Abstract
Chronic heart failure (HF) is characterized by left ventricular (LV) structural remodeling, impaired function, increased circulating noradrenaline (NA) levels and impaired responsiveness of the myocardial β-adrenoceptor (βAR)-adenylyl cyclase (AC) system. In failing hearts, inhibition of the sodium/proton-exchanger (NHE)-1 attenuates LV remodeling and improves LV function. The mechanism(s) involved in these cardioprotective effects remain(s) unclear, but might involve effects on the impaired βAR-AC system.
Therefore, we investigated whether NHE-1 inhibition with sabiporide (SABI; 30 mg kg−1 day−1 p.o.) might affect myocardial βAR density and AC activity in relation to changes in LV end-diastolic diameter (LVEDD) and LV systolic fractional shortening (LVS-FS) after 3 weeks of rapid LV pacing in rabbits.
After 3 weeks of rapid LV pacing LVEDD was significantly increased (Shams 17±0.2 mm, n=9 vs 3wksHF 20±0.5 mm, n=8; P<0.05) and LVS-FS decreased (Shams 31±1%, n=9 vs 3wksHF 10±1%, n=8; P<0.05). SABI treatment significantly improved LV function independent of whether rabbits were treated after 1 week of pacing (3wksHF+2wksSABI (n=7): LVEDD 18±1 mm; LVS-FS 16±4%) or before pacing (3wksHF+3wksSABI (n=9): LVEDD 18±1 mm; LVS-FS 18±6%). After 3 weeks of rapid LV pacing, SABI treatment significantly attenuated increases in serum NA content (Shams 0.83±0.19, 3wksHF 2.68±0.38, 3wksHF+2wksSABI 1.22±0.32, 3wksHF+3wksSABI 1.38±0.33 ng ml−1). Moreover, βAR density (Shams 64±5, 3wksHF 38±3, 3wksHF+2wksSABI 48±4, 3wksHF+3wksSABI 55±3 fmol mg−1 protein) and responsiveness (isoprenaline-stimulated AC activity. (Shams 57.6±4.9, 3wksHF 36.3±6.0, 3wksHF+2wksSABI 56.9±6.0, 3wksHF+3wksSABI 54.5±4.8 pmol cyclic AMP mg−1 protein−1 min−1) were significantly improved in SABI-treated rabbits.
From the present data we cannot address whether the improved βAR-AC system permitted improved LV function and/or whether the improved LV function resulted in less activation of the sympathetic nervous system and by this in a reduced stimulation of the βAR-AC system. Accordingly, additional studies are needed to fully establish the cause-and-effect relationship between NHE-1 inhibition and the restoration of the myocardial βAR system.
Keywords: Rapid pacing-induced heart failure, β-adrenoceptors, adenylyl cyclase activity, Na+/H+-exchanger inhibition
Introduction
One key factor that contributes to the progression of heart failure (HF), especially in mediating (mal-)adaptive ventricular remodeling and dysfunction, is the activation of the sodium/proton-exchanger isoform 1 (NHE-1) in response to pressure and volume overload (Takewaki et al., 1995; Chen et al., 2001; Baartscheer et al., 2003; 2005; Marano et al., 2004), hypertension (Camilion De Hurtado et al., 2002; Cingolani et al., 2003), ischemia and reperfusion (for reviews see Avkiran, 1999; Karmazyn et al., 1999) and, as we have recently demonstrated, in response to nonischemic rapid left ventricular (LV) pacing (Aker et al., 2004).
Conversely, we and others could demonstrate that selective NHE-1 inhibitors possess potent antiapoptotic, antihypertrophic and antifibrotic effects, attenuate the associated ventricular remodeling and preserve systolic function (Otani et al., 2000; Spitznagel et al., 2000; Yoshida & Karmazyn, 2000; Chen et al., 2001; Kusumoto et al., 2001; Engelhardt et al., 2002; Baartscheer et al., 2003; 2005; Aker et al., 2004; Marano et al., 2004).
NHE-1 represents a downstream effector of a wide variety of hypertrophic stimuli, including mechanical load and oxidative stress (Yamazaki et al., 1998; Snabaitis et al., 2002) as well as β-adrenoceptor (βAR)- and α1AR agonists (Wallert & Fröhlich, 1992; Snabaitis et al., 2000; Engelhardt et al., 2002; Schäfer et al., 2002), endothelin (Kramer et al., 1991) and angiotensin II (Gunasegaram et al., 1999).
In addition to its role in intracellular pH regulation, activation of NHE-1 results in the accumulation of intracellular sodium that, in turn, is responsible for an increase in intracellular calcium [Ca2+]i levels through Na+/Ca2+ exchange (Despa et al., 2002; Baartscheer et al., 2003). Increased [Ca2+]i, in turn, contributes to the development of cardiac hypertrophy (Wilkins & Molkentin, 2004) and it was suggested that part of the improved systolic function following inhibition of NHE-1 relates to improved calcium handling (Baartscheer et al., 2003; 2005).
Whether or not NHE-1 inhibition has direct effects on the expression or function of [Ca2+]i regulatory proteins, such as SERCA 2a, phospholamban and/or the ryanodine receptor (RyR2), which are altered in HF (Hasenfuss et al., 1997), is unknown. Likewise, it could recently be demonstrated that β1AR stimulation leads to an upregulation of both NHE-1 mRNA and protein while NHE-1 inhibition exerted an inhibitory effect on β1AR-induced cardiac hypertrophy (Engelhardt et al., 2002). However, it remains unknown whether NHE-1 inhibition possibly also affects the upstream myocardial βAR-signaling pathway, that is also impaired in HF (Brodde & Michel, 1999).
The aim of the present study was therefore to investigate the effects of NHE-1 inhibition on the myocardial βAR-signaling pathway during HF induced by rapid LV pacing, which is characterized by progressive LV remodeling and dysfunction (Ruffolo & Feuerstein, 1998; Moe & Amstrong, 1999; Aker et al., 2003; 2004; Schulz et al., 2003) and by pronounced alterations in the βAR-adenylyl cyclase (AC) system (Kawai et al., 2000; Port & Bristow, 2001).
For that purpose, we first analysed the myocardial βAR-AC system after 1, 2 and 3 weeks of rapid LV pacing to gain insight into the development and progression of LV dysfunction. Subsequently, we investigated the βAR-AC system after 3 weeks of LV pacing in rabbits treated with sabiporide (SABI, 30 mg kg−1 day−1 p.o.), a specific NHE-1 inhibitor (Aker et al., 2004); treatment was started after first signs of LV dysfunction had developed (after 1 week of pacing) or before initiation of pacing.
We determined βAR density and βAR-dependent and receptor-independent activation of AC activity in relation to LV structural (fibrosis, apoptosis and hypertrophy) and functional (LV systolic fractional shortening (LVS-FS) and LV end-diastolic diameter (LVEDD) changes.
Methods
Experimental model and protocol
This study was approved by the bioethical committee of the district of Düsseldorf, Germany, and the investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH publication no. 85-23, revised 1996).
Instrumentation of 30 Chinchilla rabbits (Charles River, Kisslegg, Germany) weighing 3.3±0.1 kg body weight (BW) was performed as described in detail elsewhere (Aker et al., 2003; Schulz et al., 2003). HF was induced in 21 rabbits by LV pacing (400 b.p.m.) for one (1wkHF, n=6), two (2wksHF, n=7) and 3 weeks (3wksHF, n=8). Nine Sham-operated rabbits (Shams) served as controls. Heart failure was evident from clinical signs, such as ascites (1wkHF: 3/6 rabbits; 2wksHF: 6/6 rabbits; 3wksHF: 8/8 rabbits) and cachexia (1wkShams/1wkHF: 3.5±0.1/3.4±0.1 kg BW; 2wksShams/2wksHF: 3.6±0.1/3.4±0.1 kg BW; 3wksShams/3wksHF: 3.6±0.1/3.2±0.1 kg BW), and echocardiographic parameters, such as a reduction of LVS-FS and an increase in LVEDD. After euthanasia of the rabbits, four to six samples (50 mg each) were taken from the LV free wall, two to three samples were frozen in liquid nitrogen and stored at −70°C until further use or were fixed in formalin and embedded in paraffin.
