Abstract
Several peptidic urotensin-II (UT) receptor antagonists exert ‘paradoxical' agonist activity in recombinant cell- and tissue-based bioassay systems, likely the result of differential urotensin-II receptor (UT receptor) signal transduction/coupling efficiency between assays. The present study has examined this phenomenon in mammalian arteries and recombinant UT-HEK (human embryonic kidney) cells.
BacMam-mediated recombinant UT receptor upregulation in HEK cells augmented agonist activity for all four peptidic UT ligands studied. The nominal rank order of relative intrinsic efficacy was U-II>urantide ([Pen5-DTrp7-Orn8]hU-II4–11)>SB-710411 (Cpa-c[DCys-Pal-DTrp-Lys-Val-Cys]-Cpa-amide)≫GSK248451 (Cin-c[DCys-Pal-DTrp-Orn-Val-Cys]-His-amide) (the relative coupling efficiency of recombinant HEK cells was cat>human≫rat UT receptor).
The present study further demonstrated that the use of high signal transduction/coupling efficiency isolated blood vessel assays (primate>cat arteries) is required in order to characterize UT receptor antagonism thoroughly. This cannot be attained simply by using the rat isolated aorta, an artery with low signal transduction/coupling efficiency in which low-efficacy agonists appear to function as antagonists.
In contrast to the ‘low-efficacy agonists' urantide and SB-710411, GSK248451 functioned as a potent UT receptor antagonist in all native isolated tissues studied (UT receptor selectivity was confirmed in the rat aorta). Further, GSK248451 exhibited an extremely low level of relative intrinsic activity in recombinant HEK cells (4–5-fold less than seen with urantide). Since GSK248451 (1 mg kg−1, i.v.) blocked the systemic pressor actions of exogenous U-II in the anaesthetized cat, it represents a suitable peptidic tool antagonist for delineating the role of U-II in the aetiology of mammalian cardiometabolic diseases.
Keywords: Urotensin-II, UT receptor, G-protein-coupled receptor, vasoconstriction, radioligand binding, BacMam, urantide, GSK248451, SB-710411, operational model
Introduction
Human urotensin-II (hU-II) induces profound haemodynamic effects in the rat (Gardiner et al., 2004), cat (Behm et al., 2004a), monkey (Ames et al., 1999; Zhu et al., 2004) and in man (Böhm & Pernow, 2002; Lim et al., 2004; Sondermeijer et al., 2005). In addition to direct vasodilator/constrictor actions, hU-II also regulates cardiorenal homeostasis by altering cardiac inotropy (Russell et al., 2003; Kompa et al., 2004), inducing natriuresis (Song et al., 2003) and exerting hypertrophic/proinflammatory actions (Zou et al., 2001; Tzanidis et al., 2003; Johns et al., 2004). Although, hU-II and its G-protein-coupled receptor, UT (formerly GPR14), are purported to be involved in the aetiology of several cardiorenal and metabolic diseases including hypertension, cardiorenal failure, atherosclerosis and diabetes (Gilbert et al., 2004; Richards & Charles, 2004; Russell, 2004), a lack of specific urotensin-II receptor (UT receptor) antagonists (Dhanak et al., 2003; Douglas et al., 2004a) has, to date, hampered efforts to delineate the true (patho)physiological significance of this hormone/receptor system.
Considerable effort has been focused recently on the development of UT receptor antagonists (Clozel et al., 2004; 2006; Douglas et al., 2005) and several nonproprietary putative peptidic inhibitors entities that are derivatives of (a) urotensin-II (e.g. [Pen5-DTrp7-Orn8]hU-II4–11 (urantide), [Orn8]hU-II, [Dab8]-derivatives including UFP-803; Camarda et al., 2002; 2006; Patacchini et al., 2003; Guerrini et al., 2005), (b) somatostatin (e.g. lanreotide, octreotide, SB-710411 (Cpa-c[DCys-Pal-DTrp-Lys-Val-Cys]-Cpa-amide), GSK248451(Cin-c[DCys-Pal-DTrp-Orn-Val-Cys]-His-amide); Coy et al., 2000; 2003; Behm et al., 2002; 2003a; Herold et al., 2002; Heller et al., 2003; Aiyar et al., 2005) or (c) neuromedin B (e.g. BIM-23127, BIM-23042; Herold et al., 2002; 2003). Interestingly, however, many of these peptidic agents exert ‘atypical' actions in selected assay systems. For example, urantide, best known as a U-II antagonist in rat aortae (Patacchini et al., 2003), exhibits ‘paradoxical' human recombinant UT receptor agonism (Camarda et al., 2004). Such a ligand may possess ‘species-specific' actions (primate UT receptors are <80% homologous to rodent UT receptor paralogues), a supposition that gains some credence from the observations that SB-710411, lanreotide and BIM-23042 are primate UT receptor agonists but function as antagonists at the rat UT receptor (Herold et al., 2002; Behm et al., 2004b). Nevertheless, a ‘species-dependent' explanation has been dismissed since [Orn8]hU-II behaves as an antagonist in the rat aorta, yet functions as a full agonist at the rat recombinant UT receptor (Camarda et al., 2002), that is, this phenomenon is ‘assay-dependent' and ‘species-independent'.
Camarda et al. (2004) have proposed that this phenomenon results from differential UT receptor expression and/or signal transduction-coupling efficiency in accord with Kenakin (2002). In order to examine the impact of this phenomenon on the pharmacodynamic properties of the U-II/UT system, the present study profiled three peptidic UT receptor ligands (SB-710411, urantide and GSK248451) in a variety of mammalian recombinant UT receptor cell lines and isolated arteries. Experiments assessed the influence of species/assay format and manipulation of UT receptor density on UT receptor agonism/antagonism.
In the present study, it is demonstrated that GSK248451 (Figure 1), SB-710411 and urantide, three putative UT receptor antagonists, are more accurately classified as ‘low-efficacy partial agonists'. Such activity is exposed in ‘high volume' UT receptor bioassay systems (Kenakin, 2003c), for example, native non-rodent (feline/primate) arteries and recombinant human embryonic kidney (HEK) cells with ‘appreciable' receptor densities. Thus, it is concluded that in order to classify a ligand as a UT receptor antagonist, studies must be performed in native mammalian tissues/assays, which possess high receptor density and/or efficient signal coupling/amplification. Unlike feline/primate arteries, the rat aorta, currently the most popular UT receptor bioassay, does not meet this criterion. The employment of this assay has led to low-efficacy partial agonists such as urantide (Patacchini et al., 2003) being erroneously classified generically as UT receptor antagonists. Such agents, it transpires, are in actual fact efficacious contractile agonists in non-rodent isolated arteries.
Figure 1.
Structure of the peptidic urotensin-II receptor ligand GSK248451 (Cin-c[DCys-Pal-DTrp-Orn-Val-Cys]-His-amide; Cin, 4-Cl-cinnamoyl; Coy et al., 2003; Aiyar et al., 2005).
Methods
Expression of recombinant mammalian UT receptors
cDNAs encoding the rat, cat or human UT receptor were cloned into the pFNcmv BacMam (recombinant baculovirus in which the polyhedrin promoter has been replaced with a mammalian promoter) transfer vector and virus stocks were generated (Ames et al., 2004a, 2004b). Radioligand (Section: Radioligand-binding studies) and intracellular calcium ([Ca2+]i) mobilization (fluorometric imaging plate reader, FLIPR; Section: [Ca2+]i mobilization (FLIPR) in mammalian recombinant cells) assays were performed in human embryonic kidney (HEK) 293 cells transiently expressing the recombinant UT receptors 48 h following transduction with 1–20% (v v−1) BacMam virus stocks.
Radioligand-binding studies
[125I]hU-II competition binding was performed (Douglas et al., 2005) in order to assess peptide ligand affinity (Ki) at mammalian UT receptors and to determine UT receptor density and affinity (Bmax, KD).
Preparation of mammalian recombinant UT receptor membranes
Membranes were prepared from rat, cat and human recombinant UT cells detached from 150 cm2 flasks with 1 mM ethylenediaminetetraacetic acid (EDTA) in Ca2+/Mg2+-free Dulbecco's phosphate-buffered saline (DPBS; Ames et al., 1999; Elshourbagy et al., 2002; Aiyar et al., 2005). Cells were washed by centrifugation (300 × g) and resuspended in ice-cold buffer (10 mM Tris-HCl (pH 7.4), 5 mM Na EDTA, 0.1 mM phenylmethylsulphonyl fluoride (PMSF), 1.0 mg ml−1 bacitracin, 0.1 mg ml−1 aprotinin). Following homogenization (Dounce homogenizer; Bellco Glass, Inc., Vineland, NJ, U.S.A.) and centrifugation (47,000 × g, 20 min, 4°C), the resultant pellets were washed twice by centrifugation (in 25 mM Tris-HCl (pH 7.4), 5 mM MgCl2, 2 mM Na ethyleneglycol-bis(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid (EGTA), 0.1 mg ml−1 bacitracin) and resuspended (5 mg ml−1) for storage (−70°C). Protein concentration was measured by the bicinchoninic acid (BCA) method (Pierce, Rockford, IL, U.S.A.).
Determination of ligand affinity (Ki) at mammalian recombinant UT receptors
Specific binding to recombinant UT membranes was assessed using 300 pM [125I(Tyr9)]hU-II (specific activity 2Ci μmol−1) in the presence/absence of increasing concentrations of cold hU-II, GSK248451, urantide or SB-710411. Nonspecific binding was defined using 1 μM excess cold hU-II. Cell membranes, precoupled to wheat germ agglutinin-SPA (scintillation proximity assay) beads (5 μg membrane protein, 0.5 mg beads; Amersham, Arlington Heights, IL, U.S.A.) were profiled in buffer (20 mM Tris-HCl (pH 7.4), 5 mM MgCl2 and 0.05% bovine serum albumin (BSA)). Assay plates were shaken gently (1 h, 25°C) and centrifuged (2000 × g, 5 min) before being counted (Packard Top Count Scintillation Counter).
Determination of UT receptor density (Bmax) and affinity (KD)
[125I]hU-II-binding density (Bmax) and affinity (KD) in rat, cat and human recombinant UT cell membranes (1–20% BacMam virus titre) were determined by competition binding as described previously (Douglas et al., 2005).
Preparation of isolated vascular tissue
GSK248451, urantide and SB-710411 were profiled in arteries from rats, cats and monkeys in accredited facilities in accordance with institutional guidelines and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources (1996), National Research Council) and with Institutional approval from the GSK Animal Care and Use Committee.
Rat isolated aortae
Male Sprague–Dawley rats (400 g; Charles River, Raleigh, NC, U.S.A.) were anaesthetized with 5% isoflurane in O2 and thoracic aortae (isolated following exsanguination) were studied under 1 g optimal resting tension (Douglas et al., 2000).
Cat isolated aortae and femoral arteries
Following sodium pentobarbital overdose, femoral arteries and thoracic aortae were isolated from adult male cats (4–5 kg; Liberty Research, Inc., Waverly, NY, U.S.A.) and studied under 2 g optimal resting tension (Behm et al., 2004a).
Cynomolgus monkey isolated renal and mesenteric arteries
Following pentobarbital overdose, renal and mesenteric arteries were isolated from male cynomolgus monkeys (4–7 kg; Primate Products, Miami, FL, U.S.A.; Covance, Alice, TX, U.S.A.; Charles River, Andover, MA, U.S.A.; Mannheimer, Homestead, FL, U.S.A.) and studied under 1 g optimal resting tension (Douglas et al., 2000).
Assessment of in vitro contractility in isolated arteries
Endothelium-denuded arterial rings (3 mm) were cleaned of adherent tissues and suspended in Krebs solution (with 10 μM indomethacin; Douglas et al., 2000). Isometric force responses (measured using MLT0201/D transducers; Letica, Barcelona, Spain) were recorded digitally (ADInstruments Chart 5.0 software, Colorado Springs, CO, U.S.A.). Following equilibration (1 h), vessels were treated with 60 mM KCl and 1 μM phenylephrine (subsequent responses were normalized to KCl). Functional endothelial loss was confirmed using 10 μM carbachol.
Antagonist studies:
Paired arteries were pretreated (30 min) with vehicle (0.1% dimethylsulphoxide (DMSO)), GSK248451, urantide or SB-710411 following which cumulative concentration–response curves to hU-II were constructed (tissues were used to generate only one concentration–response curve). In a separate series of experiments, selectivity studies were performed with GSK248451 (1 μM) in a standard (Behm et al., 2002; Clozel et al., 2004; Douglas et al., 2005; Camarda et al., 2006) rat aortic contraction assay using a selection of non-UT receptor agonists (KCl, phenylephrine, angiotensin II and endothelin-1).
Agonist studies:
If exposure of isolated arteries to the putative peptide antagonists (Section: Antagonist studies) revealed direct vasoconstrictor activity, full concentration–contraction response curves were constructed.
