Abstract
Magnolol (Mag), an active constituent isolated from the Chinese herb Hou p'u (Magnolia officinalis) has long been used to suppress inflammatory processes. Chronic inflammation is well known to be involved in vascular injuries such as atherosclerosis in which interleukin (IL)-6 may participate. Signal transducer and activator of transcription protein 3 (STAT3), a transcription factor involved in inflammation and the cell cycle, is activated by IL-6. In this study, we evaluated whether Mag can serve as an anti-inflammatory agent during endothelial injuries. The effects of Mag on IL-6-induced STAT3 activation and downstream target gene induction in endothelial cells (ECs) were examined. Pretreatment of ECs with Mag dose dependently inhibited IL-6-induced Tyr705 and Ser727 phosphorylation in STAT3 without affecting the phosphorylation of JAK1, JAK2, and ERK1/2. Mag pretreatment of these ECs dose dependently suppressed IL-6-induced promoter activity of intracellular cell adhesion molecule (ICAM)-1 that contains functional IL-6 response elements (IREs). An electrophoretic mobility shift assay (EMSA) revealed that Mag treatment significantly reduced STAT3 binding to the IRE region. Consistently, Mag treatment markedly inhibited ICAM-1 expression on the endothelial surface. As a result, reduced monocyte adhesion to IL-6-activated ECs was observed. Furthermore, Mag suppressed IL-6-induced promoter activity of cyclin D1 and monocyte chemotactic protein (MCP)-1 for which STAT3 activation plays a role. In conclusion, our results indicate that Mag inhibits IL-6-induced STAT3 activation and subsequently results in the suppression of downstream target gene expression in ECs. These results provide a therapeutic basis for the development of Mag as an anti-inflammatory agent for vascular disorders including atherosclerosis.
Keywords: Magnolol, endothelial cells, STAT3, ICAM-1, cyclin D1
Introduction
Magnolol (Mag), an active constituent isolated from the Chinese herb Hou p'u (Magnolia officinalis), is known to possess therapeutic properties such as antiplatelet aggregation, vessel dilatation, anticancer and anti-inflammatory activities (Teng et al., 1991; Fujita & Taira, 1994; Shen et al., 1998; Kong et al., 2000; Lee et al., 2001). Mag may have therapeutic potential for the treatment of chronic inflammatory diseases associated with atherosclerosis, asthma, and cancer. However, the detailed mechanisms of its anti-inflammatory effect remain to be defined. Chronic inflammation is an underlying mechanism of atherogenesis, a process in which cytokines are well known to participate. Endothelial injuries are believed to be an initial event in atherosclerosis. As Mag has been shown to have an anti-inflammatory effect, atheroprotective effects of Mag on cytokine-induced endothelial injury are being investigated. Earlier studies showed that Mag attenuated TNFα-induced vascular cell adhesion molecule-1 (VCAM-1) expression via the inhibition of NF-κB translocation (Chen et al., 2002). In contrast, TNFα-induced intracellular cell adhesion molecule (ICAM-1) expression was not affected by Mag treatment. In addition to the well-characterized NF-κB pathway, signal transducers and activators of transcription (STATs) are also known to participate in the expression of inflammation-related genes such as ICAM-1 (Kim et al., 2002; Chen et al., 2004; Yang et al., 2005) and monocyte chemotactic protein-1 (MCP-1) (Lakshminarayanan et al., 2001; Kim et al., 2002). STATs are a family of functionally related proteins that play key roles not only in the immune response but also in the actions of nonimmune mediators such as growth factors and hormones. Among those STATs, signal transducer and activator of transcription protein 3 (STAT3) can be activated by growth factors and cytokines. Interleukin (IL)-6, a proinflammatory cytokine which preferentially activates STAT3, is well recognized for its role in initiating and amplifying inflammatory processes. In addition to its role in inflammation, IL-6 also acts as an angiogenic factor by promoting VEGF expression and inducing motility of endothelial cells (ECs) (Giraudo et al., 1996; Loganadane et al., 1997). An increase in the IL-6 concentration in plasma has been shown to be associated with cardiovascular diseases including myocardial infraction and atherosclerosis (Ridker et al., 2000). We recently showed that IL-6-induced proliferation in bovine aortic ECs (Ni et al., 2004). IL-6 induces phosphorylation of Tyr705 and Ser727 in STAT3. The dimerized STAT3 is then translocated to the nucleus. This results in the induction of target genes that contain IL-6 response elements (IREs). The palindromic IRE, a cis-element required for STAT-dependent transcription, is located 115 base pairs (bp) upstream of the translation initiation site. STAT3 has been intensively studied for its role in proliferation, differentiation, transformation, inflammation, and immune response. STAT3 may possibly serve as a therapeutic target and has thus garnered a great deal of interest.
During inflammation, adhesion molecules expressed on stimulated ECs are essential for recruitment and transmigration of leukocytes to the subendothelial matrix. ICAM-1, an inducible cell surface glycoprotein, is known to participate in leukocyte adhesion and transmigration in ECs stimulated by cytokines. The induction of ICAM-1 by various cytokines is mainly regulated by transcription factors including NF-κB, AP1, IRE-binding factors, and SP1 that bind to the promoter region (Caldenhoven et al., 1994; Stratowa & Audette, 1995). IL-6-induced ICAM-1 expression is mediated via the JAK/Stat3 signaling pathway in which STAT3 phosphorylation followed by its binding to IRE is required. IREs are also found in the promoter of other inflammatory- or cell cycle-related genes including MCP-1 and cyclin D1. Cells express elevated levels of cyclin D1 when stably transfected with a dominant-active STAT3 construct (Kijima et al., 2002). In the present study, the effects of Mag on IL-6-induced STAT3 activation were analyzed along with the STAT3 downstream target genes of ICAM-1, MCP-1, and cyclin D1. Our results show that Mag treatment of ECs inhibits the expression of these genes via suppression of the STAT3 signaling pathway. Our results support the notion of the potential for developing Mag as an anti-inflammatory herbal medicine for therapeutic use particularly in cytokine-induced vascular disorders.
