Abstract
It has not been uniform to date that the Ginkgo biloba extracts enhance cognitive function in aged animals, and the mechanisms of action remain difficult to elucidate. In this study, the Morris water maze task and electrophysiological methods were used to study the effects of repeated daily administration of EGb 761, a standardized extract from G. biloba leaves, on hippocampal-dependent spatial learning and memory and synaptic plasticity of aged rats.
The adult subjects perform the Morris water maze task better than aged rats, as a cellular mechanism, the hippocampal long-term potentiation (LTP) elicited from adult animals is robust (139.29±2.7%).
In addition, the spatial learning and memory of aged rats that had been fed on an EGb 761-supplemented diet (60 mg kg−1) for 30 days were significantly better than those of control aged rats. The magnitude of LTP (116.63±3.6%) recorded in vivo from the hippocampus CA1 area of aged rats was significantly enhanced by EGb 761 (60 mg kg−1).
In conclusion, the spatial learning and memory of aged rats is worse than that of young subjects, and EGb 761, acting as a ‘cognitive enhancer', has benefit on synaptic plasticity and cognition in aged rats. The present data further confirmed that enhancement of synaptic plasticity of the hippocampus might ameliorate the deficit in spatial learning and memory in aged rats.
Keywords: Morris water maze, hippocampus, EGb 761, aged rats, LTP, spatial learning and memory
Introduction
Ginkgo biloba leaves have been used for the treatment of respiratory system disease since hundreds of years in China. In recent decades, the pharmacological actions of EGb 761, a standardized extract of G. biloba leaves, were widely studied for its effects on cerebral vascular insufficiency, cognitive deficits and Alzheimer's disease (AD) (Le Bars, 2003; Muller & Chatterjee, 2003; Gertz & Kiefer, 2004). Although the results from clinical trials examining the efficacy of EGb 761 in AD were equivocal, animal experiments demonstrated obvious effects of EGb 761 on the central nervous system. Data revealed that EGb 761 improved cerebral dysfunction including, among others, mood disorder and anxiety. However, the effects of EGb 761 on memory have not been uniform, with different results coming from different labs. In most studies, radial maze tasks, avoidance tasks, operant conditioning and delay response tasks were used to explore the effect of EGb 761 on cognitive functions. It was found that EGb 761 facilitated learning in both young and old rodents (Winter, 1991, 1998; Cohen-Salmon et al., 1997; Walesiuk et al., 2005). However, Stoll et al. (1996) reported that the long-term memory in a passive avoidance task of aged female NMRI mice was not improved by 3 weeks of daily treatment with EGb 761 (100 mg kg−1). Additionally, ‘learned helplessness', shock-suppressed licking, forced swimming-induced immobility, shock-suppressed exploration, spontaneous exploration, food consumption in a novel situation and the passive avoidance test were used to investigate the effects of EGb 761 on anxiety behavior and memory of rats and mice by Porsolt and his co-workers. The results showed that repeated administration of EGB 761 (50 and 100 mg kg−1 day−1) induced anxiolytic-like activity; however, the performance of the passive avoidance task and the animals' response to electric shock were not affected (Porsolt et al., 1990). To clarify the data, it is important to carry out more studies of EGb 761 on the cognitive function of aged animals.
The pharmacological mechanisms of EGb 761 are to date unclear. Most studies indicated that EGb 761 is an antagonist of platelet-activating factor (PAF), and a free-radical scavenger; it inhibits lipid peroxidation and, in turn, increases cell membrane fluidity (Szabo et al., 1992, 1995; Ahlemeyer et al., 1999; Bastianetto et al., 2000; Drieu et al., 2000; Eckert et al., 2003). A study of the pharmacological mechanisms of EGb 761 showed that it produces reversible inhibition of rat brain monoamine oxidase (MAO). Our lab found that an extract of G. biloba leaves reversed the alpha-2 adrenoceptor antagonist yohimbine-induced spatial working memory deficit in adult rats (Zhang & Cai, 2005). Recent studies indicated that EGb 761 upregulated the expression of several genes, such as AMPA and chloride channel proteins (Watanabe et al., 2001), and the synaptic plasticity in hippocampal slices of aged mice was improved by EGb 761 (Williams et al., 2004).
In the present study, we confirmed the effects of EGb 761 on the spatial learning and memory of aged rats, and revealed that the mechanism was related to enhanced synaptic plasticity in vivo of the hippocampus.