[Ca2+]i-mobilization (FLIPR) in mammalian recombinant cells
[Ca2+]i-mobilization was assessed using a microtitre plate-based fluorometric imaging plate reader (FLIPR, Molecular Devices, Sunnyvale, CA, U.S.A.; Ames et al., 1999) in HEK293 cells expressing varying levels of rat, cat or human recombinant UT receptor. Receptor expression was modulated using the BacMam transduction system (Bmax/KD were assessed in parallel; Sections: Expression of recombinant mammalian UT receptors and Radioligand-binding studies). UT-HEK cells were plated in 96-well black wall, clear bottom Biocoat plates (∼50,000 cells well−1; Becton Dickinson, San Jose, CA, U.S.A.). On the day of experimentation, media were replaced with ‘loading media' (Eagle's minimal essential media with Earl's salts, L-glutamine, 0.1% BSA, 2.5 mM probenecid, 4 μM fluo-3-acetoxymethyl ester indicator dye (Molecular Probes, Eugene, OR, U.S.A.)). Cells were incubated (1 h, 37°C) at which point the media were replaced with indicator-free media. Cells were incubated for a further 10 min and then washed with ‘assay media' (three times in 120 mM NaCl, 4.6 mM KCl, 1.03 mM KH2PO4, 25 mM NaHCO3, 1.0 mM CaCl2, 1.1 mM MgCl2, 11 mM glucose, 20 mM 4-(2-hydroxyethyl)-1-piperazineethane sulphonic acid (HEPES, pH 7.4), 0.1% gelatin, 2.5 mM probenecid) at which point they were exposed to ligands.
Haemodynamic measurements in the anaesthetized cat
Haemodynamic measurements were recorded in anaesthetized cats (Behm et al., 2004a). Briefly, female cats (2–4 kg), initially anaesthetized with ketamine (3 mg kg−1, i.m.) and transferred onto isoflurane, were artificially ventilated via a tracheal cannula (Model 665 ventilator, Harvard Apparatus, Holliston, MA, U.S.A.). Body temperature was maintained at 37°C (Harvard Apparatus homeothermic blanket) and blood gases were adjusted by altering tidal volume/ventilation rate (end-tidal PCO2/PO2, blood pH were monitored with a Model ALB5 blood gas analyzer, Radiometer, Copenhagen, Denmark). Femoral artery and vein catheters were used for arterial blood pressure measurement and drug administration, respectively. Blood pressure and lead II ECG (via limb lead electrodes) signals were preamplified (Model P122, Grass Astromed, Quincy, MA, U.S.A.) and recorded using a computerized data acquisition system (CA recorder version 7B21, Data Integrated Scientific Systems, Pickney, MI, U.S.A.). Following the initial surgical instrumentation, anaesthesia was maintained with α-chloralose (65 mg kg−1, i.v. bolus) and isoflurane was discontinued. Haemodynamics and blood gases were allowed to stabilize before recording basal values. At 10 min prior to hU-II administration (1 nmol kg−1, i.v. bolus), cats were pretreated with vehicle (1% DMSO in saline, v v−1) or GSK248451 (1 mg kg−1, bolus i.v.).
Drugs and materials
SB-710411 (Cpa, 4-chlorophenylalanine and Pal, 3-pyridylalanine; Coy et al., 2000; 2003; Behm et al., 2002; 2003a; Herold et al., 2002) and GSK248451 (Cin, 4-Cl-cinnamoyl; Coy et al., 2003; Aiyar et al., 2005) were custom synthesized by California Peptide Research Inc. (Napa, CA, U.S.A.) as were hU-II and urantide (Pen, L-Penicillaminyl (β,β-dimethyl-L-cysteinyl); Patacchini et al., 2003; Camarda et al., 2004). Monoiodinated hU-II was custom synthesized by Amersham (Arlington Heights, IL, U.S.A.). Carbachol, indomethacin and phenylephrine were from Sigma (St Louis, MO, U.S.A.). Ketamine, isoflurane and sodium pentobarbital were from Fort Dodge Animal Health (Fort Dodge, IA, U.S.A.), Abbott Laboratories (North Chicago, IL, U.S.A.) and Vortech Pharmaceuticals (Dearborn, MI, U.S.A.), respectively. α-chloralose was prepared as a fresh solution (40 mg ml−1 containing 25 mg ml−1 of sodium tetraborate decahydrate (Sigma)). All other reagents used were of analytical grade.
Data analysis
Competition binding curves were analysed by nonlinear regression (GraphPad Prism) and concentration–response curves were fitted to a logistic equation as previously described (Douglas et al., 2005). Antagonist affinity determinations (Kbs) were made using the Schild equation (Jenkinson et al., 1998). All values are expressed as mean±standard error of the mean (s.e.m.) and n represents either the number of independent experiments carried out in triplicate or the number of total animals from which vessels were isolated. Statistical comparisons were made using a paired, two-tailed t-test or ANOVA for repeated measures with Dunnett's post-test analysis. Differences were considered significant when P⩽0.05.
Results
Determination of ligand affinity (Ki) at mammalian recombinant UT receptors
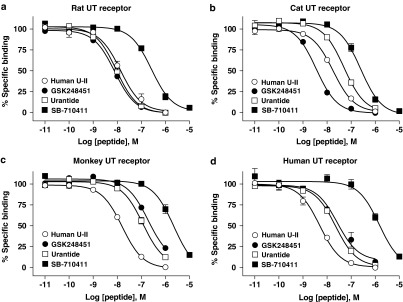
GSK248451, urantide and SB-710411 bound to all mammalian UT receptor homologues studied (Table 1; Figure 2). hU-II bound to rat, cat, monkey and human recombinant UT receptor with high affinity (Kis 2.7–8.7 nM). Similarly, GSK248451 (Kis 1.7–95.3 nM) and urantide (Kis 5.3–58.2 nM) were also high-affinity ligands across species. In contrast, SB-710411 was ⩽20-fold (cat and rat; P<0.01) to ⩽330-fold (monkey and human; P<0.05) less potent than hU-II as a UT receptor ligand (Kis 130–1400 nM).
Table 1.
Radioligand binding affinity constants (Kis) determined for human urotensin-II (hU-II), GSK248451, urantide and SB-710411 in mammalian recombinant UT receptor membranes ([125I]hU-II competition)
| |
Affinity (Ki, nM) |
Hill slope (nH) |
||||||
|---|---|---|---|---|---|---|---|---|
| Rat | Cat | Monkey | Human | Rat | Cat | Monkey | Human | |
| hU-II |
7.8±1.2 |
8.7±0.9 |
8.0±0.8 |
2.7±0.1 |
1.23±0.45 |
0.87±0.07 |
0.94±0.03 |
0.78±0.09 |
| GSK248451 |
4.5±0.6 |
1.7±0.2 |
95.3±16.2 |
12.8±1.8 |
0.95±0.10 |
1.13±0.02 |
1.04±0.08 |
0.79±0.09 |
| Urantide |
5.3±0.2 |
26.0±3.4 |
58.2±9.6 |
13.2±1.7 |
0.90±0.10 |
1.01±0.09 |
0.91±0.05 |
0.90±0.07 |
| SB-710411 | 160.0±4.2** | 135.2±25.6** | 1,382.1±491.5* | 903.4±137.1** | 1.25±0.12 | 1.24±0.05* | 1.22±0.03** | 2.54±1.33 |
All values are expressed as mean±s.e.m. (n=3 in duplicate). Statistical comparisons were performed by ANOVA analysis with a Dunnett's multiple comparison post test: *P<0.05 and **P<0.01 compared to hU-II.
Figure 2.
GSK248451 (Kis 1.7–95.3 nM), urantide (Kis 5.3–58.2 nM), SB-710411 (Kis 135–1382 nM) and hU-II (Kis 2.7–8.7 nM) compete with [125I]hU-II for binding to (a) rat, (b) cat, (c) monkey and (d) human recombinant UT receptor HEK cell membranes in a concentration-dependent manner (Table 1). Hill slopes approximate unity consistent with binding to a single homogeneous population of high affinity sites.
Assessment of antagonist properties in mammalian isolated arteries
The antagonistic properties (Table 2) of GSK248451, urantide and SB-710411 were assessed in vitro using vascular preparations from the rat (aorta), cat (aorta and femoral artery) and monkey (renal and mesenteric arteries). In summary:
GSK248451 was a potent antagonist in all species studied (no evidence of intrinsic contractile activity),
SB-710411 was a weak antagonist in rat and cat vessels but exhibited agonism (constriction) in primate vessels,
although urantide inhibited hU-II-induced contraction in rat aortae, agonism was evident in monkey and cat arteries and
GSK248451 and SB-710411 were both significantly more potent antagonists in cat femoral arteries cf. aortae (by two orders of magnitude).
Table 2.
Synopsis of agonist (induction of vasoconstriction response) and/or antagonist (inhibition of hU-II-mediated vasoconstriction) properties of hU-II, urantide, GSK248451 and SB-710411 in rat, cat and monkey isolated arteries
| Vessel | hU-II | GSK248451 | Urantide | SB-710411 |
|---|---|---|---|---|
| Rat aorta |
Agonist (full) |
Antagonist |
Antagonist |
Antagonist |
| Cat femoral artery |
Agonist (full) |
Antagonist |
Agonist (weak partial) |
Antagonist |
| Cat aorta |
Agonist (full) |
Antagonist |
Agonist (weak partial) |
Inactive |
| Monkey renal artery |
Agonist (full) |
Antagonist |
Agonist (moderate partial) |
Agonist (moderate partial) |
| Monkey mesenteric artery | Agonist (full) | Antagonist | Agonist (moderate partial) | Agonist (full) |
Weak partial agonists were defined as those exhibiting a relative efficacy [α] <0.5. Moderate partial agonists exhibited relative efficacies [α]⩾0.5 to ⩽0.9. Full agonists possessed relative efficacies [α] >0.9 (intrinsic activities are expressed relative to hU-II, considered a full agonist where relative efficacy [α] is 1.0). ‘Inactive' entities (10 μM) exhibited neither agonist nor antagonist properties in the vessels studied (see Tables 3 and 4).
Antagonist potency of GSK248451 in mammalian isolated arteries
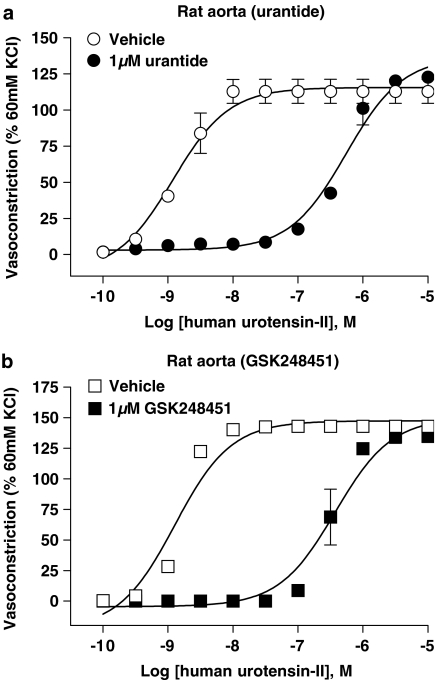
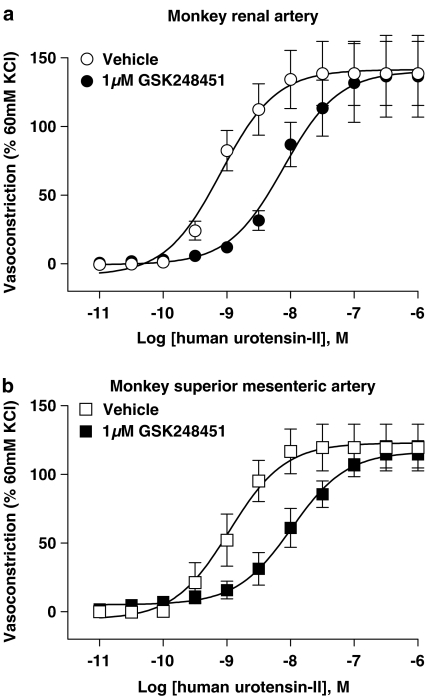
GSK248451 (1 μM) was a potent (Kb 5.9 nM), surmountable antagonist in rat aortae causing a 210-fold, rightward shift in the hU-II concentration–contraction curve without suppressing Emax (Table 3; Figure 3). GSK248451 was similarly potent in cat femoral artery (Kb 1.3 nM, no suppression of Emax; Table 3; Figure 4). Interestingly, however, a 150-fold loss in potency was observed in cat aortae (Kb 192 nM, without Emax suppression; Table 3) and monkey arteries (Kbs 146–185 nM, without Emax suppression, respectively; Table 3, Figure 5). There was no evidence of intrinsic activity even at high concentrations of GSK248451 (1 μM) in any tissue preparation.
Table 3.