Methods
Chemicals
Mag was purchased from Nacalai (Kyoto, Japan). All other reagents were purchased from Sigma Chemical (St Louis, MO, U.S.A.) except where otherwise specified.
Cell culture
Bovine aortic ECs were cultured and maintained in DMEM medium (Life Technologies, Gaithersburg, MD, U.S.A.), supplemented with 2 mM L-glutamine and 10% fetal bovine serum (FBS) (Life Technologies). The cell viability and cell number were determined by the trypan blue dye-exclusion method.
Immunoblot analysis
ECs were lysed in buffer-containing SDS and then analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). Phosphorylated STAT3 as well as total STAT3 contents were examined by Western blotting. Antibodies to STAT3 and phosphorylated STAT3 (Tyr705 or Ser727), phosphorylated JAK1 or JAK2 (pJAK1, pJAK2), and phosphorylated ERK1/2 (pERK1/2) were obtained from Biosource (Camarillo, CA, U.S.A.). Antigen–antibody complexes were detected using horseradish peroxide-labeled rabbit anti-mouse IgG. Results were analyzed using an ECL detection system (Pierce, Rockford, IL, U.S.A.).
Transient transfection and promoter activity assay
The promoter constructs of ICAM-1 (P850 (850 bp) and P137 (137 bp)) and MCP-1 (540 bps) were as described previously (Wung et al., 2001). The cyclin D1 promoter (1745 bp) construct was a kind gift from Dr Yuzuru Kanakura, Osaka, Japan (Matsumura et al., 1999). These promoter constructs all contain luciferase as a reporter gene. Different promoter constructs were cotransfected with pSV-β-galactosidase using the Lipofectamine method (GIBCO-BRL, Grand Island, NY, U.S.A.). Luciferase and β-galactosidase activities were assayed according to the manufacturer's protocol (Promega, Madison, WI, U.S.A.). Luciferase activity was normalized with β-galactosidase, and results are expressed as the mean±s.e.m. from three independent experiments. Folds of induction are shown in the treated ECs as compared with those of untreated controls.
Nuclear extract preparation and electrophoretic mobility shift assay
Nuclear proteins were extracted from ECs as described previously (Wung et al., 1997). ECs were washed with cold PBS and immediately removed by scraping in PBS. After centrifugation at 2000 r.p.m., the cell pellets were resuspended in cold buffer A (10 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride) for 15 min. Cells were lysed by adding 10% Nonidet P-40 and then centrifuged at 6000 r.p.m. to obtain a pellet of nuclei. The nucleic pellets were resuspended in cold buffer B (20 mM HEPES, 0.4 M NaCl, 1 mM EDTA, 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride), vigorously agitated, and then centrifuged. The supernatant containing the nuclear proteins was used for the electrophoretic mobility shift assay (EMSA) or stored at −70°C for later use. A double-stranded 24-mer DNA probe (5′-GATCCTTCTGGGAATTCCTAGATC-3′) containing IRE consensus sequence and the reverse complement, 3′-CTAGGAAGACCCTTAAGGATCTAG-5′, were end-labeled with [γ-32P]ATP by T4 polynucleotide kinase. The nuclear extract (10 μg) was incubated with 32P-labeled DNA (0.1 ng) for 15 min at room temperature in a final volume of 25 μl of binding buffer containing 1 μg of poly (dI-dC). The mixtures were electrophoresed on 6% nondenaturing polyacrylamide gels under high ionic strength. Gels were dried, and the results were detected by autoradiography.
Flow cytometric analysis
ECs were washed once with ice-cold PBS and harvested with versene buffer. After centrifugation, the supernatant was removed. Cells were resuspended and incubated in PBS containing an FITC-conjugated monoclonal anti-human ICAM-1 antibody. After incubation at 4°C for 30 min, cells were centrifuged at 13,000 r.p.m., washed twice with PBS, fixed in 2% paraformaldehyde, and analyzed using a fluorescence-activated cell sorter (FACScan, Becton Dickinson, NJ, U.S.A.) using 104 cells per sample. The specific mean fluorescence intensity was obtained after correction for nonspecific binding.
Cell adherence measurements
Cell adherence was measured as described previously (Cheng et al., 1996). The human monocytic cell line, THP-1, was obtained from American Type Culture Collection (Manassas, VA, U.S.A.). THP-1 cells were suspended in RPMI 1640 containing 0.1% FCS and labeled with 1 μCi 3H-labeled thymidine (with a specific activity of 23 Ci mmol−1; Amersham Biosciences, U.K.) overnight. Cells were washed three times in fresh RPMI 1640 culture medium, and 3 × 105 cells was added to each well containing ECs and incubated for 1 h. Nonadherent THP-1 cells were removed by washing with DMEM. ECs with adherent THP-1 cells were removed with lysis buffer, and radioactivities were measured using a scintillation counter.
Statistical analysis
Statistical analyses were performed using the Student's t-test. Data are presented as the mean±s.e.m. Statistical significance was defined as P<0.05.