SABI-treated animals
We were able to reanalyse from our recently published study (Aker et al., 2004) serum NA content and LV samples from seven Shams and 16 HF rabbits which had been rapidly paced for 3 weeks. Seven HF rabbits were treated with SABI (30 mg kg−1 day−1 p.o.) after 1 week of pacing when first signs of LV dysfunction had developed, while Shams and nine HF rabbits were treated with SABI before initiation of pacing for 3 weeks.
Echocardiography
Heart rate (HR) and LV function were measured (Supervision 7000, Toshiba, Neuss, Germany) as described in detail elsewhere (Aker et al., 2003; Schulz et al., 2003). LVS-FS was assessed by measurement of the LVEDD and end-systolic diameter ((LVEDD – end-systolic diameter)/LVEDD × 100) (Aker et al., 2003; 2004; Schulz et al., 2003).
Histology
Apoptosis was determined using the TdT-mediated dUTP nick end labeling (TUNEL) technique (In Situ Cell Death Detection Kit, La Roche Diagnostics, Mannheim, Germany) as described recently (Aker et al., 2003; 2004; Schulz et al., 2003). TUNEL-positive cardiomyocyte (CM) nuclei were counted using fluorescence microscopy (Leica DMLB, Bensheim, Germany).
The extent of myocardial fibrosis was determined using Masson-Goldner trichrome staining and expressed as percentage of the field of view (three fields of 0.075 mm2 each), and CM cross-sectional area (CSA) was measured in hematoxylin and eosin-stained tissue sections (three fields of 0.075 mm2 each) as described in detail elsewhere (Aker et al., 2003; 2004; Schulz et al., 2003).
Determination of serum noradrenaline (NA) content
For determination of the serum NA content peripheral arterial blood was drawn through a catheter into ice-cold tubes before euthanasia. Samples were centrifuged with 2500 × g for 10 min at 4°C, serum was removed, quickly frozen in liquid nitrogen and stored at −80°C until further use.
Serum samples were thawed on ice and 1 ng ml−1 3,4-dihydroxybenzylamine was added as an internal standard. The samples were deproteinized (trichloroacetic acid, 0.3 mol l−1 final concentration), and the serum catecholamines were adsorbed to purified alumina at pH 8.6 (2.5 M tris(hydroxymethyl)aminomethane-HCl buffer containing 10 mmol l−1 mercaptoethanol). The alumina-bound catecholamines were washed three times with 0.8 mol l−1 acetate buffer, pH 7.5, and once with water. Then the catecholamines were dissolved in 200 μl 1.0 N acetic acid. An aliquot of this solution was used for high-performance liquid chromatography. Separation was achieved on a column (Nova-Pak-C18; 3.9 × 150 mm; Waters, Eschborn, Germany), the mobile phase consisting of 150 mmol phosphoric acid, 0.1 mmol l−1 EDTA·Na2, 12 mmol l−1 1-octanesulfonic acid sodium salt, 2% (vol) methanol, pH 3.5 (adjusted with NaOH), with a flow of 1.0 ml min−1 (pump: M510, Waters; pulse dampener: LP-21, Scientific Systems, State College, PA, U.S.A.). A glassy carbon electrode set to 650 mV against Ag–AgCl was used for electrochemical detection (model 400 EC Detector; EG & G Princeton Applied Research, Princeton, NJ, U.S.A.).
βAR density
Frozen tissue was thawed on ice in ice-cold 1 mmol l−1 KHCO3 and minced with scissors and gradually homogenized with an Ultra-Turrax (Ultra-Turrax T25; Janke & Kunkel IKA® Labortechnik; Germany) at 24,000 r.p.m. for 10 s and twice at 17,500 r.p.m. for 20 s with 1 min intervals on ice. The homogenate, brought up to 15–20 ml with 1 mmol l−1 KHCO3, was centrifuged for 15 min at 1000 × g at 4°C. The pellet was resuspended in 1 ml 1 mmol l−1 KHCO3, immediately frozen in liquid nitrogen an stored at −80°C until further use (see determination of AC activity). The supernatant was filtered through four layers of cheesecloth and centrifuged for 20 min at 20,000 × g and 4°C. The resulting pellet was resuspended in 10 ml 1 mmol l−1 KHCO3 and recentrifuged again (see above). The resulting pellet was resuspended in 1 ml incubation buffer (mmol l−1: 10 Tris-HCl, pH 7.4; 154 NaCl 0.01% ascorbic acid) and protein content was determined according to Bradford (1976) using bovine γ-globulin as standard.
(−)-[125I]Iodocyanopindolol (ICYP; specific activity 2200 Ci mmol−1; NEN™, Dreieich, Germany) saturation analysis was performed as recently described (Leineweber et al., 2003) by incubating 20 μg protein/assay with six concentrations of ICYP ranging from 5 to 200 pmol l−1 in a final volume of 250 μl for 90 min at 37°C. Nonspecific binding of ICYP was defined as binding to membranes, which was not displaced by a high concentration of the nonselective βAR antagonist (±)-CGP 12177 (1 μmol l−1). Specific binding of ICYP was defined as total binding minus nonspecific binding (usually 70–80% at 50 pmol l−1 of ICYP).
AC activity
Crude LV membranes (see above) were thawed on ice and recentrifuged with 2000 × g for 15 min at 4°C. The pellet was resuspended in 5 ml ice-cold homogenisation buffer (mmol l−1: 5 Tris-HCl, pH 7.4; 1 MgCl2, 250 sucrose) and rehomogenized with an Ultra-Turrax (Ultra-Turrax T25; Janke & Kunkel IKA® Labortechnik; Germany) twice at 17,500 r.p.m. for 20 s with 1 min intervals on ice. The homogenate was recentrifuged for 15 min at 2000 × g at 4°C, the supernatant was discharged and the resulting pellet was resuspended in 1 ml ice-cold TEN buffer (mmol l−1: Tris-HCl, pH 7.4; 1 Na-ethylendiaminetetraacetic acid (Na-EDTA), 25 NaCl) and protein content was determined according to Bradford (1976) using bovine γ-globulin as standard. AC activity was assessed as described by Salomon et al. (1974) with minor modifications. Two different incubation conditions were employed. For determination of basal AC activity, membranes (30–40 μg protein) were incubated for 10 min at 30°C in incubation buffer A (mmol l−1: 40 HEPES, pH 7.4; 5 MgCl2, 1 Na-EDTA, 0.5 [α-32P]ATP (specific activity 30 Ci mmol−1; NEN™, Dreieich, Germany), 0.1 cyclic AMP) and an ATP regenerating system (5 mmol l−1 phosphocreatine and 50 U ml−1 creatine phosphokinase) in a final volume of 100 μl. For determination of GTP-, 100 μmol l−1 isoprenaline (ISO)-stimulated and 100 μmol l−1 forskolin-(Forsk)-stimulated AC activity, membranes were incubated in incubation buffer A with 10 μmol l−1 GTP. The reaction was stopped by adding 800 μl of 50 mmol l−1 Tris-HCl buffer (pH 7.4 at 25°C) containing 40 mmol l−1 ATP and 1.4 mmol l−1 cyclic AMP adjusted with [3H]cyclic AMP (5000–10,000 c.p.m., specific activity 44.5 Ci mmol−1; NEN™, Dreieich, Germany) to monitor the recovery of [32P]cyclic AMP (Salomon et al., 1974).