Inhibition of hU-II-induced contraction in rat, cat and monkey isolated arteries by GSK248451, urantide and SB-710411
| |
Vehicle-treated isolated vessels |
Antagonist-treated isolated vessels |
Antagonist potency |
||
|---|---|---|---|---|---|
| EC50 (nM) | Emax (% KCl) | EC50 (nM) | Emax (% KCl) | Kb (nM) | |
|
GSK248451 (1 μM) | |||||
| Rat aorta |
1.6±0.0 |
143±4 |
335.4±88.4* |
135±5 |
5.9±1.4 |
| Cat femoral artery |
0.3±0.0 |
386±77 |
310.0±95.3* |
366±87 |
1.3±0.3 |
| Cat thoracic aorta |
2.2±0.4 |
171±26 |
16.9±2.8** |
204±27 |
191.9±73.4 |
| Monkey renal artery |
0.9±0.1 |
138±23 |
7.1±1.0** |
138±30 |
146.0±13.7 |
| Monkey mesenteric artery |
1.3±0.3 |
119±17 |
11.4±5.5 |
118±11 |
185.2±30.3 |
| |
|
|
|
|
|
|
Urantide (1 μM) | |||||
| Rat aorta |
1.5±0.2 |
114±8 |
514.6±126.5* |
130±6 |
3.6±1.0 |
| Cat femoral arterya |
|
Kb could not be determined |
|
||
| Cat thoracic aortaa |
|
Kb could not be determined |
|
||
| Monkey renal arterya |
|
Kb could not be determined |
|
||
| Monkey mesenteric arterya |
|
Kb could not be determined |
|
||
| |
|
|
|
|
|
|
SB-710411 (10 μM) | |||||
| Rat aortaa,b |
2.7±0.8 |
66±9 |
61.5±17.4** |
83±14 |
716.7±271.0 |
| Cat femoral arterya |
0.7±0.2 |
220±28 |
9.4±2.0** |
232±16 |
962.9±233.4 |
| Cat thoracic aortaa |
1.4±0.3 |
125±9 |
1.9±0.2* |
137±3 |
⩾10,000 |
| Monkey renal arterya,b |
|
Kb could not be determined |
|
||
| Monkey mesenteric arterya,b | Kb could not be determined | ||||
All values are expressed as mean±s.e.m. (n=4–8). Statistical comparisons were performed by paired, two-tailed t-test: *P<0.05 and **P<0.01 for EC50 and Emax values from antagonist-treated tissues compared to values obtained in vehicle-treated vessels. Concentration–response parameters were determined by fitting the experimental data to a logistic equation (Douglas et al., 2005).
Antagonist potency (Kb) could not be determined for either 1 μM urantide or 10 μM SB-710411 in cat and/or monkey isolated arteries due to intrinsic contractile activity observed upon preincubation with peptidic ligand (see Table 4).
Data for SB-710411 in the rat and monkey are from Behm et al. (2002) and Behm et al. (2004b) and are included for ease of comparison.
Figure 3.
Inhibition of human hU-II-induced contraction of rat isolated aortae by 1 μM (a) urantide (Kb 3.6 nM) and (b) GSK248451 (Kb 5.9 nM; Table 3). Both peptidic ligands behaved as hU-II antagonists in this tissue and no evidence of intrinsic contractile activity was observed during 30 min antagonist preincubation prior to construction of the hU-II concentration-contraction response curve. 10 μM SB-710411 was previously reported to function as a hU-II antagonist in this preparation (Kb 716 nM; Behm et al., 2002).
Figure 4.
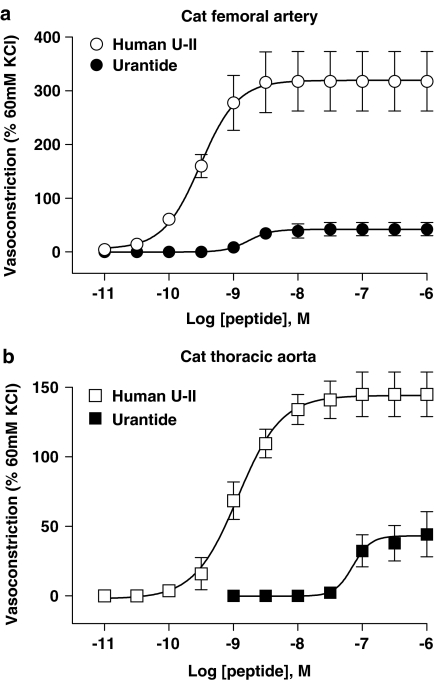
Inhibition of hU-II-induced contraction of cat isolated femoral artery and thoracic aortae by 1 μM GSK248451 (panels a and b; Kbs 1.3 and 191.9 nM, respectively; Table 3) and 10 μM SB-710411 (panels c and d; Kbs 0.9 μM and >10 μM, respectively; Table 3). Both peptidic ligands behaved as hU-II antagonists in the femoral artery (no evidence of intrinsic contractile activity was observed during 30 min antagonist preincubation prior to construction of the hU-II concentration-contraction response curve) but were >10- to 100-fold less potent in the thoracic aorta (significant antagonism was only evident with SB-710411 in isolated femoral arteries). 1 μM urantide exhibited agonist activity in both cat isolated arteries and, therefore, Kb was not determined (Tables 2 and 3 and Figure 6).
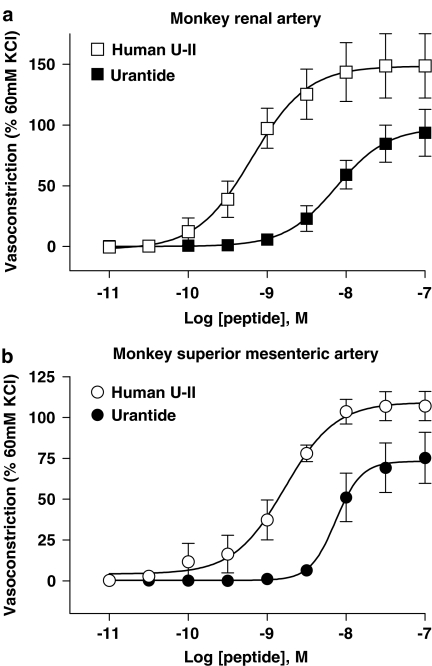
Figure 5.
Inhibition of hU-II-induced contraction of monkey isolated (a) renal artery (Kb 146 nM; Table 3) and (b) superior mesenteric artery (Kb 185 nM; Table 3) by 1 μM GSK248451. GSK248451 behaved as a hU-II antagonist in both preparations (no evidence of intrinsic contractile activity was observed during 30 min antagonist preincubation prior to construction of the hU-II concentration-contraction response curve). 1 μM urantide exhibited agonist activity in both monkey isolated arteries and, therefore, Kb was not determined (Tables 2 and 3 and Figure 7). Similarly, SB-710411 (10 μM) was also a contractile agonist in both isolated arteries (Tables 2 and 3; Behm et al., 2004b).
Antagonist potency of urantide in mammalian isolated arteries
Compared to GSK248451, urantide (1 μM) was a competitive, equipotent antagonist in rat isolated aortae (Kb 3.6 nM) causing a 340-fold, rightward shift in the hU-II concentration–contraction curve without suppressing Emax (Table 3; Figure 3). Interestingly, exposure of all cat and monkey arteries to 1 μM urantide induced an appreciable contractile response (vide infra, Table 4, Figures 6 and 7).
Table 4.
Assessment of vasoconstrictor potency and efficacy of human urotensin-II (hU-II), GSK248451, urantide and SB-710411 in cat and monkey isolated arteries
| EC50 (nM) | Emax (% KCl) | Relative potency | Relative efficacy (α) | |
|---|---|---|---|---|
|
Human urotensin-II | ||||
| Cat femoral artery |
0.3±0.0 |
320±56 |
— |
— |
| Cat thoracic aorta |
1.3±0.4 |
145±16 |
— |
— |
| Monkey renal artery |
0.7±0.1 |
148±26 |
— |
— |
| Monkey mesenteric artery |
1.6±0.5 |
107±9 |
— |
— |
| |
|
|
|
|
|
GSK248451 | ||||
| Cat femoral arterya |
>1000 |
0 |
>3330 |
0.00 |
| Cat thoracic aortaa |
>1000 |
0 |
>770 |
0.00 |
| Monkey renal arterya |
>1000 |
0 |
>1430 |
0.00 |
| Monkey mesenteric arterya |
>1000 |
0 |
>620 |
0.00 |
| |
|
|
|
|
|
Urantide | ||||
| Cat femoral artery |
1.7±0.4* |
43±12* |
6 |
0.13 |
| Cat thoracic aorta |
93.6±31.2* |
45±17*** |
72 |
0.31 |
| Monkey renal artery |
8.1±2.8 |
97±21 |
12 |
0.66 |
| Monkey mesenteric artery |
8.5±1.0 |
76±15 |
5 |
0.71 |
| |
|
|
|
|
|
SB-710411 | ||||
| Cat femoral arteryb |
>10,000 |
0 |
>33,330 |
0.00 |
| Cat thoracic aortab |
>10,000 |
0 |
>7690 |
0.00 |
| Monkey renal arteryc |
2941.3±1153.9* |
101±4 |
4202 |
0.68 |
| Monkey mesenteric arteryc | 4545.6±1263.9** | 102±17 | 2841 | 0.95 |
All values are expressed as mean±s.e.m. (n=4–5). Statistical comparisons were performed by ANOVA analysis with a Dunnett's multiple comparison post test: *P<0.05, **P<0.01 and ***P<0.001 compared to values for human urotensin-II (hU-II). Concentration–response parameters were determined by fitting the experimental data to a logistic equation (Douglas et al., 2005). Relative contractile potency and efficacy (α) is determined by comparison with hU-II.
GSK248451 (1 μM) was devoid of any contractile activity in either cat or monkey vessels, vessels in which it functioned as a potent UT receptor antagonist (see Table 3).
SB-710411 (10 μM) did not exhibit any contractile activity in the cat isolated femoral artery and thoracic aorta (Table 3).
Data for SB-710411 in the monkey isolated renal and femoral artery are from Behm et al. (2004) and are included for ease of comparison.
Figure 6.
Concentration-dependent contraction of cat isolated (a) femoral artery and (b) thoracic aorta induced by hU-II (EC50s 0.3 and 1.3 nM, Emaxs 320 and 145% KCl, respectively; Table 4) and urantide (EC50s 1.7 and 93.6 nM, Emaxs 43 and 45% KCl, respectively; Table 4). Since urantide exhibited agonist activity in both cat isolated arteries (6–72-fold less potent than hU-II with relative intrinsic activities [α] of 0.13–0.31), Kb could not be determined (see Table 3).
Figure 7.
Concentration-dependent contraction of monkey isolated (a) renal and (b) superior mesenteric artery induced by hU-II (EC50s 0.7 and 1.6 nM, Emaxs 148 and 107% KCl, respectively; Table 4) and urantide (EC50s 8.1 and 8.5 nM, Emaxs 97 and 76% KCl, respectively; Table 4). Since urantide exhibited agonist activity in both monkey isolated arteries (5–12-fold less potent than hU-II with relative intrinsic activities [α] of 0.66–0.71), Kb could not be determined (see Table 3).
Antagonist potency of SB-710411 in mammalian isolated arteries
SB-710411 (10 μM) was a weak hU-II antagonist in cat isolated femoral arteries (Kb 0.9 μM; Table 3; Figure 4), 740-fold less potent than GSK248451. SB-710411 was clearly distinguished from urantide in the feline isolated femoral artery since only the latter exhibited contractile agonism. As with GSK248451, SB-710411 was in excess of an order of magnitude more potent as an antagonist in cat femoral arteries cf. aorta (Kb>10 μM; Table 3; Figure 4). Antagonist potency could not be determined in monkey arteries since pretreatment with SB-710411 resulted in vasoconstriction.
Assessment of agonist properties in mammalian isolated arteries
All three ligands behaved as antagonists in the rat aorta. However, while GSK248451 and SB-710411 were also antagonists in cat arteries, urantide was a potent, partial agonist. Indeed, only GSK248451 was devoid of vasoconstrictor activity in primate vessels.
Agonist potency of hU-II in mammalian isolated arteries
hU-II was a potent (EC50s 0.3–1.6 nM), efficacious (Emaxs 107–320% KCl) spasmogen in all arteries studied (Table 4). Contractile responses in the cat femoral artery and aorta were qualitatively different (Table 5). Although not statistically significant, hU-II was more potent (0.3 vs 1.3 nM EC50) and efficacious (Emax 320% KCl vs 145% KCl) in femoral arteries cf. aortae (Table 4). Furthermore, contractile responses to hU-II were also more rapid in onset and less well sustained in femoral arteries.
Table 5.