Results
Mag suppresses IL-6-induced STAT3 phosphorylation in ECs
The toxicity of Mag on ECs was performed by MTT assay in which MTT-formazan adduct was detected. Cell viability was calculated as percentage of untreated control. ECs survived over 95% after 24 h treatment of Mag at 30 μM. This result showed that Mag has almost no significant toxicity to ECs at concentration up to 30 μM.
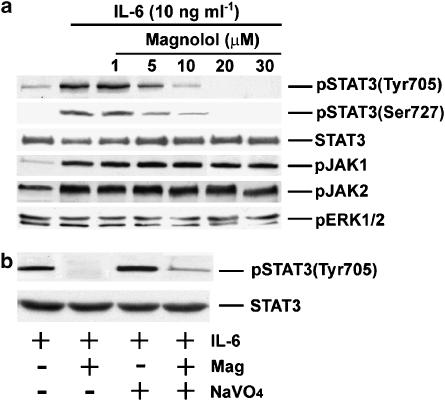
The effect of Mag on IL-6-induced STAT3 phosphorylation was examined in cultured ECs. Phosphorylation of Tyr705 and Ser727 in STAT3 by IL-6 is critical for STAT3 activity and downstream target gene induction. As shown in Figure 1, IL-6 (10 ng ml−1) treatment induced rapid phosphorylation of try705 in ECs. Mag pretreatment of ECs for 1 h dose dependently inhibited IL-6-induced try705 phosphorylation. Mag at 20 μM completely abolished the IL-6-induced try705 phosphorylation in STAT3. Similarly, Mag treatment suppressed IL-6-induced Ser727 phosphorylation in STAT3. However, Mag treatment showed neither inhibition of IL-6-induced JAK1 and JAK2 activation nor the basal ERK1/2 phosphorylation. These results show Mag specifically suppresses IL-6-induced STAT3 activity in ECs.
Figure 1.
IL-6-induced phosphorylation of Tyr705 and Ser727 in STAT3 is inhibited by Magnolol with a dose-dependent manner. (a) ECs were pretreated with Mag at indicated concentration for 1 h following with IL-6 (10 ng ml−1) treatment for 20 min in the presence of Mag. After treatment, ECs were examined for the phosphorylation of Tyr705 and Ser727 in STAT3 (pSTAT3 Tyr705 and pSTAT3 Ser727), Try 1022/1023 of JAK1 (pJAK1), Tyr 1007/1008 of JAK2 (pJAK2), and ERK1/2 (pERK1/2) by using antibody specifically reacts to each phosphorylated protein. STAT3 is shown to indicate that equal amounts of protein in each lane. (b) ECs were incubated with IL-6 (10 ng ml−1) in the presence or absence of Mag (20 μM) and/or sodium orthovanadate (NaVO4). The phosphrylation of STAT3 Tyr 705 was determined and the membranes were stripped and reprobed with antibody directed to STAT3. Similar results were obtained from three separate experiments.
The possibility whether Mag may activate protein tyrosine phosphatase(s) that subsequently dephosphorylate JAKs and STAT3 was examined. Result showed in Figure 1b indicated that protein tyrosine phosphatase did not play a major role in this suppression effects by Mag because ECs pretreaing with a tyrosine phosphatase inhibitor (sodium orthovanadate) did not reverse the Mag-dependent suppression of STAT3 phosphorylation.
Mag inhibits IL-6-induced ICAM-1 promoter activity in ECs
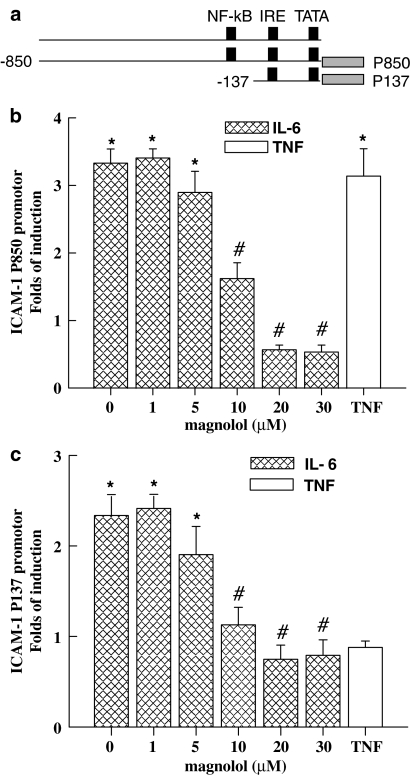
The ICAM-1 promoter construct, P850, contains cis-elements of NF-κB, AP1, and SP1. The deletion construct, P137, retains only the IRE cis-element. Promoter activities were examined to determine the functional activities of these cis-acting elements. The effect of Mag on IL-6-induced ICAM-1 expression was elucidated on ECs transfected with each ICAM-1 promoter construct. ECs were incubated with or without Mag at the indicated concentrations for 1 h prior to treatment with IL-6 (10 ng ml−1) for 16 h. As shown in Figure 2a, three-fold increases in ICAM-1 promoter activities were observed after IL-6 treatment as compared to untreated ECs (Figure 2a). Mag had no effect on the basal promoter activity of ICAM-1 (P850). In contrast, Mag dose dependently reduced IL-6-induced ICAM-1 promoter activity. Furthermore, the P137 deletion construct still responded to IL-6 stimulation despite its nonresponsiveness to TNF-α (Figure 2b). Mag pretreatment consistently suppressed the promoter activity of P137 in a similar manner to that of P850. These results suggest that the inhibitory effect of Mag on IL-6-induced ICAM-1 promoter activity is located within 137 bp of the ICAM-1 promoter.