Statistics
Experimental data given in text and figures are expressed as mean values±s.e.m. The equilibrium dissociation constant (KD) and the maximal number of binding sites (Bmax) for ICYP binding were calculated by nonlinear regression analysis (hyperbolic function: y=Bmax × x/(KD+x)) using the iterative curve-fitting Prism 2.0 program (Graph-Pad Software, San Diego, CA, U.S.A.). Linear regression analysis of data was performed by the least-squares method (model: βAR density=a × sNA+b) with 95% confidence. Pearson-correlation was calculated assuming that data were sampled from a Gaussian population with 95% confidence. The significance of differences was estimated by one-way or two-way ANOVA, where appropriate. All statistical calculations were performed using the Prism program. A P-value <0.05 was considered to be significant.
Results
Myocardial βAR density and serum NA content
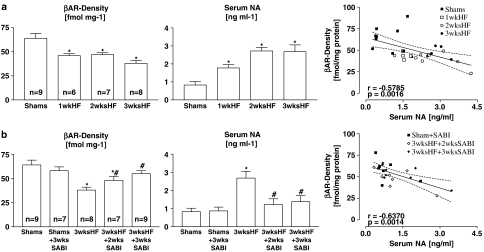
In untreated HF rabbits, βAR density significantly decreased in comparison to Shams (64±5 fmol mg−1 protein) already after 1 week of pacing (1wkHF: 46±2 fmol mg−1 protein; P<0.05 vs Shams; Figure 1a, left). βAR density persisted at this reduced level when pacing was prolonged for 2 weeks (2wksHF: 47±2 fmol mg−1 protein; P<0.05 vs Shams) but further declined after 3 weeks of pacing (3wksHF: 38±3 fmol mg−1 protein; P<0.05 vs Shams, 1wk and 2wksHF). Serum NA content increased in comparison to Shams from 0.83±0.19 ng ml−1 to 1.78±0.18 ng ml−1, 2.72±0.20 ng ml−1 and 2.68±0.38 ng ml−1 (all P<0.05 vs Shams) after 1, 2 and 3 weeks of pacing, respectively (Figure 1a, middle). Serum NA content was inversely correlated with βAR density (Figure 1a, right).
Figure 1.
Myocardial βAR density in relation to serum NA content: βAR density (left), serum NA (middle) and correlation between βAR density and serum NA (a) with progression of pacing-induced HF and (b) in SABI-treated rabbits after 3 weeks of pacing. Values are means±s.e.m. with *P<0.05 vs Shams and #P<0.05 vs 3wksHF not treated with SABI (left and middle), r represents Pearson-correlation coefficient, P represents P-value (right).
SABI treatment did not affect βAR density in Shams (Shams+SABI: 58±4 fmol mg−1 protein; Figure 1b, left). In 3wksHF rabbits, SABI treatment significantly attenuated the decrease in βAR density in comparison to untreated 3wksHF rabbits, independent of whether the treatment started after first signs of LV dysfunction had developed (3wksHF+2wksSABI: 48±4 fmol mg−1 protein; P<0.05 vs 3wksHF) or before initiation of pacing (3wksHF+3wksSABI: 55±3 fmol mg−1 protein; P<0.05 vs 3wksHF; Figure 1b, left). In addition, while SABI treatment did not affect serum NA content in Shams (Shams+SABI: 0.87±0.21 ng ml−1; Figure 1b, middle), in 3wksHF rabbits, SABI treatment significantly attenuated the increase in serum NA content measured in untreated 3wksHF rabbits, independent whether the treatment started after first signs of LV dysfunction had developed (3wksHF+2wks SABI: 1.22±0.32 ng ml−1; P<0.05 vs 3wksHF; Figure 1b, middle) or before initiation of pacing (3wksHF+3wksSABI: 1.38±0.33 ng ml−1; P<0.05 vs 3wksHF; Figure 1b, middle). Also in SABI-treated rabbits, serum NA content was inversely correlated with βAR density (Figure 1b, right).
Myocardial AC activity
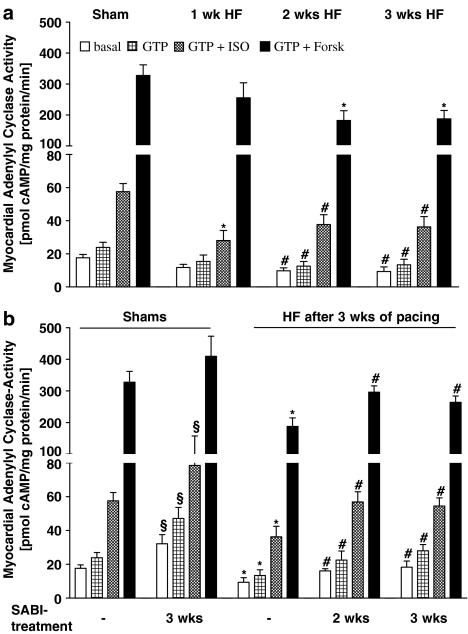
After 1 week of pacing, while basal, GTP- and Forsk-stimulated AC activities were not altered, ISO-stimulated AC activity was significantly reduced (28±6 pmol cyclic AMP (cAMP) mg−1 protein min−1) in comparison to Shams (58±5 pmol cAMP mg−1 protein min−1; Figure 2a). After 2 weeks of pacing basal, GTP-, ISO- and Forsk-stimulated AC activities were significantly reduced (basal 10±2, GTP 13±3, ISO 38±6, Forsk 183±31 pmol cAMP mg−1 protein min−1; Figure 2a) in comparison to Shams and almost persisted on these levels even when pacing was prolonged for 3 weeks (basal 9±3, GTP 13±3, ISO 36±6, Forsk 188±27 pmol cAMP mg−1 protein min−1; Figure 2a).
Figure 2.
Myocardial βAR-dependent and -independent stimulation of AC activity: (a) with progression of pacing-induced HF and (b) in SABI-treated rabbits after 3 weeks of pacing: basal, GTP 5-stimulated, 10 μmol l−1 GTP 5+100 μmol l−1 ISO-stimulated, 10 μmol l−1 GTP+100 μmol l−1 Forsk-stimulated AC activity. Values are means±s.e.m. in (a) with #P<0.05 vs Sham and *P<0.01 vs Sham; in (b) with §P<0.05 vs Shams not treated with SABI, *P<0.01 vs Shams not treated with SABI, #P<0.05 vs untreated 3wksHF rabbits.
In SABI-treated 3wksHF rabbits, AC activity (independently from the mode of activation) was significantly improved in comparison to untreated 3wksHF rabbits. Again, the functional improvement was independent of whether the treatment started after first signs of LV dysfunction had developed (3wksHF+2wksSABI: basal 16±1, GTP 23±5, ISO 57±6, Forsk 296±20 pmol cAMP mg−1 protein min−1; Figure 2b) or before initiation of pacing (3wksHF+3wksSABI: basal 18±4, GTP 28±4, ISO 54±5, Forsk 264±20 pmol cAMP mg−1 protein min−1; Figure 2b). However, also in Shams SABI treatment significantly affected basal, GTP- and ISO-stimulated AC activity (basal 32±5, GTP 47±7, ISO 89±17 pmol cAMP mg−1 protein min−1; Figure 2b) and tended to enhance Forsk-stimulated AC activity (410±64 cAMP mg−1 protein min−1; n=7, Figure 2b) in comparison to untreated Shams.