Pharmacodynamic actions of human urotensin-II (hU-II) and peptidic UT receptor ligands in cat isolated arteries: differentiation of the cat-isolated aorta from the cat femoral artery
| Cat femoral artery | Cat thoracic aorta | |
|---|---|---|
|
Pharmacodynamic properties of hU-II | ||
| Vasoconstrictor potency (EC50) |
∼0.3 nM |
∼1.3 nM |
| Contractile efficacy (Emax, % KCl) |
>300% |
<150% |
| Relative onset of contractile responsea |
Rapid (∼10 min to plateau) |
Slow (∼25 min to plateau) |
| Relative duration of established contractile responsea |
Transient (>50% loss over 1 h) |
Sustained (<10% loss over 1 h) |
| |
|
|
|
Pharmacodynamic properties of peptidic agonists | ||
| Urantide vasoconstrictor potency (EC50) |
∼1 nM |
∼100 nM |
| Urantide vasoconstrictor efficacy (% hU-II Emax) |
Weak (∼10%) |
Moderate (∼30%) |
| |
|
|
|
Pharmacodynamic properties of peptidic antagonists | ||
| GSK248451 antagonist potency (Kb) |
∼1 nM |
∼190 nM |
| SB-710411 antagonist potency (Kb) | <1 μM | >10 μM |
Data are from Behm et al. (2004a) and are included for ease of reference.
Agonist potency of GSK248451 in mammalian isolated arteries
As noted above (Section: Agonist potency of hU-II in mammalian isolated arteries), there was no evidence of contractile activity in any artery with 1 μM GSK248451. As such, full concentration–contraction curves were not constructed (Table 4).
Agonist potency of urantide in mammalian isolated arteries
Urantide was a potent (EC50 1.7 nM, only six-fold less potent than hU-II), low-efficacy partial agonist (intrinsic efficacy [α] ∼0.1) in cat femoral arteries (urantide did not contract rat aortae; Table 4, Figure 6). Urantide was significantly more efficacious as a partial agonist in cat aortae (relative efficacy [α] ∼0.3) but was 70-fold less potent than hU-II (EC50 1.3 v. 93.6 nM), an observation that further differentiated feline aorta and femoral artery (femoral vessels were previously noted to be >10- to 100-fold more sensitive to the antagonistic properties of GSK248451 and SB-710411; Table 5). Urantide was also a potent (EC50s ∼8 nM), efficacious (intrinsic activity [α] ∼0.7) spasmogen of monkey arteries (Table 4, Figure 7).
Agonist potency of SB-710411 in mammalian isolated arteries
SB-710411 (⩽10 μM) was devoid of any intrinsic activity [α] in the rat and cat arteries studied. SB-710411 did, however, exhibit contractile activity in monkey vessels (Table 4, Figure 7). As with urantide, SB-710411 was equipotent potent in both monkey arteries (EC50s∼3–5 μM). Although SB-710411 was three orders of magnitude less potent than hU-II, this analogue was an efficacious spasmogen in both vessels (where intrinsic activity [α] was ⩾0.7 relative to hU-II). Indeed, SB-710411 was a full agonist in the mesenteric artery (Emax 95% of hU-II).
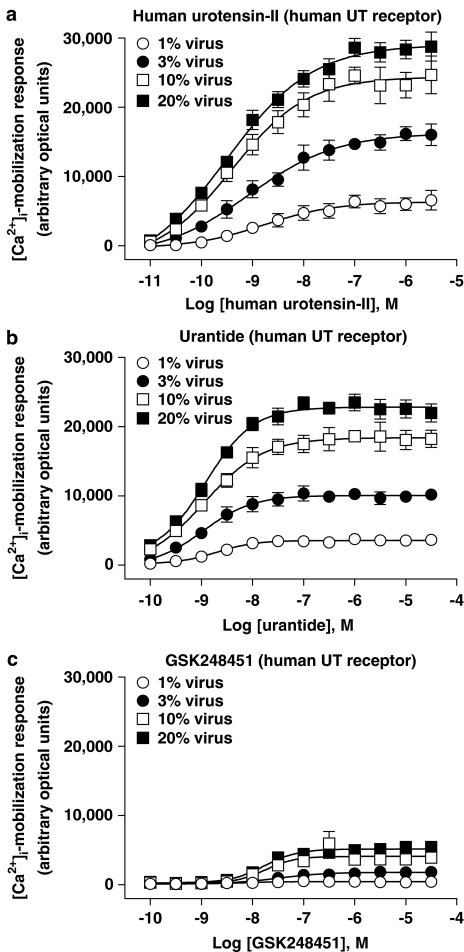
Influence of UT receptor density on [Ca2+]i-mobilization (FLIPR) in mammalian recombinant UT-HEK cells following exposure to hU-II, GSK248451 and urantide
Urantide, GSK248451 and hU-II agonism ([Ca2+]i-mobilization) was quantified in recombinant HEK293 cells expressing varying levels of rat, cat or human recombinant UT receptor. In summary:
urantide exhibited partial agonist activity in all three recombinant UT-HEK cell systems (intrinsic activity [α] ranging from 0.03 to 0.18, 0.48 to 0.89 and 0.56 to 0.79 in rat, cat and human UT-HEK cells),
while GSK248451 also exhibited partial agonist activity in recombinant cat and human UT-HEK cells (albeit with intrinsic activity [α] values lower than urantide ranging from 0.05 to 0.24 and 0.07 to 0.18), no agonism was evident in rat UT-HEK cells and
agonist responses were augmented in all three mammalian UT-HEK cell systems as a result of increased receptor expression (although relative intrinsic activity did not appear to be directly related to Bmax per se).
Influence of altered UT receptor density on ligand-induced [Ca2+]i-mobilization (FLIPR) in rat recombinant UT-HEK cells
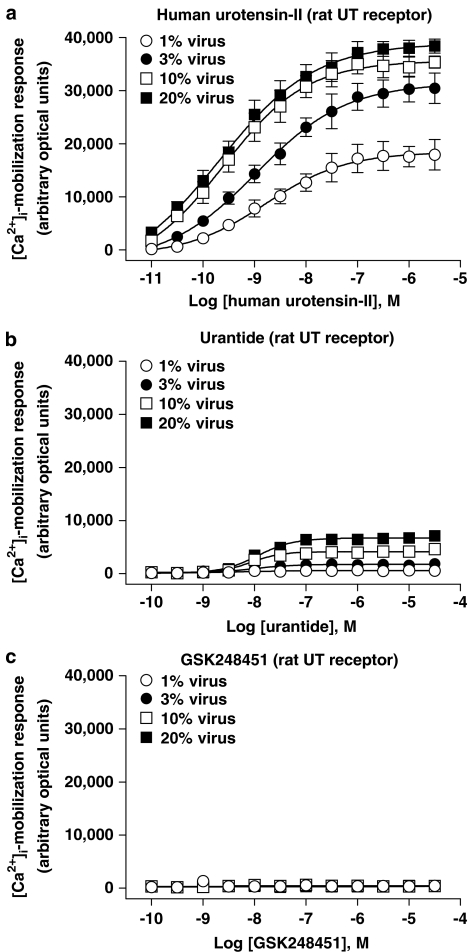
The influence of altered UT receptor density on ligand-induced [Ca2+]i-mobilization (FLIPR) was studied in HEK cells expressing increasing amounts of rat recombinant UT receptor (Figures 8 and 11c; Tables 6 and 7). In summary, intrinsic activity was recorded with both hU-II (full agonist) and urantide (partial agonist, intrinsic activity [α] ⩽0.18) at rat UT. In contrast, however, GSK248451 was devoid of any agonist activity ([Ca2+]i-mobilization) under identical conditions in the same rat UT-HEK cells.
Figure 8.
Concentration-dependent [Ca2+]i-mobilization responses (FLIPR) in HEK cells transiently expressing rat UT receptor following exposure to (a) hU-II, (b) urantide and (c) GSK248451. BacMam-mediated (1–20% virus, v v−1) upregulation of rat UT expression (Bmax increased ⩽20-fold from approximately 1.1–23.1 pmol mg−1 protein; Table 6) was associated with an increase in Emax for urantide (intrinsic activity [α] elevated from 0.03–0.17 relative to hU-II; Table 7). In contrast, GSK248451 was devoid of any agonist activity at the rat UT receptor under the experimental conditions studied.
Figure 11.
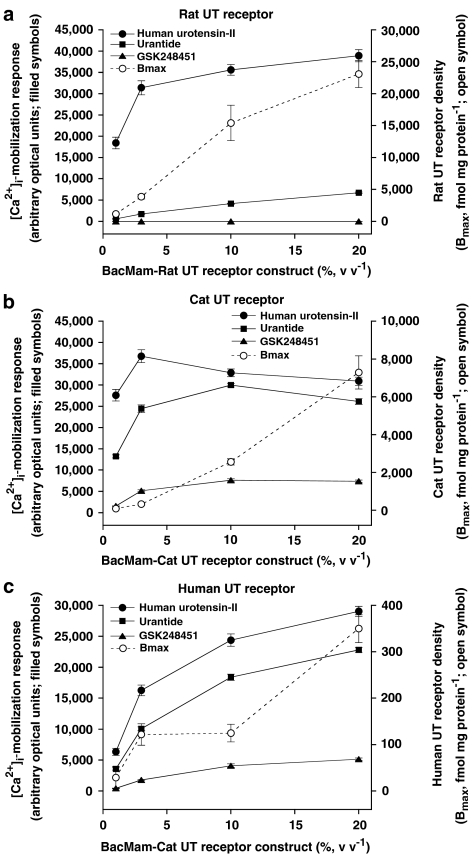
Relationship between (a) rat, (b) cat and (c) human UT receptor density (Bmax) and maximum ligand (urotensin-II, urantide and GSK248451)-induced [Ca2+]i-mobilization responses (Emax) in HEK cells (transient BacMam-mediated UT receptor expression, 1–20% virus, v v−1). UT receptor density increased for all three mammalian receptor isoforms following exposure to increasing BacMam viral titres. Upregulation of UT receptor expression increased the intrinsic activity ([α]) of the low-efficacy partial agonist urantide in the rat, cat and human UT-HEK cell assays relative to hU-II. GSK248451, in contrast, was more resistant to these changes (it was devoid of any detectable agonist activity in rat UT-HEK cells) indicating that the relative intrinsic activity of GSK248451 was significantly lower than that retained by urantide, that is, Emax 0, 28 and 22% of that seen with urantide at rat, cat and monkey UT receptor, respectively (20% BacMam virus, v v−1).
Table 6.
Radioligand ([125I]hU-II)-binding site density (Bmax) and affinity (KD) in HEK cells exposed to increasing concentrations (1–20% v v−1) of mammalian (rat, cat or human) recombinant UT receptor BacMam virus
|
Titre |
Radioligand-binding affinity (KD, nM) |
Radioligand-binding site density (Bmax, fmol mg−1 protein) |
||||
|---|---|---|---|---|---|---|
| Rat | Cat | Human | Rat | Cat | Human | |
| 1% virus (v v−1) |
5.2±1.2 |
9.3±1.0 |
11.6±1.6 |
1140±112 |
93±14 |
29±24 |
| 3% virus (v v−1) |
5.5±0.9 |
14.5±2.9 |
12.9±0.7 |
3852±349 |
329±44 |
122±23* |
| 10% virus (v v−1) |
6.7±0.8 |
19.4±9.6 |
5.3±0.7 |
15,427±2776* |
2559±165** |
125±19* |
| 20% virus (v v−1) | 8.1±1.4 | 13.7±0.5 | 5.5±0.4 | 23,104±2135** | 7292±876** | 350±30** |
All values are expressed as mean±s.e.m. (n=3 in duplicate). Statistical comparisons were performed by ANOVA (Dunnett's multiple comparisons post test: *P<0.05 and **P<0.01 compared to values obtained following exposure of HEK cells to 1% (v v−1) BacMam virus).
Table 7.
Agonist potency (EC50) and efficacy (Emax; [Ca2+]i-mobilization; FLIPR) of hU-II, urantide and GSK248451 in mammalian (rat, cat human) recombinant HEK cells at various levels of UT receptor expression (achieved following exposure to increasing concentrations (1–20% v v−1) of rat, cat or human recombinant UT receptor-BacMam virus)
| |
Agonist potency ([Ca2+]i-mobilization EC50, nM) |
Agonist efficacy ([Ca2+]i-mobilization Emax, arbitrary optical units) |
||||
|---|---|---|---|---|---|---|
| Viral titre (v v−1) | hU-II | Urantide | GSK248451 | hU-II | Urantide | GSK248451 |
|
Rat UT receptor | ||||||
| 1% |
2.0±0.3 |
ND |
— |
18,428±1372 |
587±71†† (3.2%) |
0†† (0%) |
| 3% |
1.5±0.3 |
ND |
— |
31,408±1651* |
1749±137†† (5.6%) |
0†† (0%) |
| 10% |
0.5±0.2** |
9.0±3.5†† |
— |
35,608±1246** |
4142±102†† (11.6%) |
0†† (0%) |
| 20% |
0.5±0.2** |
11.5±2.9†† |
— |
38,942±1431** |
6712±161†† (17.2%) |
0†† (0%) |
| |
|
|
|
|
|
|
|
Cat UT receptor | ||||||
| 1% |
0.4±0.2 |
4.9±0.9†† |
ND |
27,623±1355 |
13,228±362†† (47.9%) |
1552±70 (5.6%) |
| 3% |
0.3±0.1 |
6.1±1.0 |
110.8±15.4†† |
36,795±1493* |
24,470±613**,†† (66.5%) |
5157±226**,†† (14.0%) |
| 10% |
0.1±0.0 |
10.5±1.7* |
255.9±199.9 |
32,884±901 |
28,987±422** (88.1%) |
7624±373**,†† (23.2%) |
| 20% |
0.1±0.0 |
9.7±1.7* |
141.4±27.7†† |
30,918±968 |
26,147±689** (84.6%) |
7390±290**,†† (23.9%) |
| |
|
|
|
|
|
|
|
Human UT receptor | ||||||
| 1% |
2.4±0.1 |
2.1±0.5 |
ND |
6338±567 |
3577±187 (56.4%) |
442±38†† (7.0%) |
| 3% |
1.4±0.6 |
1.3±0.3 |
20.0±1.1 |
16,282±866** |
10,073±304**,†† (61.9%) |
1782±83†† (10.9%) |
| 10% |
0.6±0.1 |
1.2±0.2 |
12.3±0.7†† |
24,387±992** |
18,407±502** (75.5%) |
4088±292**,†† (16.8%) |
| 20% | 0.5±0.1 | 1.1±0.1 | 14.4±2.0†† | 29,051±820** | 22,818±399**,† (78.5%) | 5149±183**,†† (17.7%) |
Percentage (%) values shown in parentheses for GSK248451 and urantide Emax values represent relative intrinsic activity (compared to hU-II). ND; parameters not determined due to low intrinsic efficacy. Where no value is shown (‘—'), no response was observed and curve fitting was not, therefore, performed. Statistical comparisons: *P<0.05 and **P<0.01 compared to values obtained with 1% viral (v v−1) and ††P<0.01 compared to values obtained with urotensin-II (two-tailed t-test or ANOVA with Dunnett's multiple comparison post test).
