Abstract
Protease-activated receptor (PAR)-2 plays important roles in intestinal inflammatory responses. Changes in PAR-2-mediated smooth muscle function may contribute pathophysiologically to the intestinal motility disorders often observed in inflammatory bowel disease (IBD).
Stimulation of PAR-2 by trypsin-induced relaxation of carbachol- and KCl-induced contractions in normal rat colonic smooth muscle was completely resolved by tissue pretreatment with apamin, but not by pretreatment with l-NMMA or a cocktail of neuronal blockers (tetrodotoxin, hexamethonium and propranolol).
In colon inflamed by dextran sodium sulphate (DSS), trypsin-induced inhibitory effects were significantly reduced. Relaxation induced by SLIGRL-NH2, a selective PAR-2-activating peptide, was also reduced in DSS-treated rat colon. However, inhibitory effects of 1-ethylbenzimidazolin-2-one, an activator of small conductance Ca2+-activated K+ channel, were unaffected.
Expression of PAR-2 mRNA in colonic muscularis externa was significantly lower in DSS-treated rats than in control rats.
These results suggest that the PAR-2 mediated relaxation system in colonic smooth muscle is suppressed in this experimental colitis rat model, and may contribute to motility disorders in IBD.
Keywords: Protease-activated receptor-2, colonic smooth muscle, inflammatory bowel disease, motility disorder
Introduction
Protease-activated receptors (PARs) are a novel family of seven-transmembrane G-protein-coupled receptors activated by proteolytic cleavage of the extracellular N-terminal domain, resulting in the generation of a new tethered ligand activating the receptor itself (Vu et al., 1991; Nystedt et al., 1994; Hollenberg et al., 1996; Ishihara et al., 1997). To date, four members of this family have been identified and all are activated by endogenous proteinases. Among the PAR family, the function of protease-activated receptor-2 (PAR-2) has been widely studied, and this receptor is known to be involved in inflammation, allergies, haemorrhaging, exocrine activities and intestinal ion transport (Nguyen et al., 1999; Kawabata et al., 2000c; Ossovskaya & Bunnett, 2004).
Intestinal motility is reportedly impaired in patients with inflammatory bowel disease (IBD) such as Crohn's disease and ulcerative colitis (Reddy et al., 1991; Annese et al., 1997; Kinoshita et al., 2003), and alterations to intestinal motor function are often associated with indications of inflammation (Koch et al., 1988; Bossone et al., 2001). The development of intestinal dysmotility may in turn result in abnormal growth of intestinal microflora, subsequently inducing a translocation of bacteria or bacterial products through the impaired mucosal barrier. These events probably form a vicious cycle, increasing the severity of intestinal injury. Furthermore, trinitrobenzene sulphonic acid-induced colitis induces a loss of inhibitory nitrergic neurotransmission in rabbits (Depoortere et al., 2002), and dextran sulphate sodium (DSS)-induced colitis also impairs non-adrenergic, non-cholinergic inhibitory neuron-dependent relaxation in the distal colon of rats (Mizuta et al., 2000).
PAR-2 is highly expressed in cells of the gastrointestinal tract such as epithelial cells, neuronal elements and myocytes (Nystedt et al., 1994; Vergnolle, 2000; Kawabata, 2002), and activated with trypsin, mast cell tryptase and factors VIIa and Xa (Ossovskaya & Bunnett, 2004). Although the mechanisms of PAR-2-induced inflammation are not clearly understood, PAR-2 activation is thought to be linked to proinflammatory responses in intestinal tissues (Cenac et al., 2002, 2003). Moreover, PAR-2 reportedly modulates smooth muscle motility, and activation of these receptors induces relaxant, contractile or biphasic responses (Kawabata et al., 2000a, 2000b; Mule et al., 2002a, 2002b). As intestinal inflammation is associated with the generation and release of proteases that are potential activators of PAR-2, identifying the role of PAR-2 in intestinal inflammatory disease is important. However, in pathophysiological conditions involving the gastrointestinal tract, such as IBD, the behaviour of PAR-2 in smooth muscle cells and the effect of PAR-2 agonists on contraction have not been fully elucidated. The present study thus aimed to assess possible changes in PAR-2-mediated relaxation pathways in colonic smooth muscle contractions using a rat model of DSS-induced colitis.
Methods
Induction of colitis and tissue preparation
Colonic inflammation was induced in male Sprague–Dawley rats (7- to 10 weeks old) by administering 5% (wt vol−1) DSS (molecular weight, 36,000–50,000) in drinking water for 5 or 7 days (DSS-treated rats). For each control, drinking water without DSS was supplied for 5 or 7 days (control rats). Procedures and care were approved by the Animal Care and Use Committee of Yamaguchi University. Rats were anaesthetised using ether, stunned by a blow on the head and exsanguinated. The distal colon was then excised. To assess the severity of colitis, body weight and length of the colon were measured. Colonic tissue isolated from control or DSS-treated rats was cut open along the mesenteric attachment, and the mucosal layer was removed. The following experiments mainly used rats treated with DSS for 7 days. In some experiments, rats treated with DSS for 5 days were used. The remaining muscle layers prepared for mechanical experiments were placed in tris (hydroxymethyl) aminomethane (Tris)-buffered solution (NaCl, 123.7 mM; KCl, 2.7 mM; MgCl2, 1.0 mM; CaCl2 1.8 mM; glucose, 5.5 mM; Tris, 25 mM; and ethylenediaminetetraacetic acid (EDTA), 0.01 mM; pH 7.4 at 37°C).