Methods
Animals
Experiments were carried out on 12- to 13-week-old (adult) and 74- to 78-week-old (aged) male Wistar rats (inbred strain, Animal House Center, Kunming General Hospital, Kunming, PR China). The aged subjects were randomly allocated to a control (VEH) group (n=8), a 30 mg kg−1 EGb 761-supplemented diet group (n=8) or a 60 mg kg−1 EGb 761-supplemented diet group (n=8). The adult animals were assigned as a young control (Y-VEH) group (n=8). Rats were fed in separate home cages and were given free access to water in an established house having a 12 : 12 h light/dark cycle and thermoregulated environment. The experiments were conducted according to the guidelines for care and use of animals approved by the Chinese Academy of Sciences, China.
Drug administration
EGb 761 powder, commercially available, was produced by the Dr Willmar Schwabe Group (Germany) using a validated production process (Biber, 2003). Its pharmacologically active constituents, ginkgo flavone glycosides and terpene lactones (gikgolides and bilobalide), are 24 and 6% (3.1 and 2.9%), respectively. The concentration of ginkgolic acids is below 5 p.p.m. The constant production process also maintains the concentrations of other constituents such as proanthocyanidins (7%), carboxylic acids (13%) and non-flavone glycosides (20%). Standard animal chow pellets and powder were purchased from the Institute of Medical Biology, Chinese Academy of Medical Sciences and Peking Union Medical College. Food powder was blended with water and made into a steam-cooked ‘bread'. After cooking, a 30 or 60 mg kg−1 body weight dose of EGb 761 for individuals was added and knead into each piece of steamed bread. EGb 761 was distributed uniformly throughout the bread as could as possible by kneading, and then the experimental food was shaped into pellets for animals in the EGb 761 groups. The steamed bread without addition of EGb 761 was the control food pellet for both the control animals (VEH and Y-VEH group). Animals were individually fed with the pellets at 08:00 hours every day for 30 days. After the animals had consumed all the steamed bread, the standard pellets were supplied to the animals until 22:00 hours. All animals were treated in this way for 30 consecutive days.
Cognitive test
The Morris water maze was similar to that described previously (Yang et al., 2003). Briefly, the water maze was a circular pool (250 cm in diameter) filled with water (25±1°C) to a depth of 20 cm, and the water surface was covered with floating black resin beads. Yellow curtains were drawn around the pool (50 cm from the pool periphery). Swimming traces, escape latencies and distance traveled were monitored by an automatic tracking system. Following repeated daily administration of EGb 761 for 30 days, a behavior test was conducted. During the water maze behavioral testing period, either the EGb 761 pellets or control pellets were available to the animals.
Experiment 1, the effects of EGb 761 on spatial learning and memory using the classic Morris water maze task (Morris et al., 1982): a submerged Perspex platform (13 cm × 13 cm and 1.5 cm below the water surface) was placed in the center of a quadrant (‘quadrant I'). Animals were placed into the water at different starting positions, which were equally distributed around the perimeter of the maze, in a semirandom order, for the training of animals to find the hidden platform. The time spent by animals to find the platform was called ‘escape latency'. If an animal failed to climb onto the platform within 120 s, it was manually guided onto the platform. After the animal climbed on to the platform, it was allowed to remain there for 15 s. At the end of each trial, the animal was dried and put back into its home cage for 60 s before the next trial began. Four trials comprised a session, and two sessions per day were performed with a 2 h interval. The criterion of successful performance was designed for the animal to find the submerged platform in 30 s. The training process was ceased if the animals reached the criterion or experienced up to 4 training days. At 24 h after the training process, a probe test was given that consisted of a 180 s free swim period without the Perspex platform. The animals were put into the maze from the staring point opposite to the platform.
Experiment 2, the effects of EGb 761 on repeated-reversal spatial learning and memory with a Platform-switched Morris water maze task (Jankowsky et al., 2005): the day after experiment 1 was performed, the animals were subjected to a ‘platform-switched learning' (PSL) task for 3 days. The submerged platform was placed in the center of quadrant II (opposite to quadrant I) during the first training day, and transferred to the center of quadrants III and IV on the second and third days, respectively. Two sessions per day, as described in experiment 1, were given for training the subjects to find the hidden platform that was positioned at new locations.
Experiment 3, the effect of EGb 761 on visual acuity: during the next day after experiment 2, the animal was given a visual acuity test (VAT). The animal was required to perform eight consecutive trials in a training day, and the swimming starting position at the perimeter of the maze was varied quasi-randomly during the training. The location of an elevated platform, which had a visible mark in the maze, was varied quasi-randomly from trial to trial.