Exposure of HEK293 cells to rat UT receptor BacMam virus (1–20%, v v−1) resulted in a titre-dependent, ⩽20-fold increase in [125I]hU-II-binding site density (Bmax was elevated from ∼1000 fmol mg−1 protein up to ∼23,000 fmol mg−1 protein at 1 and 20% virus, v v−1, respectively; Table 6, Figure 11a). Compared to cells exposed to 1% (v v−1) virus, receptor upregulation became significant (P<0.05) at rat UT-BacMam titres of ⩾10% (v v−1). The increase in [125I]hU-II Bmax was roughly linear over the BacMam titre range studied. UT receptor upregulation occurred without any appreciable change in radioligand-binding affinity (KDs ranged from 5 to 8 nM; Table 6).
Exposure of rat UT receptor-HEK cells to hU-II resulted in concentration-dependent increases in [Ca2+]i-mobilization (Table 7, Figure 8). Prior exposure of HEK cells to increasing titres of BacMam virus (from 1% to up to 20%, v v−1, an effect associated with up to an ∼20-fold increase in UT receptor Bmax; Table 6) had no significant effect on hU-II potency (EC50s ranged from 0.5 to 2.0 nM; Table 7), consistent with the hU-II-binding affinity at rat UT (7 nM; Table 1) and the agonist potency of hU-II in the rat isolated aorta (∼2 nM; Table 3). However, UT receptor upregulation (from 1 to 4 pmol mg−1 protein; Table 6) had a pronounced effect on hU-II agonist efficacy (Emax was doubled from ∼18,000 to ∼31,000 arbitrary optical units at BacMam titres as low as 3%; P<0.05). A further increase in UT Bmax (from ∼4 to 15–23 pmol mg−1 protein) with 10 and 20% (v v−1) BacMam virus had little additional impact on the agonist efficacy of hU-II (Emax∼36–39,000 arbitrary optical units).
In contrast to hU-II, GSK248451 was devoid of any detectable agonist activity in rat UT-HEK cells (Figure 8, Table 7) under all experimental conditions studied (i.e. at concentrations ⩽30 μM, ∼4 orders of magnitude over this ligands affinity [∼4 nM Ki] for the rat UT receptor expressed in HEK cells; Table 1). Such observations were consistent with the antagonist potency described in the rat isolated aortae above (Kb 6 nM; Table 3).
In contrast to GSK248451, however, exposure of rat UT-HEK cells to urantide was associated with the observation of intrinsic activity (Figure 8; Table 7). Such an observation contrasted the antagonistic properties observed with urantide in the rat isolated aortae (Kb 4 nM; Table 3). UT receptor agonism was observed at all BacMam titres studied. Relative to hU-II, intrinsic activity [α] was ‘marginal' (⩽0.05) but detectable at low viral titres (1–3%, v v−1). This became appreciable following exposure to higher viral titres (intrinsic activity [α] of 0.12–0.17 at 10–20% BacMam virus, v v−1). At low viral titres (1–3%, v v−1), the low intrinsic activity recorded prohibited accurate concentration–response curve fitting (hence EC50s could not be accurately determined). However, agonist potency estimates at 10–20% (v v−1) BacMam virus (EC50s∼10 nM; Table 7) were entirely consistent with those affinities determined for urantide at the rat UT receptor expressed in recombinant HEK cells (Ki 5 nM; Table 1) and in the rat aortic contraction assay (Kb ∼3 nM; Table 3).
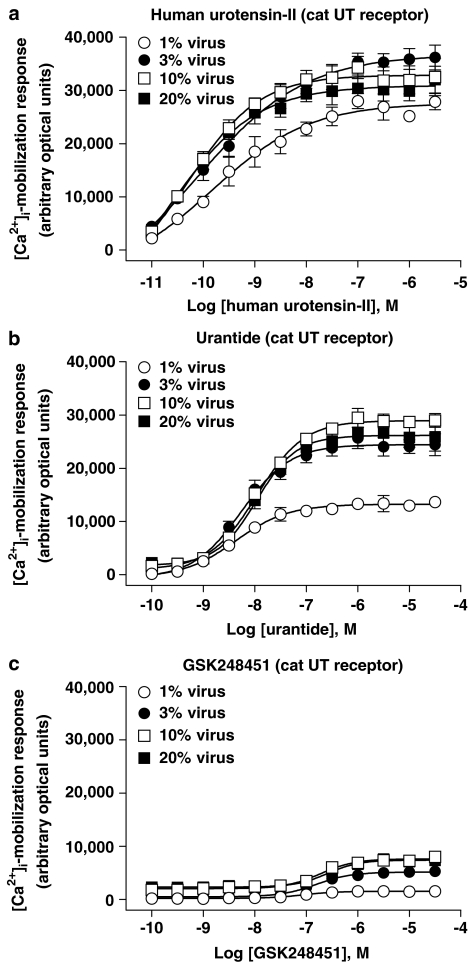
Influence of altered UT receptor density on ligand-induced [Ca2+]i-mobilization (FLIPR) in cat recombinant UT-HEK cells
The influence of altered cat recombinant UT receptor density on ligand-induced [Ca2+]i-mobilization was studied in HEK cells (Figures 9 and 11b; Tables 6 and 7). In contrast to rat UT-HEK cells, all three peptides exhibited agonist activity in the cat UT-HEK cells. Relative to hU-II (full agonist), both urantide ([α]⩽0.88) and GSK248451 ([α]⩽0.24) were partial agonists ([Ca2+]i-mobilization EC50s were commensurate with cat UT receptor Kis).
Figure 9.
Concentration-dependent [Ca2+]i-mobilization responses (FLIPR) in HEK cells transiently expressing cat UT receptor following exposure to (a) hU-II, (b) urantide and (c) GSK248451. BacMam-mediated (1–20% virus, v v−1) upregulation of cat UT expression (Bmax increased ⩽78-fold from approximately 0.1–7.3 pmol mg−1 protein; Table 6) was associated with an increase in Emax for both urantide (intrinsic activity [α] elevated from 0.48–0.85 relative to hU-II) and GSK248451 (intrinsic activity [α] elevated from 0.06–0.24 relative to hU-II; Table 7).
Exposure of HEK cells to 1–20% (v v−1) cat UT receptor BacMam virus resulted in a titre-dependent, ⩽80-fold increase in [125I]hU-II Bmax (elevated from approximately 0.1 pmol mg−1 protein up to 7.3 pmol mg−1 protein at 1 and 20% virus, v v−1, respectively; Table 6, Figure 11b). Notably, cat UT receptor density was 3–12-fold lower than the corresponding expression level observed with the rat UT receptor at similar BacMam titres. Compared to cells exposed to 1% (v v−1) virus, this upregulation became significant at cat UT BacMam titres of ⩾10% (v v−1). The increase in [125I]hU-II Bmax was roughly linear over the BacMam titre range studied. As was seen with rat UT, cat UT receptor upregulation occurred without any appreciable change in radioligand binding affinity ([125I]hU-II KD ranged from around 9 to 19 nM, similar to that observed in the rat UT-HEK cells; Table 6).
Despite the fact that cat UT receptor was expressed at a density approximately an order of magnitude lower than that recorded in rat UT-HEK cells, hU-II still evoked comparable [Ca2+]i increases in these cells, even following exposure to low viral titres (Emaxs ∼28,000–37,000 arbitrary optical units at 1–20% (v v−1) BacMam virus titres; Figure 9). The potency of hU-II in this FLIPR assay (EC50s 0.1–0.4 nM at 1–20% (v v−1) BacMam virus titres; Table 7) was similar to that observed for hU-II as a spasmogen in cat isolated arteries (EC50s 0.3–1.3 nM; Table 4).
As observed in rat UT-HEK cells, urantide was a partial agonist at the cat UT receptor (Figure 9, Table 7). However, a greater intrinsic activity was observed with urantide at the feline UT isoform (intrinsic activity [α]⩽0.88) compared to that seen at the rat receptor (5–15-fold greater intrinsic activity than that seen at the rodent UT isoform over the range of viral titres studied). The potency of urantide at inducing [Ca2+]i-mobilization (EC50s 5–11 nM) was comparable to that observed in rat UT-HEK cells and was consistent with both the affinity of urantide for the cat UT receptor (Ki 26 nM; Table 1) and the potency of this analogue as a spasmogen of cat-isolated arteries (e.g. EC50s ∼2–90 nM in femoral artery and aorta; Table 4).
In contrast to the rat UT receptor, where GSK248451 was devoid of any intrinsic activity, this analogue induced a measurable [Ca2+]i-mobilization response at cat UT receptor (where measurable, EC50s were ∼100–250 nM). However, the relative intrinsic activity [α] observed with GSK248451 was low, ranging from 0.06 to 0.24 in cells exposed to BacMam titres ranging from 1 to 20% (v v−1). These findings contrasted the observation that GSK248451 was unable to contract cat isolated arteries at concentrations ⩽1 μM (Table 4). Indeed, GSK248451 was an antagonist of hU-II in the cat isolated femoral artery and aorta (Kbs ∼1–190 nM; Table 3).
Influence of altered UT receptor density on ligand-induced [Ca2+]i-mobilization (FLIPR) in human recombinant UT-HEK cells
The influence of altered UT receptor density on ligand-induced [Ca2+]i-mobilization (FLIPR) was studied in HEK cells expressing increasing amounts of human recombinant UT receptor (Figures 10 and 11c; Tables 6 and 7). In summary, all three ligands exhibited notable intrinsic activity. As was the case in cat UT-HEK293 cells, urantide (α⩽0.79) and GSK248451 (α⩽0.18) were both partial agonists relative to hU-II. Further, as observed in cat UT-HEK cells, the ability of GSK248451 to evoke [Ca2+]i-mobilization in human UT-HEK cells was in contrast to the observations made in rat UT-HEK cells where no agonism was noted, this in spite of the fact that human UT Bmax estimates were two orders of magnitude lower than those estimated in the rat UT-HEK system.
Figure 10.
Concentration-dependent [Ca2+]i-mobilization responses (FLIPR) in HEK cells transiently expressing human UT receptor following exposure to (a) hU-II, (b) urantide and (c) GSK248451. BacMam-mediated (1–20% virus, v v−1) upregulation of human UT expression (Bmax increased ⩽12-fold from approximately 0.03–0.35 pmol mg−1 protein; Table 6) was associated with an increase in Emax for both urantide (intrinsic activity [α] elevated from 0.56–0.78 relative to hU-II) and GSK248451 (intrinsic activity [α] elevated from 0.07–0.18 relative to hU-II; Table 7).
Exposure of HEK cells to increasing (1–20%, v v−1) human UT receptor BacMam virus resulted in a titre-dependent, ⩽12-fold increase in [125I]hU-II Bmax (elevated from ∼30 fmol mg−1 protein up to ∼350 fmol mg−1 protein at 1 and 20% virus, v v−1, respectively; Table 6, Figure 11c). However, human UT receptor density was appreciably lower in HEK cells compared to those transfected with either rat (30- to 120-fold lower) or cat (3–20-fold lower) UT receptor under identical conditions. Compared to cells exposed to 1% (v v−1) virus, this upregulation became significant at human UT-BacMam titres of ⩾3% (v v−1), slightly lower than the ⩾10% observed with rat and cat UT receptor. The increase in [125I]hU-II Bmax was roughly linear over the BacMam titre range studied. As was seen with the rat and cat UT receptor, upregulation occurred without any appreciable change in radioligand-binding affinity (KDs ranged from 5 to 13 nM, similar to those values observed in the rat and cat UT-HEK cells; Table 6).