Myeloperoxidase assay
Myeloperoxidase (MPO) activity was measured as an index of tissue inflammation in tissue extracts using a slight modification of previously described methods (Bradley et al., 1982). The mucosal layer removed from the distal colon or the smooth muscle layer was homogenised in 1 ml of 50 mM potassium phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide (pH 6.0) using a Polytron homogeniser (Kinematica, Lucerne, Switzerland). Homogenates were frozen in liquid nitrogen and thawed using a sonicator (Taitec, Nagoya, Japan). These procedures were repeated three times, then homogenate was centrifuged at 40,000 × g for 30 min at 4°C. Supernatants were used to measure MPO activity. MPO in the sample was activated by 0.0005% H2O2 in potassium phosphate buffer solution containing 0.5 mM o-dianosidine dihydrochloride (pH 6.0). Optical density was recorded using a spectrophotometer (Hitachi, Tokyo, Japan) and converted to MPO activity using the standard curve for human leukeocyte MPO. Concentrations of protein in supernatants were measured using a protein assay kit (Bio-Rad, Tokyo, Japan) based on the methods of Bradford (1976). For protein assay, bovine serum albumin was used as a standard.
Measurement of muscle tension
Muscle strips isolated from the distal colon were suspended along the circular axis in a tissue bath filled with Tris-buffered solution at 37°C in an atmosphere of 100% O2 (pH 7.4). Responses of circular smooth muscle were measured isometrically under a resting tension of 10 mN and recorded. After equilibration for 15 min in a bath, each strip was repeatedly exposed to 65.4 mM KCl until responses stabilised. Concentration–response curves for KCl and carbachol were obtained using single application of agonists. To avoid the possibility of changes in tissue sensitivity during the course of this experiment, each dose of agonist was applied at random. At the end of tension measurements, wet weight of each muscle strip was measured. To evaluate absolute force induced by KCl or carbachol, area under the curve (AUC) of each agonist was calculated and normalised to wet weight of each strip.
For experiments to evaluate relaxations induced by PAR-2 agonist and 1-ethylbenzimidazolin-2-one (1-EBIO), each strip was precontracted using KCl or carbachol. PAR-2 agonists and 1-EBIO were applied at 5 min after the addition of agonists. In KCl-stimulated colon, each contraction level was compared before and after addition of PAR-2 agonists. In rat colonic circular smooth muscle, carbachol induced large phasic contractions followed by oscillatory contraction that were smaller than phasic contractions, but the relaxing effects are difficult to evaluate (Figure 3a). To evaluate the inhibitory effects of PAR-2 agonists and 1-EBIO on carbachol-induced contraction, AUCs in the presence and absence of PAR-2 agonists and 1-EBIO were compared.
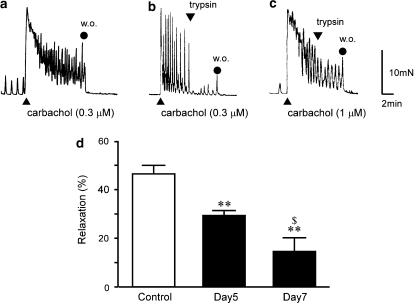
Figure 3.
Inhibitory effect of trypsin on carbachol-induced contraction in control and DSS-treated rat colon. Typical results of 0.3 μM carbachol-induced contraction for 10 min in control rat colon (a). Trypsin (1 μM) was applied at 5 min after addition of 0.3 μM carbachol in control rat colon (b) and 1 μM carbachol in rat colon treated with DSS for 7 days (c). Analytical results of relaxations in control rat colon and DSS-treated rat colon (treated with DSS for 5 days (day 5) or 7 days (day 7)) (d). AUC of carbachol in the absence of trypsin was considered 100%. Results are expressed as means±s.e.m. of 6–12 experiments. **P<0.01 vs control; $P<0.01 vs day 5; w.o., washing out.
Reverse transcriptase–polymerase chain reaction
Total RNA was extracted from colonic smooth muscle strips of control and DSS-treated rat using TRIzol reagent (Invitrogen, Tokyo, Japan). RNA concentration was adjusted to 1 μg μl−1 with RNase-free distilled water. Reverse transcriptase–polymerase chain reaction (RT–PCR) was performed using an Access Quick RT–PCR System (Promega, Tokyo, Japan), which combined cDNA synthesis and PCR in a single reaction. Next, 1 μl of total RNA was added to the 49-μl final reaction mixture solution. For single-step RT–PCR, reverse transcription (48°C for 50 min) was followed by initial denaturation at 95°C for 2 min. Cycling profiles used were: denaturing at 95°C for 30 s; annealing at 55°C for 1 min and extension at 72°C for 2 min. A total of 22–32 cycles was used, followed by a final extension step of 5 min at 72°C. The following oligonucleotide primers were designed (Kawabata et al., 2000c; Nishikawa et al., 2002):
PAR-2, 5′-CACCAGTAAGGGAGAAGTCT-3′ (sense) and 5′-GGGCAGCACGTCGTGACAGGT-3′ (antisense); and β-actin, 5′-CGTGGGCCGCCCTAGGCACCA-3′ (sense) and 5′-TTGGCCTTAGGGTTCAGGGGG-3′ (antisense).
All primers were designed to avoid significant secondary and complementary structures and to include a GC content of 50–60%. Suitable sizes of synthesised cDNA for PAR-2 and β-actin were 598 and 537 bp, respectively. PCR products in each cycle were electrophoresed on 2% agarose gel. PCR products of the predicted size were stained using ethidium bromide and visualised using an ultraviolet transilluminator (UVP, Cambridge, U.K.). Quantification of each band was performed using Scion Image densitometry analysis software (Scion Corporation, Maryland, U.S.A.). PCR signals yielded by PAR primers were normalised to the PCR signal generated by primers for β-actin.