Figure 2.
Magnolol inhibits IL-6-induced ICAM-1 promoter activity. Promoter construct (P850 or P137) of ICAM-1 containing luciferase as a reporter gene was cotransfected with pSV-β-galactosidase plasmid into ECs. ECs were pretreated with Mag at indicated dose for 1 h following with stimulation of IL-6 (10 ng ml−1) for 16 h in the presence of Mag. Luciferase activities after normalizing with β-galactosidase were expressed as folds of induction. Folds of induction are shown in the treated ECs as compared with those of untreated controls. Results are shown as mean±s.e. from three independent experiments. *P<0.05 vs control cells; #P<0.05 vs IL-6-treated cells.
Mag treatment suppresses IL-6-induced IRE activation
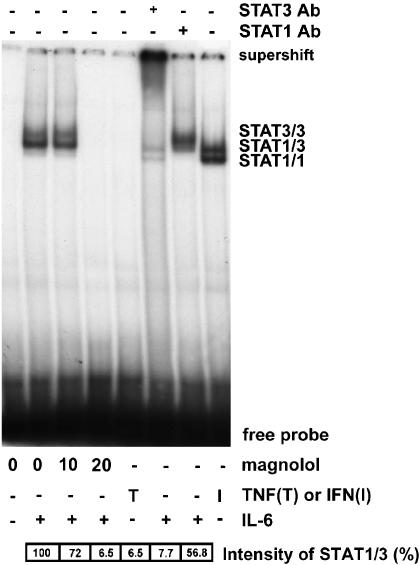
To verify whether the inhibitory mechanism of Mag towards ICAM-1 promoter activity is mediated via suppression of STAT3 transcriptional activity, nuclear extracts of ECs were prepared, and STAT3 binding to the IRE was analyzed by EMSA (Figure 3). An increase in the STAT3-binding complex was observed in nuclear extracts from ECs treated with IL-6. This binding complex was mainly composed of a STAT3/3 homodimer and STAT1/3 heterodimer. When pretreated with Mag, ECs suppressed this IL-6-induced STAT3-binding activity in a concentration-dependent manner. Furthermore, a super-shift band was noted only when an antibody to STAT3, but not one to STAT1, was preincubated with nuclear extracts. These results indicate that Mag suppresses STAT3 activation and results in a decrease in IRE-binding activity to the ICAM-1 promoter.
Figure 3.
Magnolol treatment suppresses IL-6-induced Stat3 binding to IRE. ECs were pretreated with Mag at indicated dose for 1 h following with stimulation of IL-6 (10 ng ml−1) for 20 min in the presence of Mag. Nuclear protein was extracted and electrophoretic mobility shift assay (EMSA) was performed using radiolabeled oligonucleotides containing consensus IRE-binding sequence. Supershift of EMSA was performed by preincubating nuclear extracts with antibody to Stat3 or Stat1. For Stat3 activation, ECs treated with IL-6 or TNF-α (10 ng ml−1) were used as positive or negative controls, respectively. IFN-γ-treated ECs (1 ng ml−1) were used as positive controls for Stat1 activation. Relative intensity of each STAT1/3 band was expressed as percentage as compared with IL-6 treated cells. Data shown are the representative from three independent experiments with similar results.
Mag reduces IL-6-induced ICAM-1 expression
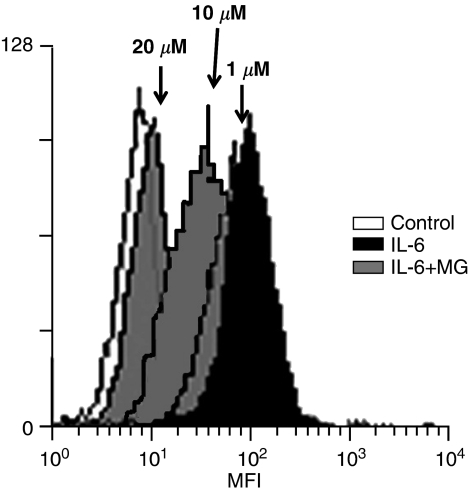
The effect of Mag on IL-6-induced ICAM-1 expression on the endothelial surface was examined (Figure 4). ECs with or without Mag treatment were cultured for 24 h in the presence of IL-6. The expression of ICAM-1 on the endothelial surface was analyzed using flow cytometry. ECs treated with IL-6 showed significant increases in the expression of ICAM-1 (Figure 4). This induction was nearly abolished after preincubating ECs with Mag (20 μM). These results clearly show that Mag pretreatment of ECs suppresses IL-6-induced ICAM-1 expression.
Figure 4.
Magnolol decreases IL-6-induced ICAM-1 expression on endothelial surface. ECs pretreated with Mag for 1 h were followed with the stimulation of IL-6 (10 ng ml−1) for 24 h in the presence of Mag. ECs were resuspended and incubated in PBS with FITC-conjugated monoclonal anti-human ICAM-1 antibody. ECs were washed and fixed and analyzed using a fluorescence-activated cell sorter (FACScan, Becton Dickinson, NJ, U.S.A.) using 104 cells per sample. ICAM-1 expression on cell surface is represented by mean fluorescence intensity (MFI). Result is a representative of three independent experiments with similar results.