Although the hUT Bmax was <5% of that observed in the rat and cat UT-HEK cell systems (20% virus, v v−1; Table 6), hU-II was still an efficacious agonist in the FLIPR assay, capable of inducing a [Ca2+]i-mobilization response (Emax ∼30,000 arbitrary optical units) comparable to that obtained in the rat and cat UT-HEK cell lines (Table 7). The potency of hU-II in this Ca2+ assay (EC50 0.5–2.4 nM) was similar to that recorded in rat and cat UT-HEK cells (Table 7) and was consistent with the (a) affinity of [125I]hU-II and cold U-II (Ki and KD ∼2–5 nM; Tables 1 and 6) for hUT in radioligand-binding studies and (b) the potency of hU-II as a spasmogen (EC50s 0.7–1.6 nM in primate isolated arteries; Table 4).
As was observed in rat and cat UT-HEK cells, urantide exhibited partial agonist activity in the human UT-HEK cell [Ca2+]i-mobilization assay (Table 7). The relative intrinsic activity observed at human UT (ranging from ∼0.56 to 0.79, values increasing with increasing viral titre/UT receptor density) was similar to that observed in HEK cells expressing cat UT (ranging from ∼0.47 to 0.89) but greater than that observed in rat UT-HEK cells ([α] ranging from ∼0.03 to 0.18) under identical conditions (despite the fact that human UT density was <2% of that in the rat UT system). The potency of urantide in this Ca2+ assay (EC50 1.1–2.1 nM) was consistent with the (a) affinity of urantide (Ki∼13 nM; Table 1) at human UT in radioligand-binding studies and (b) the potency of urantide as a spasmogen, at least in the cat femoral artery (EC50 ∼2 nM) and monkey isolated renal and mesenteric arteries (EC50s ∼8 nM; Table 4).
In contrast to the observations made in rat UT-HEK cells, GSK248451 was a partial agonist in the HEK cells expressing human UT (Table 7) in accord with those findings made in cat UT-HEK cells. The relative intrinsic activity observed at human UT was relatively low (ranging from ∼0.07 to 0.18) and similar to that observed in HEK cells expressing cat UT (ranging from ∼0.06 to 0.24) under identical conditions. The potency of GSK248451 in the [Ca2+]i-mobilization assay (EC50 12–20 nM) was consistent with the reported radioligand-binding affinity (Ki∼13 nM; Table 1) at human UT. However, these findings contrasted those observations made in the rat (aorta), cat (femoral artery/aorta)- and monkey (renal/mesenteric) isolated arteries, where GSK248451 behaved as a competitive antagonist devoid of intrinsic activity (Tables 3 and 4).
GSK248451 selectivity
Since GSK248451 was a competitive antagonist devoid of any intrinsic activity in all isolated arteries studied, attempts were made to assess the antagonist properties of this peptidic moiety in vivo (Section: In vivo cat haemodynamics). Prior to this, however, antagonist selectivity was assessed in rat isolated aortae. U-II selectivity was confirmed since 1 μM GSK248451 (∼170-fold greater than its affinity against hU-II Kb; Table 3) did not alter the contractile properties of KCl, phenylephrine, angiotensin II or endothelin-1 (Table 8).
Table 8.
GSK248451 is a selective U-II receptor antagonist since preincubation with 1 μM peptide did not alter KCl, phenylephrine, angiotensin II or endothelin-1 induced contraction in the rat isolated aorta
| |
Contractile potency (EC50, nM) |
Contractile efficacy (Emax, % KCl) |
||
|---|---|---|---|---|
| Vehicle | GSK248451 | Vehicle | GSK248451 | |
| KCla |
14.1±0.7 |
15.4±1.4 |
100±2 |
101±2 |
| Phenylephrine |
9.8±1.0 |
10.3±1.0 |
167±6 |
164±6 |
| Angiotensin II |
4.7±0.7 |
4.1±0.3 |
36±22 |
16±6 |
| Endothelin-1 | 1.4±0.2 | 1.4±0.2 | 153±8 | 154±10 |
EC50 and Emax values to KCl are expressed as mM and % phenylephrine, respectively. All values are mean±s.e.m. and n represents the number of animals from which tissues were studied. Statistical comparisons were performed by paired, two-tailed t-test. No values were determined to be statistically different from those obtained in paired vehicle-treated vessels. Concentration–response parameters were determined by fitting the experimental data to a logistic equation (Douglas et al., 2005).
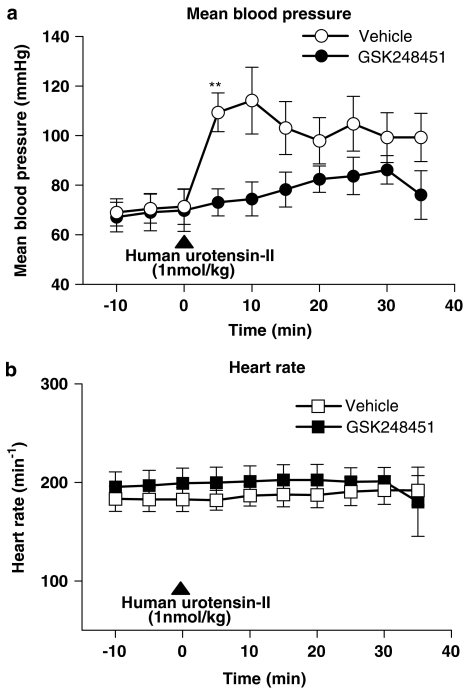
In vivo cat haemodynamics
GSK248451 (1 mg kg−1, bolus i.v.) did not alter basal heart rate or arterial blood pressure when compared to vehicle-treated cats (1% DMSO in saline, v v−1 bolus, i.v.; Table 9, Figure 12). However, the peak pressor response (∼40 mmHg, observed at 5 min) to exogenous hU-II (1 nmol kg−1, bolus i.v.) was significantly attenuated by GSK248451 (Figure 12a; P<0.01). Analysis of ‘pressure × time' products during the entire 35 min recording period post hU-II administration demonstrated that the significant inhibitory actions of GSK248451 were sustained (Table 9). Heart rate was unaffected by GSK248451 (Figure 12b).
Table 9.
Basal haemodynamic parameters in intact, anaesthetized cats prior to receiving bolus i.v. vehicle (1% DMSO in saline, v v−1) or 1 mg kg−1 GSK248451 and inhibition of arterial pressure × rate product over the recording period (35 min) immediately following U-II (1 nmol kg−1, i.v.) administration
| Vehicle-treated (1% DMSO in saline, v v−1) | GSK248451-treated (1 mg kg−1, i.v.) | |
|---|---|---|
|
Basal haemodynamic parameter | ||
| Mean arterial pressure (mmHg) |
70±6 |
69±7 |
| Systolic arterial pressure (mmHg) |
91±6 |
94±8 |
| Diastolic arterial pressure (mmHg) |
57±6 |
53±5 |
| Heart rate (min−1) |
183±12 |
197±15 |
| |
|
|
|
Arterial pressure × rate product (AUC)t=0–35 min | ||
| Mean (mmHg·min−1) |
1072±179 |
409±106* |
| Systolic (mmHg·min−1) |
1507±153 |
506±122** |
| Diastolic (mmHg·min−1) | 787±202 | 337±95 |
All values are mean±s.e.m. Statistical comparisons were performed by paired, two-tailed t-test where *P<0.05 and **P<0.01 compared to vehicle-treated cats.
Figure 12.
GSK248451 (1 mg kg−1, bolus i.v.) did not alter basal (a) mean arterial blood pressure or (b) heart rate when compared to vehicle-treated anaesthetized cats (1% DMSO in saline, v v−1, bolus i.v.). However, the peak systemic pressor response to exogenous hU-II was blocked by GSK248451 (attained 5 min following 1 nmol kg−1, bolus i.v. administration; Table 9). Heart rate changes were unaltered by GSK248451. Statistical analysis: **P<0.01 compared to time-matched vehicle control cats.
Discussion
Many of the peptidic ligands described as UT receptor antagonists exert ‘paradoxical' actions in selected assay systems. Urantide, [Orn8]U-II and UFP-803 (Camarda et al., 2002; 2004; 2006; Patacchini et al., 2003) have all been characterized as antagonists in rat isolated aortae yet behave as agonists (mobilizing [Ca2+]i) in specific recombinant UT receptor HEK/CHO cell systems. Seemingly contradictory observations have also been made with lanreotide, BIM-23042 and SB-710411 (Herold et al., 2002; Behm et al., 2004b). The proposition that such moieties retain low levels of ‘residual' intrinsic activity is, perhaps, not surprising since many such peptides are U-II analogues (developed by employing elegant peptide modelling approaches and/or traditional SAR centred around the use of the rat aorta bioassay; Grieco et al., 2005).
In accord with Kenakin (2002; 2003a, 2003b, 2003c), Camarda et al. (2002) have proposed that such ‘assay-dependent' agonism/antagonism results from differential UT receptor expression and/or signal transduction-coupling efficiency, for example, ‘…depending on the receptor density and the efficiency of receptor coupling in different organs, ligands with low levels of efficacy could be defined experimentally as being full agonists, partial agonists or antagonists; that is, the environment of the receptor dictates the apparent property of the drug…' (Kenakin, 2002). The Operational Model of agonist action developed by Black & Leff (1983) over two decades ago described this phenomenon using the concept of the ‘transducer ratio' (or tau [τ]). In any given assay system, τ relates drug-response to properties that are both ligand (efficacy, an innate property of an agonist)- and assay- (receptor density, coupling efficiency of the stimulus-response cascade) dependent. It follows, therefore, that the observed drug-response to any two agonists in any specific assay system is directly related to their relative intrinsic activities since τ is a constant. Similarly, any given ligand has the potential to act as either a full agonist (in assays with high receptor density or efficiently coupled signalling mechanisms) or a partial agonist (or, indeed, even as an apparent antagonist) depending on the receptor density/stimulus-response coupling efficiency of the assay in question. This appears to be the case with the peptidic UT receptor ligands examined in the present study. Although frequently overlooked, this phenomenon is not uncommon. For example, nociceptin/orphanin FQ receptor expression levels dictate peptide ligand agonist/antagonist activity (McDonald et al., 2003) and the low-efficacy β-adrenoceptor ligand prenalterol is an antagonist in the guinea-pig extensor digitorum longus muscle and a partial/full agonist in left and right atria (Kenakin & Beek, 1980; Kenakin, 2003b). As the present study reiterates (Table 2), terms such as antagonist or agonist cannot be applied ‘generically' across different pharmacological assay systems. As such, it is the authors contention that peptidic UT ligands such as urantide, lanreotide, BIM-23042 and SB-710411 have been erroneously labelled as antagonists. They are better categorized as ‘low-efficacy ligands'. Demonstration of UT receptor ‘antagonism' in the rat isolated aortae alone is clearly a superficial attempt to characterize ligands for this receptor system.
It follows, therefore, that an array of appropriate assays are needed in order to characterize UT receptor antagonism thoroughly, for example, a spectrum of assays where τ ranges from ‘small to large'. The present study has identified native tissue-based assays that fulfill this criterion, assays that can be considered ‘high volume control' assays (Kenakin, 2003c). Such assays are likely best represented by feline and primate arteries.
In order to define this phenomenon in more detail, the present study examined ligand-evoked UT receptor agonism under conditions of both low and high receptor density/efficient coupling and amplification. This was achieved by upregulating UT receptor density in recombinant host HEK cells exposed to increasing titres of a BacMam virus harbouring the rat, cat or human UT receptor gene. As predicted, urantide was a partial agonist in rat, cat and human recombinant UT receptor [Ca2+]i-mobilization assays. Indeed, increasing the exposure of cells from 1 to 3–20% (v v−1) BacMam virus (resulting in a titre-dependent, <20-fold increase in Bmax) resulted in a concomitant <6-fold increase in intrinsic activity relative to hU-II. Similar data were obtained in the cat and human recombinant UT receptor systems where intrinsic activity was also augmented (from 0.5–0.6 to ⩽0.8–0.9). Interestingly, despite lower UT receptor densities in the cat and human recombinant systems (<8 and <1 fmol mg−1, respectively, compared to <24 fmol mg−1 protein in the rat system), the relative intrinsic activity of urantide was appreciably greater in both the cat and human assays (∼5–18-fold greater [Ca2+]i-mobilization Emax), that is, τ was relatively high in the cat and human recombinant UT-HEK cell assays compared to the rat. For example, at 1% (v v−1) BacMam virus titres, rat UT Bmax (1,140 fmol mg−1) is 40-fold higher than that seen in the monkey UT assay (29 fmol mg−1), yet the relative [α] of urantide is 18-fold higher in the monkey UT receptor assay ([α] 0.56) compared to the rat UT assay ([α] ∼0.03). As such, intrinsic activity is not simply a function of receptor density, rather it is more closely related to the efficiency of the coupling of receptor/signalling in the host cell in this system.