Chemicals
Chemicals used were as follows: SLIGRL-NH2 (Bachem, Bubendorf, Switzerland), carbachol, o-dianosidine dihydrochloride, hexadecyltrimethylammonium bromide, human leukeocyte MPO, tetrodotoxin (TTX), hexamethonium, propranolol (Sigma, Tokyo, Japan); EDTA (Dojindo Laboratories, Tokyo, Japan); trypsin, apamin, 1-EBIO, DSS (36,000–50,000 molecular weight), Tris (Wako Pure Chemical, Osaka, Japan) and bovine serum albumin (Roche Diagnostics, Tokyo, Japan).
Statistical analyses
Results are expressed as means±standard error of the mean (s.e.m.). Statistical evaluation of data was performed using paired or unpaired Student's t-tests for comparisons between two groups and one-way analysis of variance (ANOVA) followed by the Tukey test for comparisons among ⩾3 groups with Prism software (Graph Pad Software, California, U.S.A.). Values of P<0.05 were considered statistically significant.
Results
Clinical observations in DSS-treated rat colon
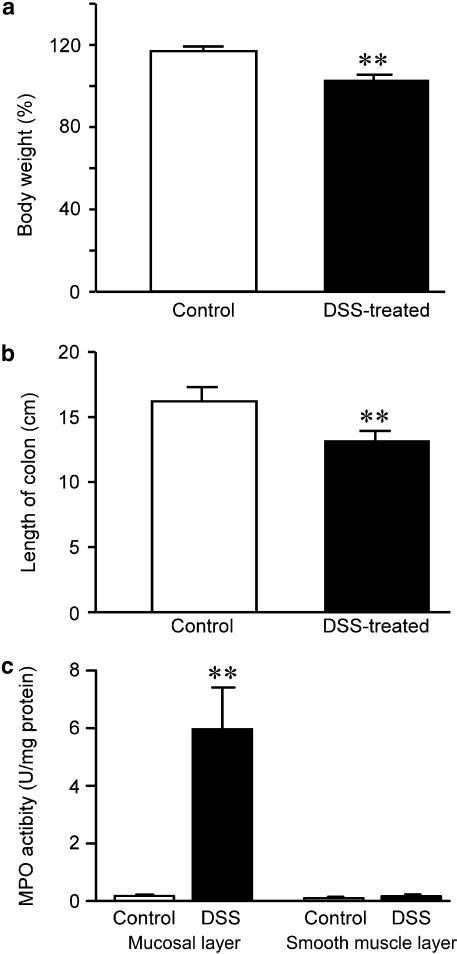
Rats treated with 5% (wt vol−1) DSS developed bloody diarrhoea 5–7 days after initiating DSS treatment. Although mean body weight for control and DSS-treated rats at 7 days was increased, rate of increments in bodyweight was significantly lower for DSS-treated rats than for control rats (Figure 1a). Mean colon length was also significantly decreased in DSS-treated rats (Figure 1b). Bloody stool and fresh blood were found in the opened colons of DSS-treated rats. Although MPO activity was significantly increased in the mucosal layer of DSS-treated rat colon, MPO activities in the smooth muscle layer of DSS-treated colon were not significantly changed (Figure 1c).
Figure 1.
Changes in body weight, colon length and myeloperoxidase (MPO) activity in control and dextran sodium sulphate (DSS)-treated rat colon. Body weight of rats treated with DSS for 7 days was significantly decreased compared to control rats. Body weight of rats at day 0 was considered 100% (a). Length of the whole colon was significantly decreased in DSS-treated rats (b). MPO activity was significantly increased in the mucosal layer of DSS-treated rat colon (c). **P<0.01, n=6–9.
KCl- and carbachol-induced contractions
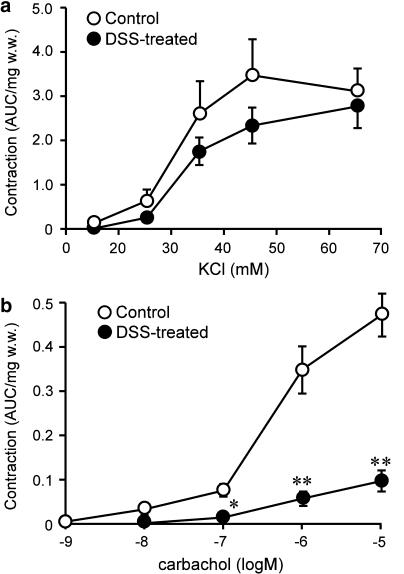
The preliminary experiments analysed length–tension relationships stimulated using 65.4 mM KCl in colonic circular smooth muscles isolated from control and DSS-treated rats, confirming that the relationship was unchanged after DSS treatment (optimal resting tension: 10 mN) (data not shown). We first examined the effects of KCl (15.4–65.4 mM) on contractions in control and DSS-treated rat colonic smooth muscle. Absolute contractile forces induced by KCl tended to be slightly smaller in DSS-treated rats than in control rats, but no significant differences were identified (Figure 2a). Next, carbachol-induced contractions were compared between control and DSS-treated rats. Carbachol (1 nM–10 μM)-induced contractions were significantly decreased in DSS-treated rat colon compared with control rat colon (Figure 2b).
Figure 2.
Analytical results of alterations in contractions induced by KCl (a) and carbachol (b) in control and DSS-treated rat colon. Contractile response was calculated as area under the curve (AUC), and AUC was normalised by wet weight of tissues (AUC mg−1 w.w.). Results are expressed as means±s.e.m. of 10–11 experiments. *P<0.05, **P<0.01.