Hemodynamics
HR tended to increase with progression of HF reaching statistical significance, however, only in 2wksHF rabbits (Shams 230±6 b.p.m., 1wkHF 261±13 b.p.m., 2wksHF 282±10 b.p.m., 3wksHF 260±15 b.p.m.). HR was not different between SABI-treated Shams (Shams+SABI: 241±28 b.p.m.) and 3wksHF rabbits, independent of whether the treatment started after first signs of LV dysfunction had developed (3wksHF+2wksSABI: 250±13 b.p.m.) or before initiation of pacing (3wksHF+3wksSABI: 268±14 b.p.m.).
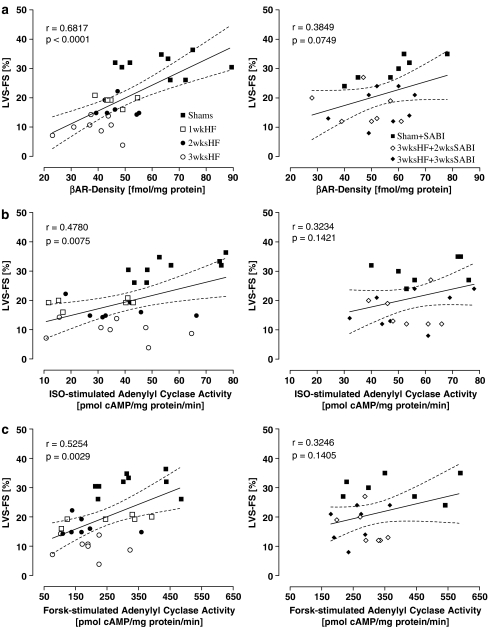
With progression of HF, LVEDD significantly increased from baseline to 3wksHF (Shams: 17±0.2 mm, 1wkHF: 18±0.3 mm, 2wksHF: 19±0.3 mm, 3wksHF: 20±0.5 mm, all HF with P<0.05 vs Shams) but remained significantly lower in SABI-treated 3wksHF rabbits, independent of whether the treatment started after first signs of LV dysfunction had developed (3wksHF+2wksSABI 18±0.6 mm with P<0.05 vs untreated 3wksHF) or before initiation of pacing (3wksHF+3wksSABI 18±0.6 mm with P<0.05 vs untreated 3wksHF). SABI treatment alone did not affect LVEDD in Shams (Shams+3wksSABI: 16±1.0 mm). With progression of HF, LVEDD was inversely correlated with βAR density (r=−0.6810, P<0.0001), ISO- (r=−0.4947, P=0.0054) and Forsk-stimulated AC-activity (r=−0.5714, P=0.001), while in SABI-treated rabbits a significant inverse correlation was only found between LVEDD and receptor-independent Forsk-stimulated AC activity (r=−0.6272, P=0.0018).
During the development of HF, LVS-FS significantly decreased from baseline to 3wksHF (Shams: 31±1%, 1wkHF: 19±1%, 2wksHF: 17±1%, 3wksHF: 10±1%, all HF with P<0.05 vs Shams) but remained significantly higher in SABI-treated 3wksHF rabbits, independent of whether the treatment started after first signs of LV dysfunction had developed (3wksHF+2wksSABI: 16±4% with P<0.05 vs untreated 3wksHF) or before initiation of pacing (3wksHF+3wksSABI: 18±6% with P<0.05 vs untreated 3wksHF) SABI treatment alone did not affect LVS-FS in Shams (Shams+SABI: 30±4%). With progression of HF, LVF-FS was inversely correlated with βAR density (Figure 3a, left), receptor-dependent (ISO-stimulated; Figure 3b, left) and -independent (Forsk-stimulated, Figure 3c, left) stimulation of AC activity; such correlations were lost following SABI-treatment (Figure 3a–c, right).
Figure 3.
LVS-FS in relation to (a) βAR density and to (b) ISO- and (c) Forsk-stimulated AC activity: Left side with progression of pacing-induced heart failure and right side in SABI-treated rabbits after 3 weeks of pacing. βAR density in fmol mg−1 protein, AC activity in pmol cAMP mg−1 protein min−1, LVS-FS in %; r represents Pearson-correlation coefficient, P represents P-value.
Histology during the progression of HF
With progression of HF, the number of TUNEL-positive CMs, the CSA of the remaining viable CMs and the extent of myocardial fibrosis tended to increase with progression of pacing-induced HF, reaching statistical significance after 3 weeks of pacing (Table 1).
Table 1.
Left ventricular structure in HF-rabbits after 1, 2 and 3 weeks of rapid left ventricular pacing and HF-rabbits after 3 weeks of rapid left ventricular pacing, treated for 2 or 3 weeks with sabiporide, and Shams, treated for 3 weeks with sabiporide
|
Groups |
CM Apoptosis |
CM hypertrophy (CSA, μm2) |
Myocardial fibrosis (% of area) |
||
|---|---|---|---|---|---|
| TUNEL-positive CM | Area (mm2) | TUNEL-positive CM (mm−2 × 10−3) | |||
|
Progression of heart failure | |||||
| Shams (n=9) |
0.6±0.2 |
116±7 |
5±2 |
251±15 |
7±1 |
| 1wkHF (n=6) |
1.8±0.8 |
127±18 |
16±6 |
282±12 |
8±1 |
| 2wksHF (n=7) |
2.1±0.3 |
118±11 |
18±3 |
295±12 |
13±1 |
| 3wksHF (n=8) |
7.5±1.6* |
139±18 |
50±10* |
317±17* |
19±2* |
| | |||||
|
Sabiporide treatment | |||||
| Shams+3wksSABI (n=7) |
1.4±0.5 |
175±5 |
9±3 |
227±11 |
5±1 |
| 3wksHF+2wksSABI (n=7) |
2.6±1.1 |
117±9 |
21±9*,# |
257±13# |
16±1*,# |
| 3wksHF+3wksSABI (n=9) | 5.8±1.3* | 169±16 | 35±6*,# | 294±9*,# | 12±1# |
Area=total analyzed surface area; CM=cardiomyocyte; CSA=cross-sectional area; HF=heart failure; SABI=sabiporide.
Values are Means±s.e.m. with *P<0.05 vs Shams and #P<0.05 vs 3wksHF.
In comparison to untreated 3wksHF rabbits, SABI-treatment attenuated these increases in TUNEL-positive CMs, CSA of the remaining viable CMs and the extent of fibrosis (Table 1).
In addition, during progression of HF the number of TUNEL-positive CMs, the CSA of the remaining viable CMs and the extent of myocardial fibrosis were negatively correlated with βAR density, whereas in rabbits treated with SABI these relations were no longer evident (Table 2). Furthermore, with progression of HF the number of TUNEL-positive CMs and CSA of the remaining viable CMs were negatively correlated with ISO- and Forsk-stimulated AC activity, whereas again in SABI-treated rabbits these relations were no longer evident (Table 2).
Table 2.
Correlation between βAR-density, isoprenaline-stimulated and forskolin-stimulated adenylyl cyclase activity and cardiomyocyte apoptosis, cardiomyocyte hypertrophy and myocardial fibrosis
| Correlation | CM apoptosis (TUNEL-positive CM mm−2) | CM hypertrophy (CSA, μm2) | Myocardial fibrosis (% of area) |
|---|---|---|---|
|
Progression of heart failure | |||
|
βAR-density |
r=−0.6930 |
r=−0.5307 |
r=−0.4490 |
| |
P<0.0001 |
P=0.0026 |
P=0.0128 |
| ISO-stimulated |
r=−0.3678 |
r=−0.0651 |
r=−0.0029 |
| AC-activity |
P=0.0455 |
P=0.7327 |
P=0.9880 |
| Forsk-stimulated |
r=−0.4385 |
r=−0.2737 |
r=−0.2212 |
| AC-activity |
P=0.0154 |
P=0.1433 |
P=0.2402 |
| | |||
|
Sabiporide treatment | |||
|
βAR-density |
r=0.0929 |
r=0.3587 |
r=−0.0925 |
| |
P=0.6810 |
P=0.1012 |
P=0.6822 |
| ISO-stimulated |
r=0.0742 |
r=0.1582 |
r=−0.0678 |
| AC-activity |
P=0.7429 |
P=0.4820 |
P=0.7644 |
| Forsk-stimulated |
r=−0.3805 |
r=0.1264 |
r=−0.1997 |
| AC-activity | P=0.0807 | P=0.5752 | P=0.3728 |
AC=adenylyl cyclase-activity in pmol cAMP mg−1 protein min−1; βAR=β-adrenoceptor in fmol mg−1 protein; CM=cardiomyocyte; ISO=isoprenaline; Forsk=forskolin.
r represents Pearson-correlation coefficient, P represents P-value.
