z., BEAVEN M.A. An essential role for phospholipase D in the activation of protein kinase C and degranulation in mast cells. J. Immunol. 2005;174:5201–5208. doi: 10.4049/jimmunol.174.9.5201. [DOI] [PubMed] [Google Scholar]
- RAZIN E., SZALLASI Z., KAZANIETZ M.G., BLUMBERG P.M., RIVERA J. Protein kinases C-β and C-ɛ link the mast cell high-affinity receptor for IgE to the expression of c-fos and c-jun. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7722–7726. doi: 10.1073/pnas.91.16.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIDLEY S.H., SARSFIELD S.J., LEE J.C., BIGG H.F., CAWSTON T.E., TAYLOR D.J., DEWITT D.L., SAKLATVALA J. Actions of IL-1 are selectively controlled by p38 mitogen-activated protein kinase: regulation of prostaglandin H synthase-2, metalloproteinases, and IL-6 at different levels. J. Immunol. 1997;158:3165–3173. [PubMed] [Google Scholar]
- RZYMKIEWICZ D.M., TETSUKA T., DAPHNA-IKEN D., SRIVASTAVA S., MORRISON A.R. Interleukin-1beta activates protein kinase C zeta in renal mesangial cells. Potential role in prostaglandin E2 up-regulation. J. Biol. Chem. 1996;271:17241–17246. doi: 10.1074/jbc.271.29.17241. [DOI] [PubMed] [Google Scholar]
- SIRAGANIAN R.P. Mast cell signal transduction from the high-affinity IgE receptor. Curr. Opin. Immunol. 2003;15:639–646. doi: 10.1016/j.coi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- SUZUKI K., HINO M., KUTSUNA H., HATO F., SAKAMOTO C., TAKAHASHI T., TATSUMI N., KITAGAWA S. Selective activation of p38 mitogen-activated protein kinase cascade in human neutrophils stimulated by IL-1beta. J. Immunol. 2001;167:5940–5947. doi: 10.4049/jimmunol.167.10.5940. [DOI] [PubMed] [Google Scholar]
- THEOHARIDES T.C.Synovial mast cells in inflammatory arthritis Encyclopedia of Molecular Cell Biology and Molecular Medicine 2005Wiley-VCH Verlag GmbH & Co.: KGaA, Weinheim; 37–63.ed. Meyers E.D. pp [Google Scholar]
- THEOHARIDES T.C., COCHRANE D.E. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J. Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- THEOHARIDES T.C., DONELAN J., KANDERE-GRZYBOWSKA K., KONSTANTINIDOU A. The role of mast cells in migraine pathophysiology. Brain Res. Rev. 2005;49:65–76. doi: 10.1016/j.brainresrev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- THEOHARIDES T., SANT G.R. Immunomodulators for the treatment of interstitial cystitis. Urology. 2005;65:633–638. doi: 10.1016/j.urology.2004.08.052. [DOI] [PubMed] [Google Scholar]
- VANDEN BERGHE W., PLAISANCE S., BOONE E., DE BOSSCHER K., SCHMITZ M.L., FIERS W., HAEGEMAN G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J. Biol. Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- WADSWORTH T.L., MCDONALD T.L., KOOP D.R. Effects of Ginkgo biloba extract (EGb 761) and quercetin on lipopolysaccharide-induced signaling pathways involved in the release of tumor necrosis factor-alpha. Biochem. Pharmacol. 2001;62:963–974. doi: 10.1016/s0006-2952(01)00734-1. [DOI] [PubMed] [Google Scholar]
- WEDEMEYER J., TSAI M., GALLI S.J. Roles of mast cells and basophils in innate and acquired immunity. Curr. Opin. Immunol. 2000;12:624–631. doi: 10.1016/s0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
- WHITE K.N., METZGER H. Translocation of protein kinase C in rat basophilic leukemic cells induced by phorbol ester or by aggregation of IgE receptors. J. Immunol. 1988;141:942–947. [PubMed] [Google Scholar]
- WINZEN R., KRACHT M., RITTER B., WILHELM A., CHEN C.Y., SHYU A.B., MULLER M., GAESTEL M., RESCH K., HOLTMANN H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XAGORARI A., ROUSSOS C., PAPAPETROPOULOS A. Inhibition of LPS-stimulated pathways in macrophages by the flavonoid luteolin. Br. J. Pharmacol. 2002;136:1058–1064. doi: 10.1038/sj.bjp.0704803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG C., HIRASAWA N., BEAVEN M.A. Antigen activation of mitogen-activated protein kinase in mast cells through protein kinase C-dependent and independent pathways. J. Immunol. 1997;158:4968–4975. [PubMed] [Google Scholar]