Coupling efficiency is influenced by many factors (host cell line, cotransfection of G-proteins, etc.). Thus, it would be interesting to see in future studies if, for example, BacMam-mediated G-protein (Gαq) co-transfection produced similar viral titre-dependent increases in relative intrinsic activity [α] for such peptidic ligands (under conditions where UT receptor Bmax remained constant). Since the ‘innate' characteristics of UT recombinant assay systems are subject to considerable inter- and intra-laboratory variation, this general phenomenon should be considered carefully when comparing results between laboratories and assays (urantide behaved as an agonist in UT-CHO cell at 37°C (presumably high τ) and an antagonist in UT-HEK cell at room temperature (presumably low τ) in experiments performed within a single laboratory; Camarda et al., 2006).
In summary, under the present experimental conditions, it can be concluded that
the relative transducer constant (τ) in recombinant HEK cell assays is cat>human≫rat UT receptor.
the relative transducer constant (τ) in native mammalian arteries is monkey>cat≫rat (τ is, perhaps, less likely to vary between tissues, cf. recombinant cell-based assays assuming laboratory animals are inherently ‘less heterogeneous' than the latter) and
the nominal rank order of ligand relative intrinsic activity [α] for peptidic UT receptor ligands would be U-II>urantide>SB-710411≫GSK248451.
It is tempting to speculate that the types of differences in receptor density/coupling efficiencies seen in the present manuscript underlie the well documented inter- and intra-species variation in vascular reactivity that has become a hallmark of U-II biology, for example, vascular reactivity to U-II is significantly attenuated when one transitions from the thoracic to abdominal aorta in the rat and although U-II is a ‘coronary-selective' spasmogen in the dog, it exerts ubiquitous contractile actions in feline and primate-isolated arteries (Douglas et al., 2000; Behm et al., 2004a). Such conjecture is worthy of subsequent investigation. Interestingly, it has been postulated that U-II lacks a significant receptor reserve in native arteries and that the signalling actions of this ‘pseudo-irreversible' ligand are regulated at the level of cell surface receptor expression and/or coupling (Douglas, 2003). It follows, therefore, that subtle changes in the degree/efficiency of receptor expression/coupling might lead to significant changes in reactivity both in preclinical species (WKY cf. SHR; Gendron et al., 2004) and in man (atherosclerosis, heart failure and hypertension; Lim et al., 2004; Maguire et al., 2004; Sondermeijer et al., 2005). Such a hypothesis is also worthy of future study.
The fact that ligands such as urantide, [Orn8]hU-II, lanreotide, BIM-23042 and SB-710411 possess appreciable low intrinsic activity [α] (partial agonists) may render them unsuitable as tool compounds. However, the present study has identified GSK248451 as a putative tool antagonist suitable for in vivo use. In summary, GSK248451:
is potent (equipotent with urantide but ⩽80-fold more potent than SB-710411),
behaves as an antagonist in all native mammalian isolated tissues studied,
retains an extremely low level of relative intrinsic activity [α] cf. urantide (Emax ⩽9-fold in recombinant UT receptor assays) and
is a selective UT receptor antagonist (concentrations 170-fold above the hU-II Kb failed to block the contractile actions of several pharmacologically distinct spasmogens in the rat aorta).
Pretreatment of the anaesthetized cat with GSK248451 (1 mg kg−1, i.v.) did not alter basal haemodynamics (consistent with basal observations made in rodents with palosuran and UFP-803; Clozel et al., 2004; Camarda et al., 2006). Upon first inspection, such data might appear to imply that U-II does not make an appreciable contribution to the maintenance of basal systemic arterial blood pressure in the normotensive intact rodent or cat. However, U-II is a ‘pseudo-irreversible' UT receptor ligand (Douglas et al., 2004b) and, as such, acute bolus i.v. administration of a peptidic antagonist may be insufficient to alter the level of chronic ‘endogenous' UT receptor occupancy.
Compared to other available UT receptor tool compounds, GSK248451 was unique in its ability to block the systemic vasopressor actions of exogenous U-II. As such, this peptidic molecule (and potentially UFP-803 since it is efficacious in a mouse U-II-induced plasma extravasation model; Camarda et al., 2006) represents a suitable tool for future investigations aimed at delineating the role of U-II in the aetiology of mammalian cardiometabolic diseases. It is interesting to note that previous studies demonstrate that urantide is unable to block the haemodynamic (vasodepressor) actions of U-II in the rat (Gendron et al., 2005).
As reported previously (Behm et al., 2004a), the contractile actions of hU-II were significantly more rapid in onset and transient in the cat isolated femoral artery compared to responses in isolated thoracic aortae (see Table 5). Although such temporal observations might, in part, result from preferential receptor access, differential internalization/recycling, etc. (e.g. relatively slow UT recycling in the femoral artery; see Giebing et al., 2005), the data suggest that cat thoracic aortae might possess an ‘atypical' UT receptor. While it is tempting to speculate upon the existence of UT receptor ‘subtypes', this should be resisted at present since the available data do not meet the minimum IUPHAR criteria (operational/functional data and structural evidence; Humphrey & Barnard, 1998). There is, to date, no compelling molecular evidence for the existence of multiple, distinct UT receptor gene products. Although the presence of UT receptor subtypes has been forwarded previously, such claims are speculative, based primarily on limited operational observations (multiple UT receptor mRNA transcripts have been identified in the mouse heart, skeletal muscle, bladder but this likely reflects alternate splicing of the UT receptor gene; Liu et al., 1999). Indeed, reactivity to hU-II is completely ablated in the UT receptor knockout mouse (Behm et al., 2003b). It is interesting, however, to note that Gendron et al. (2005) have recently reported that urantide (∼10 mg kg−1, i.v.) is unable to block the vasodilator responses to systemic U-II in the hypertensive rat. Similarly, Coy et al. (2000) claim that ‘tonic aortic contraction' to U-II is refractory to UT receptor antagonism in the rat. How such observations relate to the use of low-efficacy partial agonists (e.g. urantide) as UT receptor inhibitors and/or receptor subtypes is currently unknown.
It is clear that the responses of the cat femoral artery to hU-II differ from those seen in the aorta. Specifically, the contractile actions of hU-II were significantly more potent (∼5-fold lower EC50) and efficacious (Emax >2-fold greater when normalized to 60 mM KCl) in the cat femoral artery. Similarly, urantide was significantly (by almost two orders of magnitude) more potent as a spasmogen in femoral artery cf. aorta. This striking potency difference was also evident with the antagonists SB-710411 (<1 μM Kb in femoral artery but inactive in the aorta (Kb >10 μM)) and GSK248451 (Kbs 1 versus 190 nM in femoral artery and aorta, respectively). For now, the reason underlying such disparities remains ambiguous. Although the existence of UT receptor subtype(s) cannot be ruled out, a single UT receptor gene subject to different post-translational modification represents an alternative explanation. Equally plausible is the proposition that the cat femoral artery and aorta represent UT receptor-signalling systems with two quite different coupling/signalling efficiencies or perhaps even differences in receptor reserve. Clearly, overexpression of recombinant UT receptors in mammalian host cells (e.g. CHO, HEK cells) results in extremely high UT receptor densities (Bmax >450 fmol mg protein−1 for rodent, cat and primate UT; Ames et al., 1999; Elshourbagy et al., 2002; Aiyar et al., 2005). This creates a large population of efficiently coupled UT receptors. This contrasts native rat and cat cardiopulmonary tissue (where Bmax <20 fmol mg protein−1; Itoh et al., 1988; Ames et al., 1999; Behm et al., 2005a). This low level of native UT receptor expression also exists in human cell lines (Douglas et al., 2004b; Qi et al., 2005). Nevertheless, since [125I]U-II Bmax is low in both rat and cat cardiopulmonary tissue (Itoh et al., 1988; Behm et al., 2005a), receptor density alone is probably a lesser factor in determining apparent agonism/antagonism than inherent signal-transduction efficiency. Clearly, high-density recombinant expression systems are, by definition, ‘artefactual'. Since they create abnormally efficient receptor-effector coupling systems, they may be unrepresentative of native, physiological U-II/UT receptor systems. Such differences should be considered when selecting assay systems for profiling the operational properties (agonist versus antagonist) of UT receptor ligands.
In summary, the nonproprietary peptide GSK248451 represents a potent, surmountable tool UT receptor antagonist suitable for studying the (patho)physiological actions of U-II both in vitro and in vivo in a variety of species. It is readily differentiated from other peptidic UT receptor ligands, for example, urantide and SB-710411 (intrinsic activity [α] and/or UT receptor potency/selectivity). Use of such a ligand will hopefully advance the understanding of U-II biology.
Acknowledgments
The data described herein were presented, in part, at the Mid Atlantic Pharmacology Society meeting, University of Medicine and Dentistry of New Jersey (Behm et al., 2003a) and the American Heart Association (Behm et al., 2005b). We thank P. Buckley, W. Wixted and Dr T. Kenakin for their assistance and constructive comments made during the preparation of this manuscript.
Abbreviations
- [α]
intrinsic efficacy
- BacMam
recombinant baculovirus in which the polyhedrin promoter has been replaced with a mammalian promoter
- BCA
bicinchoninic acid
- BSA
bovine serum albumin
- [Ca2+]i
intracellular calcium
- Cin
4-Cl-cinnamoyl
- Cpa
4-chlorophenylalanine
- DMSO
dimethylsulphoxide
- DPBS
Dulbecco's phosphate-buffered saline
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethyleneglycol-bis(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid
- FLIPR
fluorometric imaging plate reader
- GSK248451
Cin-c[DCys-Pal-DTrp-Orn-Val-Cys]-His-amide
- HEK cells
human embryonic kidney cells
- HEPES
4-(2-hydroxyethyl)-1-piperazineethane sulphonic acid
- Pal
3-pyridylalanine
- Pen
L-Penicillaminyl (β,β-dimethyl-L-cysteinyl)
- PMSF
phenylmethylsulphonyl fluoride
- SB-710411
Cpa-c[DCys-Pal-DTrp-Lys-Val-Cys]-Cpa-amide
- s.e.m.