Suppression of PAR-2 agonist -induced relaxation
PAR-2 agonists such as trypsin and SLIGRL-NH2 induced relaxation in rat colonic circular smooth muscle. In the study of mechanical responses, adopting the same size of precontractions is important for comparing PAR-2-induced relaxation in control and DSS-treated rat colon. For this reason, different concentrations of carbachol and KCl were used in control and DSS-treated rats. Single application of 0.3 μM carbachol to control rat colon induced contraction at 0.11±0.03 AUC mg−1 wet weight. In the colons of rats treated with DSS for 5 and 7 days, 1 μM carbachol induced contractions at 0.12±0.04 and 0.09±0.03 AUC mg−1 wet weight, respectively. Single application of 20.4 mM KCl induced contraction at 0.22±0.02 AUC mg−1 wet weight in control rat colon, while 25.4 mM KCl induced contraction at 0.25±0.06 AUC mg−1 wet weight in rat colon treated with DSS for 7 days. No significant differences were noted in each agonist-induced contraction between control and DSS-treated rat colon.
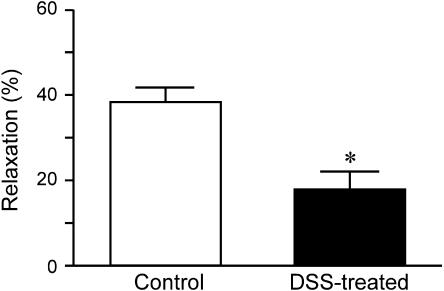
In control rat colonic circular smooth muscle, 0.3 μM carbachol induced phasic contraction followed by oscillatory contraction (Figure 3a). No differences in contractile pattern were induced by carbachol in control and DSS-treated rat colon. At 5 min after addition of 0.3 μM carbachol, addition of 1 μM trypsin induced strong and sustained relaxation in control rat colon (Figure 3b). In rat colon treated with DSS for 7 days, addition of 1 μM trypsin evoked only mild relaxation in the presence of 1 μM carbachol (Figure 3c).
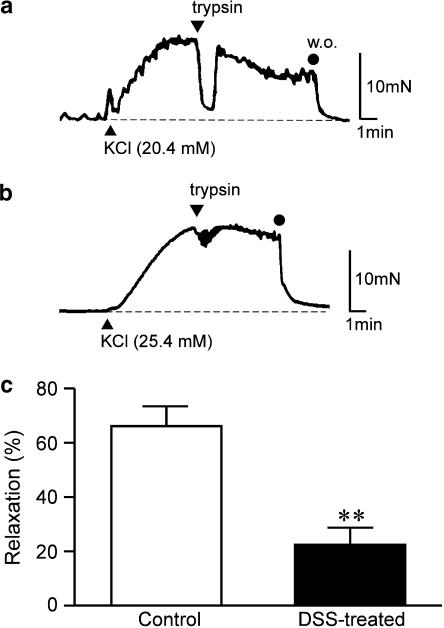
Figure 3d shows the quantitative data for relaxations, and relaxation induced by 1 μM trypsin was significantly smaller in DSS-treated rat colon than in control rat colon. This suppression was dependent on duration of DSS treatment. In the presence of 65.4 mM KCl, trypsin did not induce relaxation in control rat colon (data not shown). Although trypsin evoked a large phasic relaxation for 20.4 mM KCl-induced contraction in control rat colon (Figure 4a), only mild phasic relaxation was observed with the addition of trypsin in DSS-treated rat colon (Figure 4b). Figure 4c shows quantitative data demonstrating that relaxation induced by 1 μM trypsin was significantly smaller in DSS-treated rat colon than in control rat colon. Single application of SLIGRL-NH2 (30 μM) induced relaxation in control rat colon contracted using 0.3 μM carbachol. Amplitude of relaxation induced by SLIGRL-NH2 was almost equal to that induced by 1 μM trypsin. In DSS-treated rat colon contracted using 1 μM carbachol, relaxation induced by the addition of 30 μM SLIGRL-NH2 was also significantly smaller than that in control rat colon (Figure 5).
Figure 4.
Inhibitory effect of trypsin on KCl-induced contractions in control and DSS-treated rat colon. Trypsin (1 μM) was applied for 5 min at 5 min after the addition of 20.4 mM KCl in control rat colon (a) and 25.4 mM in DSS-treated rat colon (b). Analytical results of relaxations in control and DSS-treated rat colon (c). Contraction induced by KCl just before addition of trypsin was considered 100%. Results are expressed as means±s.e.m. of 5–6 experiments. **P<0.01; w.o., washing out.
Figure 5.
Inhibitory effect of SLIGRL-NH2 on carbachol-induced contraction in control and DSS-treated rat colon. SLIGRL-NH2 (30 μM) was applied 5 min after the addition of 0.3 μM carbachol in control and 1 μM in DSS-treated rat colon for 5 min. AUC of carbachol in the absence of SLIGRL-NH2 was considered 100%. Results are expressed as means±s.e.m. of 7–9 experiments. *P<0.05.