Discussion
In the present study, the progression of nonischemic rapid pacing-induced HF (LVEDD increased while LVS-FS decreased) was associated with increases in the extent of apoptosis, hypertrophy and fibrosis. These structural and functional alterations were associated with and correlated to increases in serum NA content and decreases in myocardial βAR densities and AC activities (independent by the mode of activation). SABI treatment significantly attenuated these LV structural and functional alterations. Moreover, SABI treatment also significantly attenuated increases in circulating NA and improved βAR density and AC activity.
Chronic HF, in humans as well as in experimental animal models, is characterized by impaired myocardial βAR responsiveness mainly caused by a decrease in βAR density (mainly β1AR) and a functional uncoupling of the remaining receptors from the Gαs-protein AC pathway (Bristow et al., 1982; Brodde & Michel, 1999). In agreement with other studies, we could demonstrate that the reduction in βAR density and function was directly related to the severity of HF and associated with an elevated activity of the sympathetic nervous system, as indicated by increased circulating NA (Delehanty et al., 1994; Kawai et al., 2000; Leineweber et al., 2002; 2005).
Within 1 week of rapid pacing LV dysfunction developed and the sympathetic nervous system was stimulated (two-fold increase in circulating NA), possibly due to an early and preferential activation of cardiac sympathetic nerves (Kawai et al., 2000). The latter results in an increased NA ‘spillover' into the synaptic cleft that is sufficient to strongly activate myocardial βARs; subsequently, G-protein-coupled receptor kinases phosphorylate and by this desensitise and/or downregulate the βARs in chronic HF (Ping & Hammond, 1994; Ping et al., 1997; Leineweber et al., 2002; 2005). Accordingly, we found in the present study a significant decrease in myocardial βAR density by about 30% and an isolated decrease in ISO-stimulated AC activity by about 50%, related, at least in part, to the uncoupling of the remaining βARs from the G-protein AC system. With progression of rapid pacing-induced HF and in association with a three-fold increase in circulating NA (after 2 and 3 weeks) myocardial βARs decreased further, accompanied by reduced AC responsiveness to ISO, GTP and GTP/Forsk and suggesting an additional defect in the G-protein AC system. This finding is in good agreement with several other studies demonstrating that prolonged rapid ventricular pacing could either result in a defect in the catalytic unit of the AC and/or a decreased AC mRNA expression and/or a decrease in the Gαs- or increase in the Gαi-protein (Calderon et al., 1991; Marzo et al., 1991; Kiuchi et al., 1993; Larosa et al., 1993; Roth et al., 1993; Ishikawa et al., 1994; Spinale et al., 1994; Ping et al., 1997; Kawai et al., 2000). However, it should be noted, that in all studies so far βAR density and AC activity were determined in crude membrane preparations that are composed not only of CMs but contain various nonmyocyte cells including fibroblasts and endothelial cells. These cells contain predominantly β2ARs and have significantly lower AC Type V and VI mRNA levels than CMs (Ishikawa et al., 1992; Ping & Hammond, 1994; Ping et al., 1997; Leineweber et al., 2003). Therefore, an alternative explanation for the pronounced reduction in βAR density and AC activity after 3 weeks of pacing could be the loss of viable CMs due to apoptosis and an interstitial replacement fibrosis.
In human chronic or end-stage HF (Narula et al., 1996; Olivetti et al., 1996; 1997) and in experimental animal models of myocardial hypertrophy or HF (Sharov et al., 1996; Li et al., 1997; Aker et al., 2003; 2004; Schulz et al., 2003), apoptosis contributes, at least in part, to cardiac remodeling, myocardial dysfunction and the alterations in the βAR-AC system, as also observed in the present study. Recent studies in isolated rat CMs or transgenic mice indicated that overstimulation and/or overexpression of the βAR system itself might contribute to promotion of apoptosis, myocardial hypertrophy and interstitial replacement fibrosis (Communal et al., 1999; Engelhardt et al., 1999; Morisco et al., 2001), possibly via β1AR-mediated cardiotoxic Ca2+ overload (Mann et al., 1992, Communal et al., 1998; Iwai-Kanai & Hasegawa, 2004). Indeed, treatment of patients with chronic HF with selective β1R-antagonists not only reversed the downregulation of β1ARs and the uncoupling of the remaining receptors but also reversed existing and prevented further remodeling and improved LV systolic function by the restoration of maladaptive defects in myocardial Ca2+-signaling (Bristow, 1997; Brodde & Michel, 1999; Reiken et al., 2001; 2003).
Recently, it became evident, that NHE-1 is one of the downstream effectors of the βAR system, that is activated secondary to βAR overexpression or βAR overstimulation (Schlüter et al., 1998; Engelhardt et al., 2002; Schäfer et al., 2002). Increased NHE-1 activity was also associated with an increase in the extent of apoptosis, fibrosis (Otani et al., 2000, Engelhardt et al., 2002; Cingolani et al., 2003, Aker et al., 2004) and hypertrophy of CMs in HF linked to increases in [Na+]i and end-diastolic [Ca2+]i and a deranged sarcoplasmatic Ca2+ handling (Baartscheer et al., 2003). Conversely, inhibition of NHE-1 by specific inhibitors, for example cariporide, has been suggested to confer structural and functional cardioprotective effects in HF (for a review see Karmazyn et al., 1999) and it was suggested that part of the improved systolic function following inhibition of NHE-1 relates to improved calcium handling (Baartscheer et al., 2003; 2005).
In good agreement with the studies mentioned above, we recently demonstrated that SABI treatment significantly attenuated the extent of apoptosis, hypertrophy and fibrosis and the increases in LVEDD and decreases in LVS-FS associated with rapid LV pacing. These structural and functional alterations were associated with an improvement in myocardial βAR density and AC activity (independent of the mode of activation), independent of whether the treatment started after first signs of HF had developed or prior to initiation of pacing. Whether NHE-1 inhibition had a direct effect on the expression or function of the βAR system is unknown, however, it should be mentioned that after 3 weeks of pacing SABI treatment significantly attenuated, in comparison to untreated 2 and 3wksHF rabbits, the increases in circulating NA by about 50%. Interestingly, most deleterious structural and functional effects of rapid LV pacing observed in the present study during progression of HF in untreated rabbits were attenuated under SABI treatment to values that we again found in the early stage of HF.
Moreover, it should be mentioned that NHE-1 is ubiquitously expressed and is activated during myocardial ischemia not only in CMs but also in sympathetic nerve endings, where it increases intraneuronal [Na+] and thus activates the Na+-dependent NA transporter uptake1 (Reid et al., 2004). Normally uptake1 is responsible for NA reuptake into sympathetic nerve endings; under ischemic conditions, however, it reverses in an outward direction (carrier-mediated release), releasing pathological amounts of NA into the synaptic cleft (Schömig & Richardt, 1990). Whether this reversed NA transport hold also true in HF is unknown. The present data, however, suggest that NHE-1 inhibition may not only restore myocardial [Ca2+]i but also the inward direction of uptake1, by restoring neuronal [Na+]i, thus leading to a reduction in NA concentrations within the synaptic cleft and by this to a reduction of the agonist-mediated deleterious effects on the myocardial βAR system.