Electrophysiology
Electrophysiological recordings were carried out 24–72 h after the behavioral test, during which EGb 761 was not administered. The method, including the surgical procedures, has been described in detail previously (Xu et al., 1997; Yang et al., 2004). Experiments were carried out under pentobarbitone sodium (50–60 mg kg−1, i.p.) anesthesia and core temperature was maintained at 37±0.5°C. Animals were ventilated with 95% O2 and 5% CO2. Recordings of the field excitatory postsynaptic potential (fEPSP) were carried out from the CA1 stratum radiatum of the hippocampus in response to ipsilateral stimulation of the Schaffer collateral/commissural pathway. The electrode implantation sites were identified using stereotaxic coordinates. Two stainless steel screws (1.5 mm diameter) were inserted into the skull through a drill-hole, without piercing the dura. One served as a ground electrode (7 mm posterior to bregma and 5 mm left of the midline), the other served as the reference electrode (8 mm anterior to bregma and 1 mm left of the midline). Recording and stimulating electrodes were glued together with a pair of twisted Teflon-coated 90% platinum/10% iridium wires (50 μm inner diameter, 75 μm outer diameter, from World Precision Instruments, Inc., U.S.A.). The recording electrode was inserted ∼3.5–4.0 mm posterior to bregma and ∼2.5–3.0 mm right of the midline, and the stimulating electrode was inserted ∼4.3–4.8 mm posterior to bregma and ∼3.5–3.8 mm right of the midline in the aged rats. The optimal depth of the electrodes in the stratum radiatum of the CA1 area of the dorsal hippocampus was determined using electrophysiological criteria and was verified by postmortem examination (Leung, 1979). In all experiments, test fEPSP was evoked by a square wave of constant current pulse 0.1 ms in duration, with a frequency of 0.033 Hz and intensity adjusted to induce an fEPSP amplitude ∼50% of the maximum response. A high-frequency stimulation (HFS) protocol for inducing long-term potentiation (LTP) consisted of 10 trains of stimulus with 20 pulses at 200 Hz. The inter-train interval was set at 2 s. The LTP was measured as mean±s.e.m. Percentage of the baseline EPSP amplitude was recorded over at least a 40 min baseline period.
Statistics
Data were presented as mean±s.e.m. Significance level was set at P<0.05. Student's t-test was used to estimate whether there was LTP after HFS stimulation in each group of animals (Microsoft Excel). Multigroup behavior data and electrophysiological results were evaluated by ANOVA with Newman–Keuls post hoc test (SPSS13.0).
Results
Effects of EGb 761 on spatial learning and memory using the classic Morris water maze task
As shown in Figure 1a, the escape latency of the aged rats (VEH) was significantly longer than that of the young animals (Y-VEH) in session 2, 3 and 4 (P<0.01), analyzed using Student's t-test. The aged subjects failed to reach the criterion, but the young rats reached the criterion after 2 days of training. As the swim speed of the young rats was significantly faster than that of the aged animals (Figure 1b, P<0.05, P<0.01; Student's t-test), the distance was also analyzed to evaluate the difference of learning and memory ability between the young and aged animals. As shown in Figure 1c, the aged subjects traveled a significantly longer distance than the young rats in session 2, 3 and 4 to reach the platform (P<0.01; Student's t-test). In the probe test, the time spent in the target quadrant (TA) of the Y-VEH was significantly longer than in the opposite quadrant (OP) (Figure 1d, P<0.05; Student's t-test) and the other two quadrants (data not shown), but there was no difference in the time spent by aged rats spent in all the four quadrants, which was consistent with the result showed in Figure 1a and c. Student's t-test reveal a significance in the time spent in the TA between Y-VEH and VEH animals (P⩽0.01). Taking together the above data, it was indicated that the spatial learning and memory of aged rats was indeed poor than young subjects. As the swim speed in the Morris water maze was significantly different between young and aged rats, the data of the young rats were not taken together in the following analysis about the results of the other behavioral tasks.
Figure 1.
The spatial learning and memory was impaired concomitant with aging in the classic Morris water maze tasks. (a) The escape latency in the Morris water maze of Y-VEH adult rats (n=8) was shorter than that of the VEH aged group (n=8) (##P<0.01). Animals in the VEH group did not reach the criterion for finding the platform in 30 s. (b) The swim speed of VEH aged animals was significantly slower than Y-VEH rats (#P<0.05, ##P<0.01). (c) The distance of Y-VEH adult rats was shorter than that of the VEH aged group (n=8) (##P<0.01). (d) The time spent in the OP of Y-VEH animals was significantly shorter compared with that in the TA on the probe test day in which the platform was removed from water maze ($P<0.05), and the searching time at TA of Y-VEH animals was also significantly longer than that of VEH aged rats (##P<0.01). The data were presented as mean±s.e.m. of each session.