Involvement of Ca2+-sensitive small conductance K+ channels
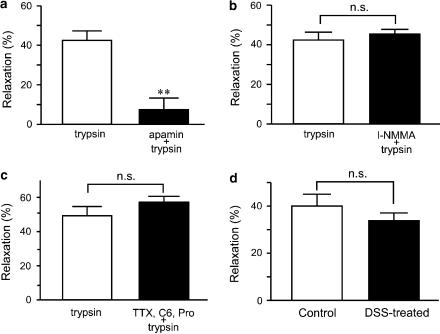
To evaluate the inhibitory mechanism of trypsin, 0.1 μM apamin was applied 20 min before addition of 0.3 μM carbachol in control rat colon. Trypsin (1 μM) was applied 5 min after addition of carbachol. The inhibitory effects of trypsin on carbachol-induced contraction were almost completely suppressed in the presence of apamin (Figure 6a). We next examined the effect of l-NMMA, a NO synthetase inhibitor, or a cocktail of neural blockers such as TTX, hexamethonium and propranolol on trypsin-induced relaxation. Either l-NMMA (200 μM) or a cocktail of TTX (1 μM), hexamethonium (100 μM) and propranolol (5 μM) was applied 20 min before the addition of 0.3 μM carbachol in control rat colon. Trypsin (1 μM) was applied 5 min after addition of carbachol, and neither l-NMMA nor neural blockers exerted any effect on trypsin-induced relaxation (Figure 6b and c). Suppression of PAR-2 agonist-induced relaxation in DSS-treated rat colon may be due to dysfunction of apamin-sensitive small conductance Ca2+-activated K+ (SKCa) channels. We therefore investigated the relaxing effects of 1-EBIO, an SKCa channel activator, on DSS-treated rat colon. At 5 min after carbachol addition, 1-EBIO (30 μM) was applied. No significant differences in the inhibitory effects of 1-EBIO on carbachol-induced contraction were seen in control or DSS-treated rat colon (Figure 6d).
Figure 6.
Effect of apamin, l-NMMA and cocktail of neural blockers on inhibitory effect of trypsin, and effect of 1-EBIO on carbachol-induced contraction. Analytical results of 1 μM trypsin-induced relaxation after preincubation without or with 0.1 μM apamin (a), without or with 200 μM l-NMMA (b) and without or with neural blockers (cocktail of 1 μM TTX, 1 μM hexamethonium and 10 μM propranolol) (c) in control rat colon. (d) Application of 1-EBIO (30 μM) was performed 5 min after addition of 0.3 μM carbachol in controls and 1 μM in DSS-treated rat colon for 5 min. Analytical results of relaxation in control and DSS-treated rat colon. AUC of carbachol in the absence of trypsin or 1-EBIO was considered 100%. Results are expressed as means±s.e.m. of 6–8 experiments. **P<0.01; n.s.; not significant, C6; hexamethonium, Pro; propranolol.
Expression of PAR-2 mRNA detected by RT–PCR
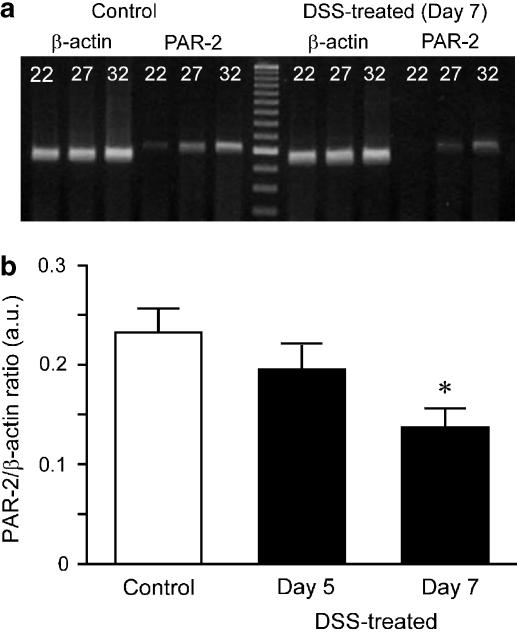
Finally, expression of PAR-2 mRNA in rat colonic muscularis externa was assessed in a semiquantitative manner using RT–PCR. PCR products of PAR-2 and β-actin were amplified at 22, 27 and 32 cycles. In control rat colon, PCR product for PAR-2 was detected at 22 cycles of amplification, but no PCR product was found in DSS-treated rat colon (Figure 7a; 7 days treatment with DSS). In contrast, expression levels of β-actin mRNA in DSS-treated rat colon were no different from those in control rat colon. Expression level of PAR-2 mRNA at 27 cycles of PCR amplification was slightly smaller in rat colon treated with DSS for 5 days and significantly smaller in rat colon treated with DSS for 7 days compared to control rat colon at a constant level of housekeeping gene β-actin (Figure 7b). Decreased expression of mRNA was thus dependent on the duration of DSS treatment.
Figure 7.
Semiquantitative analysis of RT–PCR for expression of PAR-2 mRNA in control and DSS-treated rat colon. (a) Typical RT–PCR products for β-actin (537 bp) or PAR-2 (598 bp) at 22, 27 and 32 cycles of amplification in colonic smooth muscle of control and DSS-treated rats (7 days treatment). (b) Summarised data for control rats and rats treated with DSS for 5 days (day 5) and 7 days (day 7) at 27 cycles are shown. Results are expressed as means±s.e.m. of 4–6 experiments. *P<0.05.
Discussion
IBD is associated with abnormal motility, often leading to diarrhoea or constipation. In IBD patients or animal models of intestinal inflammation induced by treatment with DSS, trinitrobenzenesulphonic acid or acetic acid, contractions of colonic smooth muscle are reportedly decreased (van Bergeijk et al., 1998; Akbarali et al., 2000; Shi & Sarna, 2000; Al-Saffar & Hellstrom, 2001; Kinoshita et al., 2003). The present experiments first confirmed that carbachol-induced contraction, but not KCl-induced contraction, of colonic smooth muscle is significantly smaller in DSS-treated rats than in control rats (Figure 2). Recent studies have clarified the molecular mechanisms responsible for motility disorders in the inflamed gut. These include increased activity of myosin light chain phosphatase, which is responsible for the Ca2+-sensitising mechanism (Ohama et al., 2003).