However, from the present data we cannot address whether the improved βAR-AC system permitted improved LV function and/or whether the improved LV function resulted in less activation of the sympathetic nervous system and by this to a reduced stimulation of the βAR-AC system. Accordingly, additional studies are needed to fully establish the cause-and-effect relationship between NHE-1 inhibition and the restoration of the myocardial βAR system.
Acknowledgments
This work was supported by a grant (SCHU 843/6-1) from the German Research Foundation.
Abbreviations
- AR
adrenoceptor
- BW
body weight
- cAMP
cyclic AMP
- CM
cardiomyocyte
- CSA
cross-sectional area
- Forsk
forskolin
- HF
heart failure
- HR
heart rate
- ICYP
(−)-[125I]Iodocyanopindolol
- ISO
isoprenaline
- LV
left ventricle
- LVEDD
LV end-diastolic diameter
- LVS-FS
LV systolic fractional shortening
- NA
noradrenaline
- NHE
sodium/proton-exchanger
- RyR2
ryanodine receptor
- SABI
sabiporide
- TUNEL
TdT-mediated dUTP nick end labeling
References
- AKER S., BELOSJOROW S., KONIETZKA I., DUSCHIN A., MARTIN C., HEUSCH G., SCHULZ R. Serum but not myocardial TNF-α concentration is increased in pacing-induced heart failure in rabbits. Am. J. Physiol. Integr. Comp. Physiol. 2003;285:R463–R469. doi: 10.1152/ajpregu.00153.2003. [DOI] [PubMed] [Google Scholar]
- AKER S., SNABAITIS A.K., KONIETZKA I., VAN DE SAND A., BÖNGLER K., AVKIRAN M., HEUSCH G, SCHULZ R. Inhibition of the Na+/H+ exchanger attenuates the deterioration of ventricular function during pacing-induced heart failure in rabbits. Cardiovasc. Res. 2004;63:273–282. doi: 10.1016/j.cardiores.2004.04.014. [DOI] [PubMed] [Google Scholar]
- AVKIRAN M. Rational basis for use of sodium-hydrogen exchange inhibitors in myocardial ischemia. Am. J. Cardiol. 1999;83:10G–17G. doi: 10.1016/s0002-9149(99)00215-5. [DOI] [PubMed] [Google Scholar]
- BAARTSCHEER A., SCHUMACHER C.A., VAN BORREN M.M., BELTERMAN C.N., CORONEL R., FIOLET J.W. Increased Na+/H+-exchange activity is the cause of increased [Na+]i and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Cardiovasc. Res. 2003;57:1015–1024. doi: 10.1016/s0008-6363(02)00809-x. [DOI] [PubMed] [Google Scholar]
- BAARTSCHEER A., SCHUMACHER C.A., VAN BORREN M.M., BELTERMAN C.N., CORONEL R., OPTHOF T., FIOLET J.W. Chronic inhibition of Na+/H+-exchanger attenuates cardiac hypertrophy and prevents cellular remodeling in heart failure. Cardiovasc. Res. 2005;65:83–92. doi: 10.1016/j.cardiores.2004.09.024. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRISTOW M.R., GINSBURG R., MINOBE W., CUBICCIOTTI R.S., SAGEMAN W.S., LURIE K., BILLINGHAM M.E., HARRISON D.C., STINSON E.B. Decreased catecholamines sensitivity and beta-adrenergic-receptor density in failing human hearts. N. Engl. J. Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- BRISTOW M.R. Mechanism of action of beta-blocking agents in heart failure. Am. J. Cardiol. 1997;80:26L–40L. doi: 10.1016/s0002-9149(97)00846-1. [DOI] [PubMed] [Google Scholar]
- BRODDE O-E., MICHEL M.C. Adrenergic and muscarinic receptors in the human heart. Pharmacol. Rev. 1999;51:651–690. [PubMed] [Google Scholar]
- CALDERON A., BOUVIER M., LI K., JUNEAU C., DE CAMPLAIN J., ROULEAU J. Dysfunction of the β- and α-adrenergic systems in a model of congestive heart failure: the pacing overdrive dog. Circ. Res. 1991;69:332–343. doi: 10.1161/01.res.69.2.332. [DOI] [PubMed] [Google Scholar]
- CAMILION DE HURTADO M.C., PORTIANSKY E.L., PEREZ N.G., REBOLLEDO O.R., CINGOLANI H.E. Regression of cardiomyocyte hypertrophy in SHR following chronic inhibition of the Na(+)/H(+) exchanger. Cardiovasc. Res. 2002;53:862–868. doi: 10.1016/s0008-6363(01)00544-2. [DOI] [PubMed] [Google Scholar]
- CHEN L., GAN X.T., HAIST J.V., FENG Q., LU X., CHAKRABARTI S., KARMAZYN M. Attenuation of compensatory right ventricular hypertrophy and heart failure following monocrotaline-induced pulmonary vascular injury by the Na+-H+ exchange inhibitor cariporide. J. Pharmacol. Exp. Ther. 2001;298:469–476. [PubMed] [Google Scholar]
- CINGOLANI H.E., REBOLLEDO O.R., PORTIANSKY E.L., PEREZ N.G., CAMILION DE HURTADO M.C. Regression of hypertensive myocardial fibrosis by Na(+)/H(+) exchange inhibition. Hypertension. 2003;41:373–377. doi: 10.1161/01.hyp.0000051502.93374.1c. [DOI] [PubMed] [Google Scholar]
- COMMUNAL C., SINGH K., PIMENTEL D.R., COLUCCI W.S. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the β-adrenergic pathway. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- COMMUNAL C., SINGH K., SAWYER D.B., COLUCCI W.S. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis : role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- DELEHANTY J.M., HIMURA Y., ELAM H., HOOD W.B., JR, LIANG C.S. Beta-adrenoceptor downregulation in pacing-induced heart failure is associated with increased interstitial NE content. Am. J. Physiol. 1994;266:H930–H935. doi: 10.1152/ajpheart.1994.266.3.H930. [DOI] [PubMed] [Google Scholar]
- DESPA S., ISLAM M.A., WEBER C.R., POGWIZD S.M., BERS D.M. Intracellular Na(+) concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation. 2002;105:2543–2548. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- ENGELHARDT S., HEIN L., KELLER U., KLAMBT K., LOHSE M.J. Inhibition of Na(+)-H(+) exchange prevents hypertrophy, fibrosis, and heart failure in beta(1)-adrenergic receptor transgenic mice. Circ. Res. 2002;90:814–819. doi: 10.1161/01.res.0000014966.97486.c0. [DOI] [PubMed] [Google Scholar]
- ENGELHARDT S., HEIN L., WIESMANN F., LOHSE M.J. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNASEGARAM S., HAWORTH R.S., HEARSE D.J., AVKIRAN M. Regulation of sarcolemmal Na(+)/H(+) exchanger activity by angiotensin II in adult rat ventricular myocytes: opposing actions via AT(1) versus AT(2) receptors. Circ. Res. 1999;85:919–930. doi: 10.1161/01.res.85.10.919. [DOI] [PubMed] [Google Scholar]
- HASENFUSS G., MEYER M., SCHILLINGER W., PREUSS M., PIESKE B., JUST H. Calcium handling proteins in the failing human heart. Basic. Res. Cardiol. 1997;92 (Suppl 1):87–93. doi: 10.1007/BF00794072. [DOI] [PubMed] [Google Scholar]