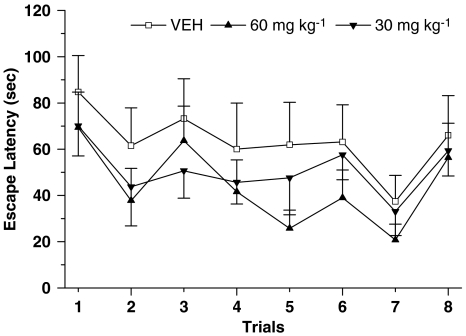
Administration of 60 mg kg−1, but not 30 mg kg−1, of EGb 761 significantly improved spatial learning in aged animals. As shown in Figure 2a, the escape latency of animals treated with 60 mg kg−1 EGb 761 group was significantly shorter than that of VEH animals on training day 3 (F(2,21)=3.68, P<0.05; F(2,21)=6.89, P<0.01) and day 4 (F(2,21)=3.36, P<0.05; F(2,21)=7.13, P<0.01). Animals administered 60 mg kg−1 EGb 761 reached the criterion after 4 days of training, but VEH animals did not reach the criterion after the same training process. The escape latency of animals in the 30 mg kg−1 EGb 761 group showed no significant difference as compared with VEH animals on all training days (F(2,21)=0.14–1.63; P>0.05). The swim speed of aged rats did not show significant differences between the VEH, 30 mg kg−1 and 60 mg kg−1 groups during all training days (P>0.05 compared with VEH; Figure 2b). So, the distance of animals treated with 60 mg kg−1 EGb 761 group was significantly shorter than that of VEH animals on training days 3 and 4 (Figure 2c). In the probe test, the animals in the 60 mg kg−1 EGb 761 group showed a longer search time in the TA than did the animals in the VEH group (F(2,21)=3.53; P<0.05; Figure 2d), and the search time in the OP of animals given 60 mg kg−1 EGb 761 was significantly shorter than that of the TA (n=8; Student's t-test: P<0.01). However, 30 mg kg−1 EGb 761 did not produce this effect (F(2,21)=0.18; P>0.05; Figure 2d). Additionally, there was no significant difference about the time spent in the other two quadrants compared across the treatment groups (data not show). The effects of EGb 761 on spatial learning and memory showed a dose-dependent pattern.
Figure 2.
Repeated daily administration of EGb 761 improved the spatial learning and memory in the classic Morris water maze tasks in aged rats. (a) The escape latency in the water maze of animals administered EGb 761 at a dose of 60 mg kg−1 (n=8), not 30 mg kg−1 (n=8), was shorter than that of the VEH group (n=8) (*P<0.05, **P<0.01 vs VEH). Animals in the VEH group did not reach the criterion for finding the platform in 30 s. (b) The swimming speed of aged animals was not affected by EGb 761 (P>0.05). (c) The distance of EGb 761 (60 mg kg−1) group animals was significantly shorter than that of VEH animals on training days 3 and 4 (*P<0.05, **P<0.01 vs VEH). (d) The searching time of EGb 761 (60 mg kg−1) group animals was significantly longer in the TA than that in the OP on the probe test day in which the platform was removed from water maze ($$P<0.01), and the searching time at TA of EGb 761 (60 mg kg−1) group animals was also significantly longer than that of VEH animals (*P<0.05). The data were presented as mean±s.e.m. of each session.
Although the animals were randomly assigned to the treatments to minimize the pre-existing factors, we further addressed this issue by analyzing the data of the first trial. The latency of Y-VEH, VEH, 60 and 30 mg kg−1 EGb 761 group was 100.56±12.3, 104.22±12.0, 101.96±10.9 and 101.69±11.1, respectively. There is no significant difference in the latency (as well as the travel distance, data not shown).
Effects of EGb 761 on repeated-reversal spatial learning and memory in the platform-switched Morris water maze task in aged rats
All groups were examined with the PSL task, which revealed that oral administration of EGb 761 markedly improved the performance of PSL task in aged rats. The escape latencies of animals in the 30 and 60 mg kg−1 EGb 761 groups were shorter than that of animals in the VEH group on all training days (F(2,21)=3.02, P<0.05; F(2,21)=11.48, P<0.01; Figure 3a). There were no significant differences in swim speeds of animals in all groups on any training day (P>0.05 compared with VEH; Figure 3b). This result suggests that EGb 761 improved the flexibility of higher brain function of aged rats.
Figure 3.