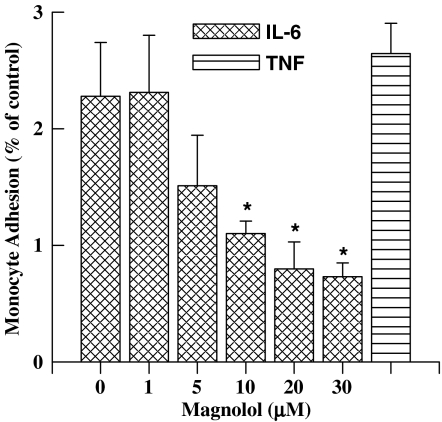
Mag pretreatment of ECs suppresses IL-6-induced THP-1 cell adhesion
Increased endothelial expression of ICAM-1 results in an increase of monocyte adhesion to ECs (Cheng et al., 1996). To address whether the inhibitory effects of Mag on ICAM-1 expression decreased the monocytic THP-1 adhesion to ECs, the adhesiveness of THP-1 cells to IL-6-stimulated ECs with or without Mag pretreatment was examined (Figure 5). ECs were incubated with or without Mag at various concentrations for 1 h prior to the treatment of IL-6. As shown, Mag pretreatment dose dependently reduced IL-6-induced THP-1 cell adhesion. ECs treated with Mag at concentrations up to 20 μM suppressed THP-1 adhesion to near control levels. Therefore, the inhibitory effect of Mag on ICAM-1 expression results in a decrease of THP-1 cell adhesion to IL-6-treated ECs.
Figure 5.
Magnolol pretreatment inhibits IL-6-induced monocyte adhesion to ECs. ECs were incubated with or without Mag for 1 h prior to IL-6 (10 ng ml−1) treatment for 24 h. Treated ECs were then incubated with 3H-labeled THP-1 monocytic cells for 1 h. Adherent THP-1 cells were lysed, and the radioactivity was counted. Results are shown as folds of induction of radioactivity from experimental groups compared with those of untreated controls. ECs treated with TNF-α (10 ng ml−1) for 24 h were used as a positive control. Data are shown as mean±s.e.m. from five separate experiments. *P<0.05 vs IL-6-treated ECs.
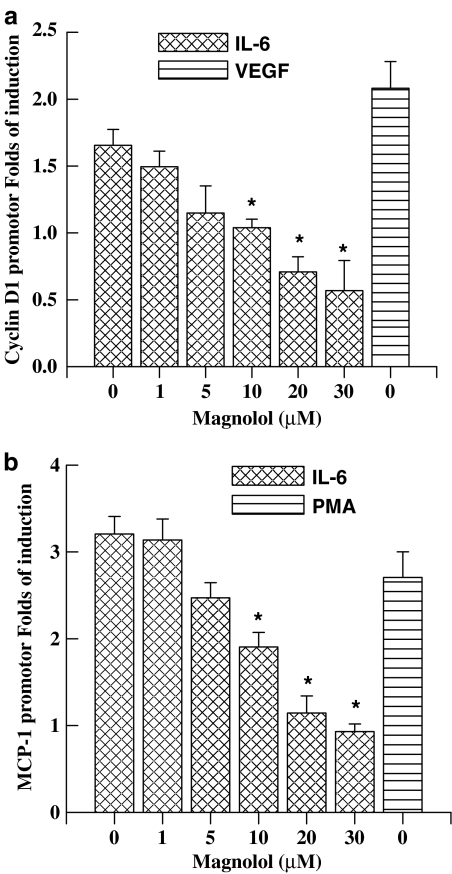
Mag inhibits IL-6-induced MCP-1 and cyclin D1 promoter activities
The above results indicate that the inhibitory effect of Mag on IL-6-induced ICAM-1 gene expression is mediated, at least in part, via the JAK/STAT signaling pathway. We further examined other downstream target genes that contain functional IREs in their promoter region and which are inducible by cytokine stimulation, namely cyclin D1 (Stratowa & Audette, 1995) and MCP-1 (Kijima et al., 2002; Stratowa & Audette, 1995; Zhou et al., 1998). The inhibitory effects of Mag on each promoter activity were examined. ECs pretreated with Mag were followed by IL-6 stimulation for 16 h. As shown, IL-6 treatment of ECs increased promoter activities of both cyclin D1 and MCP-1, similar to those of ECs with VEGF or PMA treatment (Figure 6). However, Mag pretreatment of ECs suppressed IL-6-induced both promoter activities in a dose-dependent manner. These results further confirm that Mag is able to inhibit STAT3-dependent transcriptional activation that leads to suppression of downstream target genes in ECs. Our results suggest the potential for the therapeutic development of Mag as an anti-inflammatory remedy for suppressing STAT3-mediated responses induced by cytokine.
Figure 6.
Magnolol treatment suppresses IL-6-induced cyclin D1 and MCP-1 promoter activities. Promoter construct of cyclin D1 (1745 bps) or MCP-1 (540 bps) containing luciferase as a reporter gene was cotransfected with pSV-β-galactosidase plasmid into ECs. ECs were pretreated with Mag at indicated concentration for 1 h followed with the stimulation of IL-6 (10 ng ml−1) for 16 h in the presence of Mag. ECs treated with VEGF (10 ng ml−1) or PMA (100 ng ml−1) were used as positive controls for cyclin D1 and MCP-1, respectively. Luciferase activities were normalized with β-galactosidase and results were expressed as mean±s.e. from three independent experiments. *P<0.05 vs IL-6-treated ECs without Mag.