- ISHIKAWA Y., KATSUSHIKA S., CHEN L., HALNON N.J., KAWABE J., HOMCY C.J. Isolation and characterization of a novel cardiac adenylyl cyclase cDNA. J. Biol. Chem. 1992;267:13553–13557. [PubMed] [Google Scholar]
- ISHIKAWA Y., SOROTA S., KIUCHI K., SHANNON R.P., KOMAMURA K., KATSUSHIKA S., VATNER D.E., VATNER S.F., HOMCY C.J. Downregulation of adenylyl cyclase types V and VI mRNA levels in pacing-induced heart failure in dogs. J. Clin. Invest. 1994;93:2224–2229. doi: 10.1172/JCI117219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IWAI-KANAI E., HASEGAWA K. Intracellular signaling pathways for norepinephrine- and endothelin-1-mediated regulation of myocardial cell apoptosis. Mol. Cell. Biochem. 2004;259:163–168. doi: 10.1023/b:mcbi.0000021368.80389.b9. [DOI] [PubMed] [Google Scholar]
- KARMAZYN M., GAN X.T., HUMPHREYS R.A., YOSHIDA H., KUSUMOTO K. The myocardial Na(+)-H(+) exchange: structure, regulation, and its role in heart disease. Circ. Res. 1999;85:777–786. doi: 10.1161/01.res.85.9.777. [DOI] [PubMed] [Google Scholar]
- KAWAI H., MOHAN A., HAGEN J., DONG E., ARMSTRONG J., STEVENS S.Y., LIANG C-S. Alterations in cardiac adrenergic terminal function and β-adrenoceptor density in pacing-induced heart failure. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H1708–H1716. doi: 10.1152/ajpheart.2000.278.5.H1708. [DOI] [PubMed] [Google Scholar]
- KIUCHI K., SHANNON R.P., KOMAMURA K., COHEN D.J., BIANCHI C., HOMCY C.J., VATNER S.F., VATNER D.E. Myocardial β-adrenergic receptor function during development of pacing-induced heart failure. J. Clin. Invest. 1993;91:907–914. doi: 10.1172/JCI116312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAMER B.K., SMITH T.W., KELLY R.A. Endothelin and increased contractility in adult rat ventricular myocytes. Role of intracellular alkalosis induced by activation of the protein kinase C-dependent Na(+)-H+ exchanger. Circ. Res. 1991;68:269–279. doi: 10.1161/01.res.68.1.269. [DOI] [PubMed] [Google Scholar]
- KUSUMOTO K., HAIST J.V., KARMAZYN M. Na(+)/H(+) exchange inhibition reduces hypertrophy and heart failure after myocardial infarction in rats. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H738–H745. doi: 10.1152/ajpheart.2001.280.2.H738. [DOI] [PubMed] [Google Scholar]
- LAROSA G., ARMSTRONG P.W., SEEMAN P., FORSTER C. β Adrenoceptor recovery after heart failure in the dog. Cardiovasc. Res. 1993;27:489–493. doi: 10.1093/cvr/27.3.489. [DOI] [PubMed] [Google Scholar]
- LEINEWEBER K., BEILFUß A., WOLF Ch., SPORKMANN H., ROHE P., JAKOB H-G., HEUSCH G., PHILIPP T., BRODDE O-E. Reversal of the increase in G protein-coupled-receptor-kinase activity by β-adrenoceptor blockade in human heart failure. Cardiovasc. Res. 2005;66:512–519. doi: 10.1016/j.cardiores.2005.01.025. [DOI] [PubMed] [Google Scholar]
- LEINEWEBER K., BRANDT K., WLUDYKA B., BEILFUSS A., PONICKE K., HEINROTH-HOFFMANN I., BRODDE O.E. Ventricular hypertrophy plus neurohumoral activation is necessary to alter the cardiac beta-adrenoceptor system in experimental heart failure. Circ. Res. 2002;91:1056–1062. doi: 10.1161/01.res.0000045088.59360.b7. [DOI] [PubMed] [Google Scholar]
- LEINEWEBER K., SEYFARTH T., ABRAHAM G., GERBERSHAGEN H.P., HEINROTH-HOFFMANN I., PONICKE K., BRODDE O.E. Cardiac beta-adrenoceptor changes in monocrotaline-treated rats: differences between membrane preparations from whole ventricles and isolated ventricular cardiomyocytes. J. Cardiovasc. Pharmacol. 2003;41:333–342. doi: 10.1097/00005344-200303000-00001. [DOI] [PubMed] [Google Scholar]
- LI Z., BING O.H., LONG X., ROBINSON K.G., LAKATTA E.G. Increased cardiomyocyte apoptosis during the transition to heart failure in the spontaneously hypertensive rat. Am. J. Physiol. 1997;272:H2313–H2319. doi: 10.1152/ajpheart.1997.272.5.H2313. [DOI] [PubMed] [Google Scholar]
- MANN D.L., KENT R.L., PARSONS B., COOPER G. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992;85:790–804. doi: 10.1161/01.cir.85.2.790. [DOI] [PubMed] [Google Scholar]
- MARANO G., VERGARI A., CATALANO L., GAUDI S., PALAZZESI S., MUSUMECI M., STATI T., FERRARI A.U. Na+/H+ exchange inhibition attenuates left ventricular remodeling and preserves systolic function in pressure-overloaded hearts. Br. J. Pharmacol. 2004;141:526–532. doi: 10.1038/sj.bjp.0705631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARZO K.P., FREY M.J., WISON J.R., LIANG B.T., MANNING D.R., LANOCE V., MOLINOFF P.B. β Adrenergic receptor-G-protein-adenylyl cyclase complex in experimental canine congestive heart failure produced by rapid ventricular pacing. Circ. Res. 1991;69:1546–1556. doi: 10.1161/01.res.69.6.1546. [DOI] [PubMed] [Google Scholar]
- MOE G.W., AMSTRONG P. Pacing-induced heart failure: a model to study the mechanism of disease progression and novel therapy in heart failure. Cardiovasc. Res. 1999;42:591–599. doi: 10.1016/s0008-6363(99)00032-2. [DOI] [PubMed] [Google Scholar]
- MORISCO C., ZEBROWSKI D.C., VATNER D.E., VATNER S.F., SADOSHIMA J. Beta-adrenergic cardiac hypertrophy is mediated primarily by the beta(1)-subtype in the rat heart. J. Mol. Cell. Cardiol. 2001;33:561–573. doi: 10.1006/jmcc.2000.1332. [DOI] [PubMed] [Google Scholar]
- NARULA J., HAIDER N., VIRMANI R., DISALVO T.G., KOLODGIE F.D., HAJJAR R.J., SCHMIDT U., SEMIGRAN M.J., DEC G.W., KHAW B.A. Apoptosis in myocytes in end-stage heart failure. New Engl. J. Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- OLIVETTI G., ABBI R., QUAINI F., KAJSTURA J., CHENG W., NITAHARA J.A., QUAINI E., DI LORETO C., BELTRAMI C.A., KRAJEWSKI S., REED J.C., ANVERSA P. Apoptosis in the failing human heart. New Engl. J. Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- OLIVETTI G., QUAINI F., SALA R., LAGRASTA C., CORRADI D., BONACINA E., GAMBERT S.R., CIGOLA E., ANVERSA P. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J. Mol. Cell. Cardiol. 1996;28:2005–2016. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- OTANI H., UCHIYAMA T., YAMAMURA T., NAKAO Y., HATTORI R., NINOMIYA H., KIDO M., KAWAGUCHI H., OSAKO M., IMAMURA H. Effects of the Na+/H+ exchange inhibitor cariporide (HOE 642) on cardiac function and cardiomyocyte cell death in rat ischaemic-reperfused heart. Clin. Exp. Pharmacol. Physiol. 2000;27:387–393. doi: 10.1046/j.1440-1681.2000.03248.x. [DOI] [PubMed] [Google Scholar]