Improvement of repeated-reversal spatial learning and memory in the Platform-switched Morris water maze task by EGb 761. (a) Animals administered either 30 (n=8) or 60 mg kg−1 (n=8) EGb 761 spent less time finding the location of the switched platform than control animals (*P<0.05, **P<0.01). (b) EGb 761 did not affect the motor activation of aged rats as the swim speeds of the VEH and EGb 761 groups showed no significant difference (P>0.05). The data were presented as mean±s.e.m. of each session.
Effects of EGb 761 on the visual acuity task in aged rats
The visual acuity of aged rats was not affected by either dose of EGb 761. As shown in Figure 3, there were no significant differences in the escape latency of animals in all groups in any training trial (F(2,189)=0.52; P>0.05; Figure 4).
Figure 4.
The visual acuity of aged rats was not affected by EGb 761. The time spent finding a visible marker platform did not show a significant difference between the VEH (n=8) and EGb 761 groups (n=16) (P>0.05). The data were presented as mean±s.e.m. of each trial.
Effects of EGb 761 on synaptic plasticity in the hippocampus CA1 area of aged rats
To examine the effects of EGb 761 on synaptic plasticity in the hippocampus CA1 area of aged rats, the HFS was used for inducing the LTP in vivo. There was a large LTP induced by HFS from the hippocampus of Y-VEH rats (139.29±2.7% of baseline 50 min after HFS, n=5, Student's t-test: P<0.01 compared with baseline; Figure 5a), but HFS failed to induce the LTP from the hippocampus of VEH animals (101.11±2.3% of baseline 50 min after HFS, n=5, Student's t-test: P>0.05 compared with baseline; Figure 5b). Student's t-test revealed a significant difference in the LTP induction between the Y-VEH and VEH animals (P<0.01). HFS could successfully induce a robust LTP from the hippocampus of rats in the 60 mg kg−1 EGb 761 group (116.63±3.6% of baseline, 50 min after HFS, n=6, Student's t-test: P<0.01 compared with baseline; Figure 5c). There was no improvement in hippocampal fEPSP after HFS delivery in animals administered the 30 mg kg−1 dose (97.98±4.2% of baseline, 50 min after HFS, n=5, Student's t-test: P>0.05 compared with baseline; Figure 5d). The data show that repeated EGb 761 administration significantly enhances the hippocampal LTP of aged rats at the 60 mg kg−1, but not the 30 mg kg−1 dose (F(2,13)=10.12; P<0.01: 60 mg kg−1 EGb 761 group compared with VEH group; P>0.05: 30 mg kg−1 EGb 761 group compared with VEH group).
Figure 5.
EGb 761 enhanced the augmentation of hippocampal LTP in the CA1 area of aged rats. (a) The LTP of the hippocampus of Y-VEH adult rats was large compared with baseline after HFS (139.29±2.7%, n=5, P<0.01, down triangle). (b) The changes of hippocampal fEPSP of VEH aged rats were unreliable after HFS compared with baseline (101.11±2.3%, n=5, open square). (c) The LTP of the hippocampus of aged rats in the EGb 761 (60 mg kg−1) group was obvious compared with baseline after HFS (116.63±3.6%, n=6, P<0.01, down triangle). (d) The magnitude of hippocampal fEPSP in the EGb 761 (30 mg kg−1) group showed no significant changes compared with baseline after HFS (97.98±4.2%, n=5, up triangle), and there was no significant difference between EGb 761 (30 mg kg−1) and VEH in the amplitude changes of fEPSP following HFS (P>0.05 vs VEH).
Discussion
The present data demonstrate, for the first time, that EGb 761 ameliorates deficits in spatial learning and memory of aged rats in the Morris water maze. It is well known that the spatial learning and memory of aged rats is worse than that of young rats (Ye et al., 2000; Topic et al., 2002). The present data from the classic Morris water maze task show that the VEH animals could not reach the criterion of finding the submerged platform in 30 s after undergoing 4 days of ‘extensive' training (total 32 training trials), but the Y-VEH rats could reach the same criterion after 2 days of training. Although the Y-VEH rats swim faster than VEH animals, the VEH aged rats yet traveled longer distance to reach the platform than Y-VEH adult rats. The impairment effect of aging was likewise reflected by the different time spent in the TA between the VEH and Y-VEH animals in the probe test. In the present study, the EGb 761 60 mg kg−1 group animals could reach the criterion after 4 days of ‘extensive' training, and their search time at the TA was significantly longer than that of the OP when the platform was taken away following the 4th day of training. There was a significant difference in search time at TA between the EGb 761 60 mg kg−1 group and VEH group. In the repeated-reversal PSL task, the aged rats must learn new information and eliminate previous experience to find the platform that was repeatedly changed daily. The aged rats in the 30 and 60 mg kg−1 EGb 761 groups needed a much shorter time to find the location of the changed platform than the aged rats in the VEH group. In this task, the rats not only need the spatial information but also a strategy to modulate the behavior, which might be a reason for 30 mg kg−1 EGb 761 to have an efficacy on cognition in PSL but not in classic Morris water maze task. Although Fies & Dienel (2002) reported that EGb 761 altered vision and motor function, in the current study, escape latency in the visual acuity test and swim speed in the Morris water maze experiments were not affected by EGb 761 at the 30 and 60 mg kg−1 doses. Therefore, we believe that EGb 761 acted directly on learning and memory functions. Our data support the position of Winter E. (1991), Winter J.C. (1998) and Cohen-Salmon et al. (1997) that EGb 761 is a ‘cognition enhancer'.