Discussion
Mag is used as a blood-quickening and stasis-dispelling agent in traditional Chinese medicine. Mag possesses anti-inflammatory properties that are believed to contribute to its therapeutic effects. In this study, we have shown that Mag inhibits the IL-6-induced STAT3 signaling pathway that results in suppression of downstream inflammation-associated genes. Several lines of evidence support this notion. First, Mag inhibited the IL-6-induced responses by suppressing Stat3 phosphorylation without affecting its upstream kinase JAK1 and JAK2 phosphorylation. Second, it suppressed the IL-6-induced ICAM-1 promoter activity. Third, IL-6-enhanced STAT3 binding to the IRE in the promoter region was decreased by Mag pretreatment. Fourth, IL-6-induced ICAM-1 expression on the endothelial surface was suppressed by Mag pretreatment. Fifth, Mag pretreatment reduced the adhesion of monocytes to IL-6-treated ECs. Finally, IL-6-induced cyclin D1 and MCP-1 expressions in ECs were also attenuated by Mag pretreatment. These results provide direct evidence that Mag exerts an anti-inflammtory effect on ECs by suppressing IL-6-induced STAT3 activation.
JAK-STAT signaling is closely associated with inflammation and accounts for various cellular responses to a number of cytokines, growth factors, and hormones. Phosphorylated STAT3 binds to the IRE and functionally regulates gene expression (Gao et al., 1997; Ehret et al., 2001). We have shown that STAT3 is the dominant transcriptional factor binding to the GAS/ISRE in IL-6-treated ECs (Ni et al., 2004). In the present study, IL-6-induced phosphorylation of Tyr705 and Ser727 in STAT3, which is crucial for its transcriptional activity, was inhibited by Mag treatment. As a result, Mag treatment decreased Stat3 binding to DNA. Furthermore, this inhibition by Mag was shown to be concentration dependent. The detailed mechanisms underlying the inhibiting mechanism of Mag on STAT3 activation are not clear. The phosphorylation of STAT3 upstream kinases, JAK1 and JAK2, was not affected by Mag treatment. Our results also indicate that protein tyrosine phosphatase does not play a major role in the suppressive effect by Mag. It is known that cytokine response and the activation of STATs can be negatively regulated. Among the negative regulators are the suppressor of cytokine signaling (SOCS) proteins (Naka et al., 1997). While SOCS proteins interact with JAKs and very probably reduce their tyrosine kinase activity, other inhibitors called PIAS (protein inhibitor of activated STAT) bind to activated STAT dimmers and block their DNA-binding activity (Chung et al., 1997). The possibility that PIAS and SOCSs contribute to the downregulation of STAT3 activation cannot be ruled out. The molecular mechanisms of this inhibiting effect by Mag on ECs reward for further investigation.
IL-6 has been shown to be associated with cardiovascular diseases (Schindler, 2002). IL-6-induced promoter activity of the promoter constructs, P850 and P137, of ICAM-1. ICAM-1 with the deletion of the NF-κB binding site (P137) caused ICAM-1 to lose its promoter activity toward TNFα, but to retain its activity toward IL-6. However, Mag dose dependently attenuated the promoter activities of both P850 and P137, indicating that Mag reducing STAT3 binding to IRE contributes to the decreased ICAM-1 promoter activity. It has been reported that Mag attenuates TNFα-induced expression of VCAM-1, but not ICAM-1 (Chen et al., 2002), via suppression of NF-κB activity (Chen et al., 2002). However, our results suggested that STAT3 suppression by Mag may be also involved in ICAM-1 inhibition. We recently showed that ICAM-1 induction by TNF and IL-6 is mediated via a distinct pathway via Rac in ECs (Wung et al., 2005). Rac appears to be upstream of NF-κB and Stat3 and can, respectively, be attributed to TNF- and IL-6-induced ICAM-1 expression. Rac activation has been reported to participate in the regulation of IL-6-induced Stat3 activity (Faruqi et al., 2001). Whether Mag inhibits IL-6-induced Rac activity and then results in suppression of Stat3 activity is an interesting question and remains to be determined.
ECs under inflammatory stimulation may produce cytokines, chemokines, and cell adhesion molecules, which participate in vascular remodeling and/or injuries such as atherosclerosis (Yu et al., 2004). Suppression of the release of these mediators is important for controlling inflammation. In this study, we examined those inflammation- and growth-related genes that contain functional GAS/IREs in their promoter region including ICAM-1, MCP-1, and cyclin D1 (Stratowa & Audette, 1995; Zhou et al., 1998; Kijima et al., 2002). The Mag-induced suppression of monocyte-EC adhesion via the inhibition of ICAM-1 and MCP-1 expression may have therapeutic implications in treating vascular inflammations. It has been reported that STAT3 activation upregulates target genes such as c-myc, cyclin D1, and Bcl-xL that leads to cell cycle progression and/or prevention of apoptosis (Sinibaldi et al., 2000; Turkson, 2004). The stable transfection of cells with a dominant-active mutant of STAT3 results in elevated levels of Bcl-xL and cyclin D1 and exhibits an increased proliferation associated with tumor growth (Turkson, 2004). We recently showed that IL-6-treated ECs increased endothelial proliferation, while the dominant-negative mutant of Stat3 suppressed this response (Ni et al., 2004). Thus, Mag inhibits IL-6-induced cyclin D1 promoter activity and consequently may suppress IL-6-induced endothelial proliferation. Mag may exert its function of maintaining endothelial growth arrest by inhibiting IL-6-induced STAT3 activation.
In conclusion, our findings clearly show that Mg inhibits the JAK/STAT3 signaling pathway and results in the inhibition of downstream target genes for which STAT3-binding activity is crucial. Multiple recurrent inflammatory events contribute to the initiation and progression of atherosclerosis. It is possible that IL-6 produced during inflammation activates JAK2/STAT3 and initiates EC proliferation and inflammatory response. The impaired STAT3 activation following Mag treatment shows that Mag exerts an atheroprotective effect by attenuating the JAK/STAT3 signaling process. Further elucidation of the detailed mechanisms is crucial for the therapeutic development of Mag for treating vascular disorders such as atherosclerosis and inflammation-related injuries.