- PING P., ANZAI T., GAO M., HAMMOND K. Adenylyl cyclase and G protein receptor kinase expression during development of heart failure. Am. J. Physiol. Heart Circ. Physiol. 1997;42:H707–H717. doi: 10.1152/ajpheart.1997.273.2.H707. [DOI] [PubMed] [Google Scholar]
- PING P., HAMMOND H.K. Diverse G protein and beta-adrenergic receptor mRNA expression in normal and failing porcine hearts. Am. J. Physiol. 1994;267:H2079–H2089. doi: 10.1152/ajpheart.1994.267.5.H2079. [DOI] [PubMed] [Google Scholar]
- PORT J.D., BRISTOW M.R. Altered beta-adrenergic receptor gene regulation and signalling in chronic heart failure. J. Mol. Cell. Cardiol. 2001;33:887–905. doi: 10.1006/jmcc.2001.1358. [DOI] [PubMed] [Google Scholar]
- REID A.C., MACKINS C.J., SEYEDI N., LEVI R., SILVER R.B. Coupling of angiotensin II AT1 receptors to neuronal NHE activity and carrier-mediated norepinephrine release in myocardial ischemia. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1448–H1454. doi: 10.1152/ajpheart.01062.2003. [DOI] [PubMed] [Google Scholar]
- REIKEN S., GABURJAKOVA M., GABURJAKOVA J., HE KL K.L., PRIETO A., BECKER E., YI GH G.H., WANG J., BURKHOFF D., MARKS A.R. β Adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation. 2001;104:2843–2848. doi: 10.1161/hc4701.099578. [DOI] [PubMed] [Google Scholar]
- REIKEN S., WEHRENS X.H.T., VEST J.A., BARBONE A., KLOTZ S., MANCINI D., BURKHOFF D., MARKS A. β Blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003;107:2459–2466. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- ROTH D.A., URASAWA K., HELMER G.A., HAMMOND H.K. Downregulation of cardiac guanosine 5′-triphosphate-binding proteins in right atrium and left ventricle in pacing-induced congestive heart failure. J. Clin. Invest. 1993;91:939–949. doi: 10.1172/JCI116315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUFFOLO R.R., FEUERSTEIN G.Z. Neurohumoral activation, oxygen free radicals, and apoptosis in the pathogenesis of congestive heart failure. J. Cardiovasc. Pharmacol. 1998;32:S22–S30. doi: 10.1097/00005344-199800003-00005. [DOI] [PubMed] [Google Scholar]
- SALOMON Y., LONDOS C., RODBELL M. A highly sensitive adenylate cyclase assay. Anal. Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- SCHÄFER M., SCHÄFER C., MICHAEL PIPER H., SCHLÜTER K.D. Hypertrophic responsiveness of cardiomyocytes to alpha- or beta-adrenoceptor stimulation requires sodium-proton-exchanger-1 (NHE-1) activation but not cellular alkalization. Eur. J. Heart Fail. 2002;4:249–254. doi: 10.1016/s1388-9842(02)00016-8. [DOI] [PubMed] [Google Scholar]
- SCHLÜTER K.D., SCHÄFER M., BALSER C., TAIMOR G., PIPER H.M. Influence of pHi and creatine phosphate on alpha-adrenoceptor-mediated cardiac hypertrophy. J. Mol. Cell Cardiol. 1998;30:763–771. doi: 10.1006/jmcc.1998.0640. [DOI] [PubMed] [Google Scholar]
- SCHÖMIG A., RICHARDT G. Cardiac sympathetic activity in myocardial ischemia: release and effects of noradrenaline. Basic Res. Cardiol. 1990;85 (Suppl 1):9–30. doi: 10.1007/978-3-662-11038-6_2. [DOI] [PubMed] [Google Scholar]
- SCHULZ R., AKER S., BELOSJOROW S., KONIETZKA I., RAUEN U., HEUSCH G. Stress kinase phosphorylation is increased in pacing-induced heart failure in rabbits. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2084–H2090. doi: 10.1152/ajpheart.01038.2002. [DOI] [PubMed] [Google Scholar]
- SHAROV V.G., SABBAH H.N., SHIMOYAMA H., GOUSSEV A.V., LESCH M., GOLDSTEIN S. Evidence of cardiocyte apoptosis in myocardium of dogs with chronic heart failure. Am. J. Pathol. 1996;148:141–149. [PMC free article] [PubMed] [Google Scholar]
- SNABAITIS A.K., HEARSE D.J., AVKIRAN M. Regulation of sarcolemmal Na(+)/H(+) exchange by hydrogen peroxide in adult rat ventricular myocytes. Cardiovasc. Res. 2002;53:470–480. doi: 10.1016/s0008-6363(01)00464-3. [DOI] [PubMed] [Google Scholar]
- SNABAITIS A.K., YOKOYAMA H., AVKIRAN M. Roles of mitogen-activated protein kinases and protein kinase C in alpha(1A)-adrenoceptor-mediated stimulation of the sarcolemmal Na(+)-H(+) exchanger. Circ. Res. 2000;86:214–220. doi: 10.1161/01.res.86.2.214. [DOI] [PubMed] [Google Scholar]
- SPINALE F.G., TEMPEL G.E., MUKHERJEE R., EBLE D.M., BROWN R., VACCIANO C., ZILE M.R. Cellular and molecular alterations in the β adrenergic system with cardiomyopathy induced by tachycardia. Cardiovasc. Res. 1994;28:1243–1250. doi: 10.1093/cvr/28.8.1243. [DOI] [PubMed] [Google Scholar]
- SPITZNAGEL H., CHUNG O., XIA Q., ROSSIUS B., ILLNER S., JAHNICHEN G., SANDMANN S., REINECKE A., DAEMEN M.J., UNGER T. Cardioprotective effects of the Na(+)/H(+)-exchange inhibitor cariporide in infarct-induced heart failure. Cardiovasc. Res. 2000;46:102–110. doi: 10.1016/s0008-6363(99)00428-9. [DOI] [PubMed] [Google Scholar]
- TAKEWAKI S., KURO-O M., HIROI Y., YAMAZAKI T., NOGUCHI T., MIYAGISHI A., NAKAHARA K., AIKAWA M., MANABE I., YAZAKI Y., NAGAI R. Activation of Na(+)-H+ antiporter (NHE-1) gene expression during growth, hypertrophy and proliferation of the rabbit cardiovascular system. J. Mol. Cell. Cardiol. 1995;27:729–742. doi: 10.1016/s0022-2828(08)80063-6. [DOI] [PubMed] [Google Scholar]
- WALLERT M.A., FRÖHLICH O. Alpha 1-adrenergic stimulation of Na-H exchange in cardiac myocytes. Am. J. Physiol. 1992;263:C1096–C1102. doi: 10.1152/ajpcell.1992.263.5.C1096. [DOI] [PubMed] [Google Scholar]
- WILKINS B.J., MOLKENTIN J.D. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem. Biophys. Res. Commun. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI T., KOMURO I., YAZAKI Y. Signalling pathways for cardiac hypertrophy. Cell. Signal. 1998;10:693–698. doi: 10.1016/s0898-6568(98)00036-9. [DOI] [PubMed] [Google Scholar]
- YOSHIDA H., KARMAZYN M. Na(+)/H(+) exchange inhibition attenuates hypertrophy and heart failure in 1-wk postinfarction rat myocardium. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H300–H304. doi: 10.1152/ajpheart.2000.278.1.H300. [DOI] [PubMed] [Google Scholar]