Activation of PAR-2 induces biphasic responses (contraction and relaxation) in gastrointestinal tissues, depending on tissue-specific differences (Ossovskaya & Bunnett, 2004). In addition, PAR-1 and -2 reportedly play dual roles in the contraction of rat colon, with activation of PARs producing contraction in longitudinal muscle and suppressing contraction in circular muscle (Mule et al., 2002a). In the present study, neither trypsin nor SLIGRL-NH2, a selective PAR-2-activating peptide, induced any contraction when added under resting conditions (data not shown). In the presence of 20.4 mM of KCl, trypsin induced a transient inhibitory effect in control rat colon (Figure 4a). However, trypsin did not inhibit contraction under more depolarised conditions in the presence of 65.4 mM of KCl. Inhibitory effects of trypsin on carbachol-induced contraction in control rat colon were almost completely suppressed by preincubation with apamin (Figure 6a). These data suggest that the relaxing effect of trypsin is mainly due to membrane hyperpolarisation through the activation of apamin-sensitive SKCa channels. These findings are consistent with previous reports using rat circular and longitudinal smooth muscle (Mule et al., 2002a, 2002b).
In rat colon, relaxation induced by PAR-2 agonists is reportedly produced through NO release (Mule et al., 2003). In the present study, however, trypsin-induced relaxation was unchanged in the presence of l-NMMA (Figure 6b). PAR-2 is also apparently expressed on a subset of myenteric neurons (Corvera et al., 1999) and the activation of PAR-2 elicits prolonged depolarisation and excitation of myenteric neurons in guinea pig ileum (Linden et al., 2001). The possibility thus remains that enteric neuron activation may account for some of the present findings. We therefore treated tissue with a cocktail of neural blockers including TTX, hexamethonium and propranolol to verify neural involvement in PAR-2 responses. In the presence of the cocktail of neural blockers, the inhibitory effects of PAR-2 agonist on carbachol-induced contraction remained unchanged (Figure 6c). These results suggest that the inhibitory effect of PAR-2 agonist is not due to the release of inhibitory neurotransmitters.
The present study found that the inhibitory effects of trypsin on carbachol- and KCl-induced contraction are significantly reduced in DSS-treated rat colonic smooth muscle (Figures 3 and 4). SLIGRL-NH2-induced relaxation was also significantly decreased in DSS-treated rat colon (Figure 5). These findings suggest that rat colonic smooth muscle relaxation is mediated by the PAR-2/SKCa channel system, but not by NO production or the release of inhibitory neurotransmitters, and this system is suppressed under DSS-induced inflammatory conditions. Regarding suppression of the PAR-2/SKCa channel system, one possible mechanism would involve reductions in the activity of SKCa channels in DSS-treated rats. To solve this problem, we used a selective activator of SKCa channel, 1-EBIO (Matsumoto et al., 2004). In DSS-treated rat colon, 1-EBIO inhibited carbachol-induced contractions, but the inhibitory effects of this compound were unchanged after colonic inflammation (Figure 6d).
Another possible mechanism to explain suppression of PAR-2-agonist-induced relaxation in DSS-treated rats would be a reduction in PAR-2 expression in colonic smooth muscle. Trypsin removes or destroys the tethered ligand or cleaves the binding domain in the extracellular loop (Nystedt et al., 1994). PARs are endocytosed and trafficked to lysosomes once cleaved, irrevocably terminating signalling (Bohm et al., 1996). As the recovery of PAR-2 requires synthesis or mobilisation of new receptors, expression of PAR-2 mRNA is important for the maintenance of this system. We analysed expression levels of PAR-2 mRNA in the smooth muscle layer using semiquantified RT–PCR experiments, and found that PAR-2 mRNA was significantly less expressed in DSS-treated rat colon than in control rat colon (Figure 7). The present study did not examine the mechanisms of suppression for PAR-2 mRNA, and further investigations are therefore warranted.
An alternative possibility remained that reductions in PAR-2 effect are due to the impairment of the myogenic relaxation system itself in DSS-treated rat colon. To exclude this possibility, we examined the effect of forskolin, an adenylate cyclase activator, on carbachol-induced contraction. Addition of 0.3 μM forskolin induced vigorous relaxation both in control and DSS-treated rat colons (unpublished data). Relaxation induced by sodium nitroprusside, a guanylate cyclase activator, in colonic smooth muscle reportedly remains unchanged in DSS-treated rats (Mizuta et al., 2000). These results suggest that the myogenic relaxing system remains intact in DSS-induced colitis.
Under normal circumstances, physiological concentrations of trypsin in the intestinal tract can stimulate enterocytes by activating PAR-2, and bacterial proteases can also signal the intestinal lumen. Under pathophysiological conditions during inflammation, high levels of protease and trypsin released from inflammatory cells such as mast cells are found in the intestinal tract and enhance PAR-2 activation. Coagulation factors VIIa and Xa are also capable of activating PAR-2 (Camerer et al., 2000). Since the population of mast cells increases in intestinal tissues and massive bleeding is usually observed in ulcerative colitis patients and animal models of colitis, PAR-2 may be chronically activated by proteases and coagulation factors, and such chronic activation would lead to the downregulation of PAR-2. An argument could be made that mRNA of PAR-2 should be increased in DSS animals due to stimulated receptor turnover. Indeed, stimulation with cytokines such as tumour necrosis factor-α and interleukin-1α and lipopolysaccharide reportedly elevates expression of PAR-2 in endothelial cells (Nystedt et al., 1996). However, lysophosphatidic acid and the injury condition of hypoglycaemia induce significant decreases in PAR-1 mRNA expression in neuronal cell lines (Weinstein et al., 1998). The present study did not analyse the mechanisms of PAR mRNA downregulation and the physiological meaning of this mechanism remains unclear, but we speculate that an excess of protease-mediated inflammatory responses would be counterbalanced by downregulation of PAR-2.