Acknowledgments
This work was supported in part by a Grant (NSC92-2320-B-077-013) from the National Science Council, Taiwan, and a Grant (NRICM-93-DBCMR–08) from the National Research Institute of Chinese Medicine, Taiwan. We thank Mr D.P. Chamberline for critical reading the manuscript.
Abbreviations
- ICAM-1
intracellular cell adhesion molecule-1
- IRE
IL-6 response element
- Mag
magnolol
- MCP-1
monocyte chemotactic protein-1
- STAT3
signal transducer and activator of transcription protein 3
References
- CALDENHOVEN E., COFFER P., YUAN J., VAN DE STOLPE A., HORN F., KRUIJER W., VAN DER SAAG P.T. Stimulation of the human intercellular adhesion molecule-1 promoter by interleukin-6 and interferon-gamma involves binding of distinct factors to a palindromic response element. J. Biol. Chem. 1994;269:21146–21154. [PubMed] [Google Scholar]
- CHEN C.C., CHOW M.P., HUANG W.C., LIN Y.C., CHANG Y.J. Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kappaB: structure-activity relationships. Mol. Pharmacol. 2004;66:683–693. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- CHEN Y.H., LIN S.J., CHEN J.W., KU H.H., CHEN Y.L. Magnolol attenuates VCAM-1 expression in vitro in TNF-alpha-treated human aortic endothelial cells and in vivo in the aorta of cholesterol-fed rabbits. Br. J. Pharmacol. 2002;135:37–47. doi: 10.1038/sj.bjp.0704458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG J.J., WUNG B.S., CHAO Y.J., WANG D.L. Cyclic strain enhances adhesion of monocytes to endothelial cells by increasing intercellular adhesion molecule-1 expression. Hypertension. 1996;28:386–391. doi: 10.1161/01.hyp.28.3.386. [DOI] [PubMed] [Google Scholar]
- CHUNG C.D., LIAO J., LIU B., RAO X., JAY P., BERTA P., SHUAI K. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- EHRET G.B., REICHENBACH P., SCHINDLER U., HORVATH C.M., FRITZ S., NABHOLZ M., BUCHER P. DNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J. Biol. Chem. 2001;276:6675–6688. doi: 10.1074/jbc.M001748200. [DOI] [PubMed] [Google Scholar]
- FARUQI T.R., GOMEZ D., BUSTELO X.R., BAR-SAGI D., REICH N.C. Rac1 mediates STAT3 activation by autocrine IL-6. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9014–9019. doi: 10.1073/pnas.161281298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJITA S., TAIRA J. Biphenyl compounds are hydroxyl radical scavengers: their effective inhibition for UV-induced mutation in Salmonella typhimurium TA102. Free Radic. Biol. Med. 1994;17:273–277. doi: 10.1016/0891-5849(94)90083-3. [DOI] [PubMed] [Google Scholar]
- GAO J., MORRISON D.C., PARMELY T.J., RUSSELL S.W., MURPHY W.J. An interferon-gamma-activated site (GAS) is necessary for full expression of the mouse iNOS gene in response to interferon-gamma and lipopolysaccharide. J. Biol. Chem. 1997;272:1226–1230. doi: 10.1074/jbc.272.2.1226. [DOI] [PubMed] [Google Scholar]
- GIRAUDO E., ARESE M., TONIATTI C., STRASLY M., PRIMO L., MANTOVANI A., CILIBERTO G., BUSSOLINI F. IL-6 is an in vitro and in vivo autocrine growth factor for middle T antigen-transformed endothelial cells. J. Immunol. 1996;157:2618–2623. [PubMed] [Google Scholar]
- KIJIMA T., NIWA H., STEINMAN R.A., DRENNING S.D., GOODING W.E., WENTZEL A.L., XI S., GRANDIS J.R. STAT3 activation abrogates growth factor dependence and contributes to head and neck squamous cell carcinoma tumor growth in vivo. Cell Growth Differ. 2002;13:355–362. [PubMed] [Google Scholar]
- KIM O.S., PARK E.J., JOE E.H., JOU I. JAK-STAT signaling mediates gangliosides-induced inflammatory responses in brain microglial cells. J. Biol. Chem. 2002;277:40594–40601. doi: 10.1074/jbc.M203885200. [DOI] [PubMed] [Google Scholar]
- KONG C.W., TSAI K., CHIN J.H., CHAN W.L., HONG C.Y. Magnolol attenuates peroxidative damage and improves survival of rats with sepsis. Shock. 2000;13:24–28. doi: 10.1097/00024382-200013010-00005. [DOI] [PubMed] [Google Scholar]
- LAKSHMINARAYANAN V., LEWALLEN M., FRANGOGIANNIS N.G., EVANS A.J., WEDIN K.E., MICHAEL L.H., ENTMAN M.L. Reactive oxygen intermediates induce monocyte chemotactic protein-1 in vascular endothelium after brief ischemia. Am. J. Pathol. 2001;159:1301–1311. doi: 10.1016/S0002-9440(10)62517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE Y.M., HSIAO G., CHEN H.R., CHEN Y.C., SHEU J.R., YEN M.H. Magnolol reduces myocardial ischemia/reperfusion injury via neutrophil inhibition in rats. Eur. J. Pharmacol. 2001;422:159–167. doi: 10.1016/s0014-2999(01)01069-x. [DOI] [PubMed] [Google Scholar]