Several studies have demonstrated, both in vivo and in vitro, that NMDA receptor-dependent LTP of the CA1 area of the hippocampus diminishes in aged rats (Norris et al., 1996; Lanahan et al., 1997; Rosenzweig & Barnes, 2003; Xiong et al., 2004), and synaptic plasticity, neuronal network dynamics and hippocampal-dependant cognitive functions of aged rats become worse. Our data further demonstrated that the synaptic plasticity was changed concomitant with aging. As the reduction of LTP has been extensively postulated as a cellular and molecular base for the deficit of spatial learning and memory in aged animals, our data show, for the first time, that the deficit in hippocampal LTP of aged rats that had repeated daily doses of EGb 761 was ameliorated in vivo, and as a result, the deficit in spatial learning and memory could be rescued. Similarly, Williams & his colleagues (2004) found that EGb 761 enhanced the augmentation of hippocampal LTP in vitro. However, there are differences between these studies in the magnitude of LTP in aged animals. Williams and his co-workers elicited a large LTP on the slices of hippocampus in aged mice, but we did not find LTP from the CA1 area in aged VEH rats. The contrasting results might relate to the different protocols for eliciting LTP. Some studies documented a shift in LTP from NMDA receptor-dependent mechanisms to voltage-dependent calcium channel (VDCC)-dependent mechanisms; even the magnitude of the compound LTP in vitro showed no difference between young and aged animals (Shankar et al., 1998). Recent studies showed that there was a deficit of in vivo LTP induction in aged animals with population spikes or fEPSP (Xiong et al., 2004). The protocols used to elicit LTP differ between these in vivo and in vitro experiments, and different protocols could induce different forms of LTP. The protocol used in the present study was same as that of Xiong et al. (2004), and consistent with his results, we did not induce in vivo LTP from aged VEH rats using that protocol. Nevertheless, together with findings from Williams et al. (2004), it can be concluded that EGb 761 enhanced hippocampal LTP in the CA1 area of aged rodents in vitro and in vivo.
In this study, our data further indicate that the spatial learning and memory is deficit in aged rats. There is a robust in vivo hippocampal LTP elicited by HFS from adult rats, but the hippocampal synaptic plasticity was changed in aged animals. Interestingly, EGb 761 (60 mg kg−1) significantly increased the in vivo hippocampal LTP in aged brains, and as a result, the deficit of spatial learning and memory of aged animals might be ameliorated.
Acknowledgments
We thank Professor Lin Xu and Xintian Hu for excellent technical support and constructive suggestions. This work was supported by Cooperative Program between Yunnan Province and Chinese Academy of Sciences (2000yk-02) and National Basic Research Program (G1999054000).