In summary, this study shows for the first time that PAR-2 agonist-induced relaxation mediated by apamin-sensitive potassium channel, but not by NO production or release of inhibitory neurotransmitters, in colonic circular smooth muscle is suppressed in a rat model of colitis after DSS treatment. This mechanism could contribute to gastrointestinal dysmotility in IBD.
Acknowledgments
This work was supported by a Program for the Promotion of Basic Research Activities for Innovative Biosciences (BRAIN), Morinaga Hoshi-kai, and a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture and Science.
Abbreviations
- 1-EBIO
1-ethylbenzimidazolin-2-one
- DSS
dextran sodium sulphate
- IBD
inflammatory bowel disease
- PAR-2
protease-activated receptor-2
- SKCa
small conductance Ca2+-activated K+
- TTX
tetrodotoxin
References
- AKBARALI H.I., POTHOULAKIS C., CASTAGLIUOLO I. Altered ion channel activity in murine colonic smooth muscle myocytes in an experimental colitis model. Biochem. Biophys. Res. Commun. 2000;275:637–642. doi: 10.1006/bbrc.2000.3346. [DOI] [PubMed] [Google Scholar]
- AL-SAFFAR A., HELLSTROM P.M. Contractile responses to natural tachykinins and selective tachykinin analogs in normal and inflamed ileal and colonic muscle. Scand. J. Gastroenterol. 2001;36:485–493. [PubMed] [Google Scholar]
- ANNESE V., BASSOTTI G., NAPOLITANO G., USAI P., ANDRIULLI A., VANTRAPPEN G. Gastrointestinal motility disorders in patients with inactive Crohn's disease. Scand. J. Gastroenterol. 1997;32:1107–1117. doi: 10.3109/00365529709002989. [DOI] [PubMed] [Google Scholar]
- BOHM S.K., KHITIN L.M., GRADY E.F., APONTE G., PAYAN D.G., BUNNETT N.W. Mechanisms of desensitization and resensitization of proteinase-activated receptor-2. J. Biol. Chem. 1996;271:22003–22016. doi: 10.1074/jbc.271.36.22003. [DOI] [PubMed] [Google Scholar]
- BOSSONE C., HOSSEINI J.M., PINEIRO-CARRERO V., SHEA-DONOHUE T. Alterations in spontaneous contractions in vitro after repeated inflammation of rat distal colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G949–G957. doi: 10.1152/ajpgi.2001.280.5.G949. [DOI] [PubMed] [Google Scholar]
- BRADFORD M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRADLEY P.P., PRIEBAT D.A., CHRISTENSEN R.D., ROTHSTEIN G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- CAMERER E., HUANG W., COUGHLIN S.R. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CENAC N., COELHO A.M., NGUYEN C., COMPTON S., ANDRADE-GORDON P., MACNAUGHTON W.K., WALLACE J.L., HOLLENBERG M.D., BUNNETT N.W., GARCIA-VILLAR R., BUENO L., VERGNOLLE N. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am. J. Pathol. 2002;161:1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CENAC N., GARCIA-VILLAR R., FERRIER L., LARAUCHE M., VERGNOLLE N., BUNNETT N.W., COELHO A.M., FIORAMONTI J., BUENO L. Proteinase-activated receptor-2-induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J. Immunol. 2003;170:4296–4300. doi: 10.4049/jimmunol.170.8.4296. [DOI] [PubMed] [Google Scholar]
- CORVERA C.U., DERY O., MCCONALOGUE K., GAMP P., THOMA M., AL-ANI B., CAUGHEY G.H., HOLLENBERG M.D., BUNNETT N.W. Thrombin and mast cell tryptase regulate guinea-pig myenteric neurons through proteinase-activated receptors-1 and -2. J. Physiol. 1999;517:741–756. doi: 10.1111/j.1469-7793.1999.0741s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEPOORTERE I., THIJS T., PEETERS T.L. Generalized loss of inhibitory innervation reverses serotonergic inhibition into excitation in a rabbit model of TNBS-colitis. Br. J. Pharmacol. 2002;135:2011–2019. doi: 10.1038/sj.bjp.0704648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL-ANI B. Proteinase-activated receptor-2 in rat aorta: structural requirements for agonist activity of receptor-activating peptides. Mol. Pharmacol. 1996;49:229–233. [PubMed] [Google Scholar]
- ISHIHARA H., CONNOLLY A.J., ZENG D., KAHN M.L., ZHENG Y.W., TIMMONS C., TRAM T., COUGHLIN S.R. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- KAWABATA A. PAR-2: structure, function and relevance to human diseases of the gastric mucosa. Expert Rev. Mol. Med. 2002;2002:1–17. doi: 10.1017/S1462399402004799. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., KUROKI N., NISHIKAWA H., KAWAI K. Dual modulation by thrombin of the motility of rat oesophageal muscularis mucosae via two distinct protease-activated receptors (PARs): a novel role for PAR-4 as opposed to PAR-1. Br. J. Pharmacol. 2000a;131:578–584. doi: 10.1038/sj.bjp.0703590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., KUROKI N., NISHIKAWA H., KAWAI K., ARAKI H. Characterization of the protease-activated receptor-1-mediated contraction and relaxation in the rat duodenal smooth muscle. Life Sci. 2000b;67:2521–2530. doi: 10.1016/s0024-3205(00)00835-3. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., NISHIKAWA H., KURODA R., KAWAI K., HOLLENBERG M.D. Proteinase-activated receptor-2 (PAR-2): regulation of salivary and pancreatic exocrine secretion in vivo in rats and mice. Br. J. Pharmacol. 2000c;129:1808–1814. doi: 10.1038/sj.bjp.0703274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINOSHITA K., SATO K., HORI M., OZAKI H., KARAKI H. Decrease in activity of smooth muscle L-type Ca2+ channels and its reversal by NF-κB inhibitors in Crohn's colitis model. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G483–G493. doi: 10.1152/ajpgi.00038.2003. [DOI] [PubMed] [Google Scholar]