- LOGANADANE L.D., BERGE N., LEGRAND C., FAUVEL-LAFEVE F. Endothelial cell proliferation regulated by cytokines modulates thrombospondin-1 secretion into the subendothelium. Cytokine. 1997;9:740–746. doi: 10.1006/cyto.1997.0229. [DOI] [PubMed] [Google Scholar]
- MATSUMURA I., KITAMURA T., WAKAO H., TANAKA H., HASHIMOTO K., ALBANESE C., DOWNWARD J., PESTELL R.G., KANAKURA Y. Transcriptional regulation of the cyclin D1 promoter by STAT5: its involvement in cytokine-dependent growth of hematopoietic cells. EMBO J. 1999;18:1367–1377. doi: 10.1093/emboj/18.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKA T., NARAZAKI M., HIRATA M., MATSUMOTO T., MINAMOTO S., AONO A., NISHIMOTO N., KAJITA T., TAGA T., YOSHIZAKI K., AKIRA S., KISHIMOTO T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- NI C.W., HSIEH H.J., CHAO Y.J., WANG D.L. Interleukin-6-induced JAK2/STAT3 signaling pathway in endothelial cells is suppressed by hemodynamic flow. Am. J. Physiol. Cell Physiol. 2004;287:C771–C780. doi: 10.1152/ajpcell.00532.2003. [DOI] [PubMed] [Google Scholar]
- RIDKER P.M., RIFAI N., STAMPFER M.J., HENNEKENS C.H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- SCHINDLER C.W. Series introduction. JAK-STAT signaling in human disease. J. Clin. Invest. 2002;109:1133–1137. doi: 10.1172/JCI15644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN Y.C., SUNG Y.J., CHEN C.F. Magnolol inhibits Mac-1 (CD11b/CD18)-dependent neutrophil adhesion: relationship with its antioxidant effect. Eur. J. Pharmacol. 1998;343:79–86. doi: 10.1016/s0014-2999(97)01519-7. [DOI] [PubMed] [Google Scholar]
- SINIBALDI D., WHARTON W., TURKSON J., BOWMAN T., PLEDGER W.J., JOVE R. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene. 2000;19:5419–5427. doi: 10.1038/sj.onc.1203947. [DOI] [PubMed] [Google Scholar]
- STRATOWA C., AUDETTE M. Transcriptional regulation of the human intercellular adhesion molecule-1 gene: a short overview. Immunobiology. 1995;193:293–304. doi: 10.1016/S0171-2985(11)80558-9. [DOI] [PubMed] [Google Scholar]
- TENG C.M., YU S.M., KO F.N., CHEN C.C., WANG W.C., CHEN K.Y., HUANG Y.L., HUANG T.F. Comparison of the actions of some platelet-activating factor antagonists on platelets and aortic smooth muscles. Eur. J. Pharmacol. 1991;205:151–156. doi: 10.1016/0014-2999(91)90813-6. [DOI] [PubMed] [Google Scholar]
- TURKSON J. STAT proteins as novel targets for cancer drug discovery. Expert. Opin. Ther. Targets. 2004;8:409–422. doi: 10.1517/14728222.8.5.409. [DOI] [PubMed] [Google Scholar]
- WUNG B.S., CHENG J.J., HSIEH H.J., SHYY Y.J., WANG D.L. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ. Res. 1997;81:1–7. doi: 10.1161/01.res.81.1.1. [DOI] [PubMed] [Google Scholar]
- WUNG B.S., CHENG J.J., SHYUE S.K., WANG D.L. NO modulates monocyte chemotactic protein-1 expression in endothelial cells under cyclic strain. Arterioscler. Thromb. Vasc. Biol. 2001;21:1941–1947. doi: 10.1161/hq1201.099428. [DOI] [PubMed] [Google Scholar]
- WUNG B.S., NI C.W., WANG D.L. ICAM-1 induction by TNF-α and IL-6 is mediated by distinct pathways via Rac in endothelial cells. J. Biomed. Sci. 2005;12:1–11. doi: 10.1007/s11373-004-8170-z. [DOI] [PubMed] [Google Scholar]
- YANG X.P., IRANI K., MATTAGAJASINGH S., DIPAULA A., KHANDAY F., OZAKI M., FOX-TALBOT K., BALDWIN W.M., III, BECKER L.C. Signal transducer and activator of transcription 3{alpha} and specificity protein 1 interact to upregulate intercellular adhesion molecule-1 in ischemic-reperfused myocardium and vascular endothelium. Arterioscler. Thromb. Vasc. Biol. 2005;25,:1395–1400. doi: 10.1161/01.ATV.0000168428.96177.24. [DOI] [PubMed] [Google Scholar]
- YU R., KIM C.S., KAWADA T., KWON T.W., LIM T.H., KIM Y.W., KWON B.S. Involvement of leukotactin-1, a novel CC chemokine, in human atherosclerosis. Atherosclerosis. 2004;174:35–42. doi: 10.1016/j.atherosclerosis.2003.11.024. [DOI] [PubMed] [Google Scholar]
- ZHOU Z.H., CHATURVEDI P., HAN Y.L., ARAS S., LI Y.S., KOLATTUKUDY P.E., PING D., BOSS J.M., RANSOHOFF R.M. IFN-gamma induction of the human monocyte chemoattractant protein (hMCP)-1 gene in astrocytoma cells: functional interaction between an IFN-gamma-activated site and a GC-rich element. J. Immunol. 1998;160:3908–3916. [PubMed] [Google Scholar]