Abbreviations
- fEPSP
field excitatory postsynaptic potential
- LTP
long-term potentiation
- MAO
monoamine oxidase
- NMDA
N-methyl-D-asparate
- OP
opposite quadrant
- PAF
platelet-activating factor
- PSL
platform-switched learning
- TA
training quadrant
- VAT
visual acuity test
- VDCC
voltage-dependent calcium channel
References
- AHLEMEYER B., MOWES A., KRIEGLSTEIN J. Inhibition of serum deprivation- and staurosporine-induced neuronal apoptosis by Ginkgo biloba extract and some of its constituents. Eur. J. Pharmacol. 1999;367:423–430. doi: 10.1016/s0014-2999(98)00903-0. [DOI] [PubMed] [Google Scholar]
- BASTIANETTO S., RAMASSAMY C., DORE S., CHRISTEN Y., POIRIER J., QUIRION R. The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur. J. Neurosci. 2000;12:1882–1890. doi: 10.1046/j.1460-9568.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- BIBER A. Pharmacokinetics of Ginkgo biloba extracts. Pharmacopsychiatry. 2003;36 (Suppl. 1):S32–S37. doi: 10.1055/s-2003-40446. [DOI] [PubMed] [Google Scholar]
- COHEN-SALMON C., VENAULT P., MARTIN B., RAFFALLI-SEBILLE M.J., BARKATS M., CLOSTRE F., PARDON M.C., CHRISTEN Y., CHAPOUTHIER G. Effects of Ginkgo biloba extract (EGb 761) on learning and possible actions on aging. J. Physiol. Paris. 1997;91:291–300. doi: 10.1016/s0928-4257(97)82409-6. [DOI] [PubMed] [Google Scholar]
- DRIEU K., VRANCKX R., BENASSAYAD C., HAOURIGI M., HASSID J., YOA R.G., RAPIN J.R., NUNEZ E.A. Effect of the extract of Ginkgo biloba (EGb 761) on the circulating and cellular profiles of polyunsaturated fatty acids: correlation with the anti-oxidant properties of the extract. Prostaglandins Leukot. Essent. Fatty Acids. 2000;63:293–300. doi: 10.1054/plef.2000.0217. [DOI] [PubMed] [Google Scholar]
- ECKERT A., KEIL U., KRESSMANN S., SCHINDOWSKI K., LEUTNER S., LEUTZ S., MULLER W.E. Effects of EGb 761 Ginkgo biloba extract on mitochondrial function and oxidative stress. Pharmacopsychiatry. 2003;36 (Suppl. 1):S15–S23. doi: 10.1055/s-2003-40449. [DOI] [PubMed] [Google Scholar]
- FIES P., DIENEL A. Ginkgo extract in impaired vision – treatment with special extract EGb 761 of impaired vision due to dry senile macular degeneration. Wien. Med. Wochenschr. 2002;152:423–426. doi: 10.1046/j.1563-258x.2002.02066.x. [DOI] [PubMed] [Google Scholar]
- GERTZ H.J., KIEFER M. Review about Ginkgo biloba special extract EGb 761 (Ginkgo) Curr. Pharm. Des. 2004;10:261–264. doi: 10.2174/1381612043386437. [DOI] [PubMed] [Google Scholar]
- JANKOWSKY J.L., MELNIKOVA T., FADALE D.J., XU G.M., SLUNT H.H., GONZALES V., YOUNKIN L.H., YOUNKIN S.G., BORCHELT D.R., SAVONENKO A.V. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer's disease. J. Neurosci. 2005;25:5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANAHAN A., LYFORD G., STEVENSON G.S., WORLEY P.F., BARNES C.A. Selective alteration of long-term potentiation-induced transcriptional response in hippocampus of aged, memory-impaired rats. J. Neurosci. 1997;17:2876–2885. doi: 10.1523/JNEUROSCI.17-08-02876.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LE BARS P.L. Magnitude of effect and special approach to Ginkgo biloba extract EGb 761 in cognitive disorders. Pharmacopsychiatry. 2003;36 (Suppl. 1):S44–S49. doi: 10.1055/s-2003-40458. [DOI] [PubMed] [Google Scholar]
- LEUNG L.W. Orthodromic activation of hippocampal CA1 region of the rat. Brain Res. 1979;176:49–63. doi: 10.1016/0006-8993(79)90869-2. [DOI] [PubMed] [Google Scholar]
- MORRIS R.G., GARRUD P., RAWLINS J.N., O'KEEFE J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- MULLER W.E., CHATTERJEE S.S. Cognitive and other behavioral effects of EGb 761 in animal models. Pharmacopsychiatry. 2003;36 (Suppl. 1):S24–S31. doi: 10.1055/s-2003-40459. [DOI] [PubMed] [Google Scholar]
- NORRIS C.M., KOROL D.L., FOSTER T.C. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J. Neurosci. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORSOLT R.D., MARTIN P., LENEGRE A., FROMAGE S., DRIEU K. Effects of an extract of Ginkgo biloba (EGB 761) on ‘learned helplessness' and other models of stress in rodents. Pharmacol. Biochem. Behav. 1990;36:963–971. doi: 10.1016/0091-3057(90)90107-s. [DOI] [PubMed] [Google Scholar]