- KOCH T.R., CARNEY J.A., GO V.L., SZURSZEWSKI J.H. Spontaneous contractions and some electrophysiologic properties of circular muscle from normal sigmoid colon and ulcerative colitis. Gastroenterology. 1988;95:77–84. doi: 10.1016/0016-5085(88)90293-4. [DOI] [PubMed] [Google Scholar]
- LINDEN D.R., MANNING B.P., BUNNETT N.W., MAWE G.M. Agonists of proteinase-activated receptor 2 excite guinea pig ileal myenteric neurons. Eur. J. Pharmacol. 2001;431:311–314. doi: 10.1016/s0014-2999(01)01447-9. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO T., YOSHIYAMA S., WAKABAYASHI K., KOBAYASHI T., KAMATA K. Effect of chronic insulin on cromakalim-induced relaxation in established streptozotocin-diabetic rat basilar artery. Eur. J. Pharmacol. 2004;504:129–137. doi: 10.1016/j.ejphar.2004.09.031. [DOI] [PubMed] [Google Scholar]
- MIZUTA Y., ISOMOTO H., TAKAHASHI T. Impaired nitrergic innervation in rat colitis induced by dextran sulfate sodium. Gastroenterology. 2000;118:714–723. doi: 10.1016/s0016-5085(00)70141-7. [DOI] [PubMed] [Google Scholar]
- MULE F., BAFFI M.C., CAPPARELLI A., PIZZUTI R. Involvement of nitric oxide and tachykinins in the effects induced by protease-activated receptors in rat colon longitudinal muscle. Br. J. Pharmacol. 2003;139:598–604. doi: 10.1038/sj.bjp.0705273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULE F., BAFFI M.C., CERRA M.C. Dual effect mediated by protease-activated receptors on the mechanical activity of rat colon. Br. J. Pharmacol. 2002a;136:367–374. doi: 10.1038/sj.bjp.0704746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULE F., BAFFI M.C., FALZONE M., CERRA M.C. Signal transduction pathways involved in the mechanical responses to protease-activated receptors in rat colon. J. Pharmacol. Exp. Ther. 2002b;303:1265–1272. doi: 10.1124/jpet.102.041301. [DOI] [PubMed] [Google Scholar]
- NGUYEN T.D., MOODY M.W., STEINHOFF M., OKOLO C., KOH D.S., BUNNETT N.W. Trypsin activates pancreatic duct epithelial cell ion channels through proteinase-activated receptor-2. J. Clin. Invest. 1999;103:261–269. doi: 10.1172/JCI2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIKAWA H., KAWAI K., NISHIMURA S., TANAKA S., ARAKI H., AL-ANI B., HOLLENBERG M.D., KURODA R., KAWABATA A. Suppression by protease-activated receptor-2 activation of gastric acid secretion in rats. Eur. J. Pharmacol. 2002;447:87–90. doi: 10.1016/s0014-2999(02)01892-7. [DOI] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYSTEDT S., RAMAKRISHNAN V., SUNDELIN J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J. Biol. Chem. 1996;271:14910–14915. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- OHAMA T., HORI M., SATO K., OZAKI H., KARAKI H. Chronic treatment with interleukin-1β attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J. Biol. Chem. 2003;278:48794–48804. doi: 10.1074/jbc.M310166200. [DOI] [PubMed] [Google Scholar]
- OSSOVSKAYA V.S., BUNNETT N.W. Protease-activated receptors: contribution to physiology and disease. Physiol. Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- REDDY S.N., BAZZOCCHI G., CHAN S., AKASHI K., VILLANUEVA-MEYER J., YANNI G., MENA I., SNAPE W.J., Jr Colonic motility and transit in health and ulcerative colitis. Gastroenterology. 1991;101:1289–1297. doi: 10.1016/0016-5085(91)90079-z. [DOI] [PubMed] [Google Scholar]
- SHI X.Z., SARNA S.K. Impairment of Ca2+ mobilization in circular muscle cells of the inflamed colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G234–G242. doi: 10.1152/ajpgi.2000.278.2.G234. [DOI] [PubMed] [Google Scholar]
- VAN BERGEIJK J.D., VAN WESTREENEN H., ADHIEN P., ZIJLSTRA F.J. Diminished nitroprusside-induced relaxation of inflamed colonic smooth muscle in mice. Mediators Inflamm. 1998;7:283–287. doi: 10.1080/09629359890974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N. Review article: Proteinase-activated receptors – novel signals for gastrointestinal pathophysiology. Aliment Pharmacol. Ther. 2000;14:257–266. doi: 10.1046/j.1365-2036.2000.00690.x. [DOI] [PubMed] [Google Scholar]
- VU T.K., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- WEINSTEIN J.R., LAU A.L., BRASS L.F., CUNNINGHAM D.D. Injury-related factors and conditions down-regulate the thrombin receptor (PAR-1) in a human neuronal cell line. J. Neurochem. 1998;71:1034–1050. doi: 10.1046/j.1471-4159.1998.71031034.x. [DOI] [PubMed] [Google Scholar]