- ROSENZWEIG E.S., BARNES C.A. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- SHANKAR S., TEYLER T.J., ROBBINS N. Aging differentially alters forms of long-term potentiation in rat hippocampal area CA1. J. Neurophysiol. 1998;79:334–341. doi: 10.1152/jn.1998.79.1.334. [DOI] [PubMed] [Google Scholar]
- STOLL S., SCHEUER K., POHL O., MULLER W.E. Ginkgo biloba extract (EGb 761) independently improves changes in passive avoidance learning and brain membrane fluidity in the aging mouse. Pharmacopsychiatry. 1996;29:144–149. doi: 10.1055/s-2007-979561. [DOI] [PubMed] [Google Scholar]
- SZABO M.E., DROY-LEFAIX M.T., DOLY M. Modification of reperfusion-induced ionic imbalance by free radical scavengers in spontaneously hypertensive rat retina. Free Radic. Biol. Med. 1992;13:609–620. doi: 10.1016/0891-5849(92)90035-f. [DOI] [PubMed] [Google Scholar]
- SZABO M.E., DROY-LEFAIX M.T., DOLY M. EGb 761 and the recovery of ion imbalance in ischemic reperfused diabetic rat retina. Ophthalmic Res. 1995;27:102–109. doi: 10.1159/000267606. [DOI] [PubMed] [Google Scholar]
- TOPIC B., TANI E., TSIAKITZIS K., KOUROUNAKIS P.N., DERE E., HASENOHRL R.U., HACKER R., MATTERN C.M., HUSTON J.P. Enhanced maze performance and reduced oxidative stress by combined extracts of zingiber officinale and Ginkgo biloba in the aged rat. Neurobiol. Aging. 2002;23:135–143. doi: 10.1016/s0197-4580(01)00241-x. [DOI] [PubMed] [Google Scholar]
- WALESIUK A., TROFIMIUK E., BRASZKO J.J. Gingko biloba extract diminishes stress-induced memory deficits in rats. Pharmacol. Rep. 2005;57:176–187. [PubMed] [Google Scholar]
- WATANABE C.M., WOLFFRAM S., ADER P., RIMBACH G., PACKER L., MAGUIRE J.J., SCHULTZ P.G., GOHIL K. The in vivo neuromodulatory effects of the herbal medicine Ginkgo biloba. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6577–6580. doi: 10.1073/pnas.111126298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS B., WATANABE C.M., SCHULTZ P.G., RIMBACH G., KRUCKER T. Age-related effects of Ginkgo biloba extract on synaptic plasticity and excitability. Neurobiol. Aging. 2004;25:955–962. doi: 10.1016/j.neurobiolaging.2003.10.008. [DOI] [PubMed] [Google Scholar]
- WINTER E. Effects of an extract of Ginkgo biloba on learning and memory in mice. Pharmacol. Biochem. Behav. 1991;38:109–114. doi: 10.1016/0091-3057(91)90597-u. [DOI] [PubMed] [Google Scholar]
- WINTER J.C. The effects of an extract of Ginkgo biloba, EGb 761, on cognitive behavior and longevity in the rat. Physiol. Behav. 1998;63:425–433. doi: 10.1016/s0031-9384(97)00464-2. [DOI] [PubMed] [Google Scholar]
- XIONG W., WEI H., XIANG X., CAO J., DONG Z., WANG Y., XU T., XU L. The effect of acute stress on LTP and LTD induction in the hippocampal CA1 region of anesthetized rats at three different ages. Brain Res. 2004;1005:187–192. doi: 10.1016/j.brainres.2004.01.051. [DOI] [PubMed] [Google Scholar]
- XU L., ANWYL R., ROWAN M.J. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- YANG Y., CAO J., XIONG W., ZHANG J., ZHOU Q., WEI H., LIANG C., DENG J., LI T., YANG S., XU L., XU L. Both stress experience and age determine the impairment or enhancement effect of stress on spatial memory retrieval. J. Endocrinol. 2003;178:45–54. doi: 10.1677/joe.0.1780045. [DOI] [PubMed] [Google Scholar]
- YANG Y., ZHENG X., WANG Y., CAO J., DONG Z., CAI J., SUI N., XU L. Stress enables synaptic depression in CA1 synapses by acute and chronic morphine: possible mechanisms for corticosterone on opiate addiction. J. Neurosci. 2004;24:2412–2420. doi: 10.1523/JNEUROSCI.5544-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YE J.W., SHANG Y.Z., WANG Z.M., TANG X.C. Huperzine A ameliorates the impaired memory of aged rat in the Morris water maze performance. Acta. Pharmacol. Sin. 2000;21:65–69. [PubMed] [Google Scholar]
- ZHANG M., CAI J. Extract of Ginkgo biloba leaves reverses yohimbine-induced spatial working memory deficit in rats. Behav. Pharmacol. 2005;16:651–656. doi: 10.1097/00008877-200512000-00008. [DOI] [PubMed] [Google Scholar]