standard error of the mean
- SPA
scintillation proximity assay
- hU-II
human urotensin-II
- UT receptor
urotensin-II receptor
- urantide
[Pen5-DTrp7-Orn8]hU-II4–11
References
- AIYAR N.V., JOHNS D.G., AO Z., DISA J., BEHM D.J., FOLEY J.J., BUCKLEY P.T., SARAU H.M., VAN DER KEYL H.K., ELSHOURBAGY N.A., DOUGLAS S.A. Cloning and pharmacological characterization of the cat urotensin-II receptor (UT) Biochem. Pharmacol. 2005;69:1069–1079. doi: 10.1016/j.bcp.2004.12.016. [DOI] [PubMed] [Google Scholar]
- AMES R.S., FORNWALD J.A., NUTHULAGANTI P., TRILL J.J., FOLEY J.F., BUCKLEY P.T., KOST T.A., WU Z., ROMANOS M.A. BacMam recombinant baculovirus in G protein-coupled receptor drug discovery. Receptors Channels. 2004a;10:99–107. doi: 10.1080/10606820490514969. [DOI] [PubMed] [Google Scholar]
- AMES R.S., NUTHULAGANTI P., FORNWALD J.A., SHABON U., VAN DER KEYL H.K., ELSHOURBAGY N.A. Heterologous expression of G protein-coupled receptors in U-2 OS osteosarcoma cells. Receptors Channels. 2004b;10:117–124. doi: 10.1080/10606820490515012. [DOI] [PubMed] [Google Scholar]
- AMES R.S., SARAU H.M., CHAMBERS J.K., WILLETTE R.N., AIYAR N.V., ROMANIC A.M., LOUDEN C.S., FOLEY J.J., SAUERMELCH C.F., COATNEY R.W., AO Z., DISA J., HOLMES S.D., STADEL J.M., MARTIN J.D., LIU W.S., GLOVER G.I., WILSON S., MCNULTY D.E., ELLIS C.E., ELSHOURBAGY N.A., SHABON U., TRILL J.J., HAY D.W., OHLSTEIN E.H., BERGSMA D.J., DOUGLAS S.A. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- BEHM D.J., AO Z., CAMARDA V., AIYAR N.V., JOHNS D.G., DOUGLAS S.A. Inhibitory effects of putative peptidic urotensin-II receptor antagonists on urotensin-II-induced contraction of cat isolated respiratory smooth muscle. Eur. J. Pharmacol. 2005a;516:276–281. doi: 10.1016/j.ejphar.2005.04.043. [DOI] [PubMed] [Google Scholar]
- BEHM D.J., DOE C.P., JOHNS D.G., MANISCALCO K., STANKUS G.P., WIBBERLEY A., WILLETTE R.N., DOUGLAS S.A. Urotensin-II: a novel systemic hypertensive factor in the cat. Naunyn Schmiedeberg's Arch. Pharmacol. 2004a;369:274–280. doi: 10.1007/s00210-004-0873-1. [DOI] [PubMed] [Google Scholar]
- BEHM D.J., HARRISON S.M., AO Z., MANISCALCO K., PICKERING S.J., GRAU E.V., WOODS T.N., COATNEY R.W., DOE C.P., WILLETTE R.N., JOHNS D.G., DOUGLAS S.A. Deletion of the UT receptor gene results in the selective loss of urotensin-II contractile activity in aortae isolated from UT receptor knockout mice. Br. J. Pharmacol. 2003b;139:464–472. doi: 10.1038/sj.bjp.0705254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEHM D.J., HEROLD C.L., CAMARDA V., AIYAR N.V., DOUGLAS S.A. Differential agonistic and antagonistic effects of the urotensin-II ligand SB-710411 at rodent and primate UT receptors. Eur. J. Pharmacol. 2004b;25:113–116. doi: 10.1016/j.ejphar.2004.03.059. [DOI] [PubMed] [Google Scholar]
- BEHM D.J., HEROLD C.L., OHLSTEIN E.H., KNIGHT S.D., DHANAK D., DOUGLAS S.A. Pharmacological characterization of SB-710411 (Cap-c[D-Cys-Pal-D-Trp-Lys-Val-Cys]-Cpa-amide), a novel peptidic urotensin-II receptor antagonist. Br. J. Pharmacol. 2002;137:449–458. doi: 10.1038/sj.bjp.0704887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEHM D.J., JOHNS D.J., CAMARDA V., AIYAR N.V., DOUGLAS S.A. Differential antagonistic properties of novel peptidic UT receptor antagonists in feline isolated arteries: evidence for the existence of UT receptor subtypes. Pharmacologist. 2003a;45:138–139. [Google Scholar]
- BEHM D.J., STANKUS G.P., DOE C.P., WILLETTE R.N., SARAU H.M., FOLEY J.J., BUCKLEY P.T., SCHMIDT D.B., WIXTED W.E., NUTHULAGANTI P., FORNWALD J.A., AMES R.S., CAMARDA V., AIYAR N.V., DOUGLAS S.A. GSK248451, a novel peptidic urotensin-II receptor antagonist, potently inhibits urotensin-II-induced vasoconstriction both in vitro and in vivo. Circulation. 2005b;112:II-147. [Google Scholar]
- BLACK J.W., LEFF P. Operational models of pharmacological agonists. Proc. R. Soc Lond. (Biol. Sci.) 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- BÖHM F., PERNOW J. Urotensin II evokes potent vasoconstriction in humans in vivo. Br. J. Pharmacol. 2002;135:25–27. doi: 10.1038/sj.bjp.0704448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMARDA V., GUERRINI R., KOSTENIS E., RIZZI A., CALO' G., HATTENBURGER A., ZUCCHINI M., SALVADORI S., REGOLI D. A new ligand for the urotensin II receptor. Br. J. Pharmacol. 2002;137:311–314. doi: 10.1038/sj.bjp.0704895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMARDA V., SPAGNOL M., SONG W., VERGURA R., ROTH A.L., THOMPSON J.P., ROWBOTHAM D.J., GUERRINI R., MARZOLA E., SALVADORI S., CAVANNI P., REGOLI D., DOUGLAS S.A., LAMBERT D.G., CALÒ G. In vitro and in vivo pharmacological characterization of the novel UT receptor ligand [Pen5, DTrp6, Dab8]urotensin II(4–11) (UFP-803) Br. J. Pharmacol. 2006;147:92–100. doi: 10.1038/sj.bjp.0706438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMARDA V., SONG W., MARZOLA E., SPAGNOL M., GUERRINI R., SALVADORI S., REGOLI D., THOMPSON J.P., ROWBOTHAM D.J., BEHM D.J., DOUGLAS S., CALO' G., LAMBERT D.G. Urantide mimics urotensin-II induced calcium release in cells expressing recombinant UT receptors. Eur. J. Pharmacol. 2004;498:83–86. doi: 10.1016/j.ejphar.2004.07.089. [DOI] [PubMed] [Google Scholar]
- CLOZEL M., BINKERT C., BIRKER-ROBACZEWSKA M., BOUKHADRA C., DING S.S., FISCHLI W., HESS P., MATHYS B., MORRISON K., MULLER C., MULLER C., NAYLER O., QIU C., REY M., SCHERZ M.W., VELKER J., WELLER T., XI J.F., ZILTENER P. Pharmacology of the urotensin-II receptor antagonist palosuran (ACT-058362: 1-[2-(4-benzyl-4-hydroxy-piperidin-1-yl)-ethyl]-3-(2-methyl-quinolin-4-yl)-urea sulfate salt): first demonstration of a pathophysiological role of the urotensin system. J. Pharmacol. Expl Ther. 2004;311:204–212. doi: 10.1124/jpet.104.068320. [DOI] [PubMed] [Google Scholar]
- CLOZEL M., HESS P., QUI C., DING S.-S., REY M. The urotensin receptor antagonist palosuran improves pancreatic and renal function in diabetic rats. J. Pharmacol. Expl Ther. 2006;316:1115–1121. doi: 10.1124/jpet.105.094821. [DOI] [PubMed] [Google Scholar]
- COY D.H., ROSSOWSKI W.J., CHENG B.L., HOCARD S.J., TAYLOR J.E. Novel urotensin-II (UII) antagonists point to multiple receptor involvement in UII bioactivity. Regul. Pept. 2000;94:P48. [Google Scholar]
- COY D.H., ROSSOWSKI W.J., CHENG B.L., TAYLOR J.E.Highly potent heptapeptide antagonists of the vasoactive peptide urotensin II Abstracts of papers, 225th ACS National meeting 2003Washington, DC: American Chemical Society; New Orleans, LA, United States, March 23–27 [Google Scholar]
- DHANAK D., NEEB M.J., DOUGLAS S.A. Urotensin-II receptor modulators. Ann. Rep. Med. Chem. 2003;38:99–110. [Google Scholar]
- DOUGLAS S.A. Human urotensin-II as a novel cardiovascular target: ‘heart' of the matter or simply a fishy ‘tail'. Curr. Opin. Pharmacol. 2003;3:159–167. doi: 10.1016/s1471-4892(03)00012-2. [DOI] [PubMed] [Google Scholar]
- DOUGLAS S.A., BEHM D.J., AIYAR N.V., NASELSKY D., DISA J., BROOKS D.P., OHLSTEIN E.H., GLEASON J.G., SARAU H.M., FOLEY J.J, BUCKLEY P.T., SCHMIDT D.B., WIXTED W.E., WIDDOWSON K., RILEY G., JIN J., GALLAGHER T.F., SCHMIDT S.J., RIDGERS L., CHRISTMANN L.T., KEENAN R.M., KNIGHT S.D., DHANAK D. Non-peptidic urotensin-II receptor (UT) antagonists I: In vitro pharmacological characterisation of SB-706375. Br. J. Pharmacol. 2005;145:620–635. doi: 10.1038/sj.bjp.0706229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS S.A., DHANAK D., JOHNS D.G. From ‘gills to pills': urotensin-II as a regulator of mammalian cardiorenal function. Trends Pharmacol. Sci. 2004a;25:76–85. doi: 10.1016/j.tips.2003.12.005. [DOI] [PubMed] [Google Scholar]
- DOUGLAS S.A., NASELSKY D., AO Z., DISA J., HEROLD C.L., LYNCH F., AIYAR N.V. Identification and pharmacological characterization of native, functional human urotensin-II receptors in rhabdomyosarcoma cell lines. Br. J. Pharmacol. 2004b;142:921–932. doi: 10.1038/sj.bjp.0705743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS S.A., SULPIZIO A.C., PIERCY V., SARAU H.M., AMES R.S., AIYAR N.V., OHLSTEIN E.H., WILLETTE R.N. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br. J. Pharmacol. 2000;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELSHOURBAGY N.A., DOUGLAS S.A., SHABON U., HARRISON S., DUDDY G., SECHLER J.L., AO Z., MALEEFF B.E., NASELSKY D., DISA J., AIYAR N.V. Molecular and pharmacological characterization of genes encoding urotensin-II peptides and their cognate G-protein-coupled receptors from the mouse and monkey. Br. J. Pharmacol. 2002;136:9–22. doi: 10.1038/sj.bjp.0704671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., MARCH J.E., KEMP P.A., BENNETT T. Bolus injection of human UII in conscious rats evokes a biphasic haemodynamic response. Br. J. Pharmacol. 2004;143:422–430. doi: 10.1038/sj.bjp.0705954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENDRON G., GOBEIL F., JR, BÉLANGER S., GAGNON S., REGOLI D., D'ORLEANS-JUSTE P. Urotensin II-induced hypotensive responses in Wistar–Kyoto (Wky) and spontaneously hypertensive (Shr) rats. Peptides. 2005;26:1468–1474. doi: 10.1016/j.peptides.2005.03.012. [DOI] [PubMed] [Google Scholar]
- GENDRON G., SIMARD B., GOBEIL F., JR, SIROIS P., D'ORLEANS-JUSTE P., REGOLI D. Human urotensin-II enhances plasma extravasation in specific vascular districts in Wistar rats. Can. J. Physiol. Pharmacol. 2004;82:16–21. doi: 10.1139/y03-122. [DOI] [PubMed] [Google Scholar]
- GIEBING G., TÖLLE M., JÜRGENSEN J., EICHHORST J., FURKERT J., BEYERMANN M., NEUSCHÄFER-RUBE F., ROSENTHAL W., ZIDEK W., VAN DER GIET M., OKSCHE A. Arrestin-independent internalization and recycling of the urotensin receptor contribute to long-lasting urotensin II-mediated vasoconstriction. Circ. Res. 2005;97:707–715. doi: 10.1161/01.RES.0000184670.58688.9F. [DOI] [PubMed] [Google Scholar]
- GILBERT R.E., DOUGLAS S.A., KRUM H. Urotensin-II as a novel therapeutic target in the clinical management of cardiorenal disease. Curr. Opin. Invest. Drugs. 2004;5:276–282. [PubMed] [Google Scholar]
- GRIECO P., CAROTENUTO A., CAMPIGLIA P., MARINELLI L., LAMA T., PATACCHINI R., SANTICIOLI P., MAGGI C.A., ROVERO P., NOVELLINO E. Urotensin-II receptor ligands. From agonist to antagonist activity. J. Med. Chem. 2005;48:7290–7297. doi: 10.1021/jm058043j. [DOI] [PubMed] [Google Scholar]
- GUERRINI R., CAMARDA V., MARZOLA E., ARDUIN M., CALO G., SPAGNOL M., RIZZI A., SALVADORI S., REGOLI D. Structure–activity relationship study on human urotensin II. J. Pept. Sci. 2005;11:85–90. doi: 10.1002/psc.590. [DOI] [PubMed] [Google Scholar]
- HELLER J., FISCHER N., SCHEPKE M., NEEF M., SAUERBRUCH T. Octreotide antagonizes urotensin II (U II) induced aortic contractions in normal and cirrhotic rats. Hepatolology. 2003;38 (Suppl. 2):59. [Google Scholar]
- HEROLD C.L., BEHM D.J., BUCKLEY P.T., FOLEY J.J., DOUGLAS S.A. The peptidic somatostatin analogs lanreotide, BIM-23127 and BIM-23042 are urotensin-II receptor ligands. Pharmacologist. 2002;44:170–171. [Google Scholar]
- HEROLD C.L., BEHM D.J., BUCKLEY P.T., FOLEY J.J., WIXTED W.E., SARAU H.M., DOUGLAS S.A. The neuromedin B receptor antagonist, BIM-23127, is a potent antagonist at human and rat urotensin-II receptors. Br. J. Pharmacol. 2003;139:203–207. doi: 10.1038/sj.bjp.0705251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMPHREY P.P.A., BARNARD E.A. International Union of Pharmacology. XIX. The IUPHAR receptor code: a proposal for an alphanumeric classification system. Pharmacol. Rev. 1998;50:271–277. [PubMed] [Google Scholar]
- ITOH H., MCMASTER D, LEDERIS K. Functional receptors for fish neuropeptide urotensin II in major rat arteries. Eur. J. Pharmacol. 1988;149:61–66. doi: 10.1016/0014-2999(88)90042-8. [DOI] [PubMed] [Google Scholar]
- JENKINSON D.H., BARNARD E.A., HOYER D., HUMPHREY P.P.A., LEFF P., SHANKLEY N.P.Terms and symbols in quantitative pharmacology The IUPHAR Compendium of Receptor Characterization and Classification 1998London: IUPHAR Media; 6–20.ed. Girdlestone, D. pp [Google Scholar]
- JOHNS D.G., AO A., NASELSKY D., HEROLD C.L., MANISCALCO K., SAROV-BLAT L., STEPLEWSKI K., AIYAR N., DOUGLAS S.A. Urotensin-II-mediated cardiomyocyte hypertrophy: effect of receptor antagonism and role of inflammatory mediators. Naunyn Schmiedeberg's Arch. Pharmacol. 2004;370:238–250. doi: 10.1007/s00210-004-0980-z. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. Efficacy at G-protein-coupled receptors. Nat. Rev. Drug Disc. 2002;1:103–110. doi: 10.1038/nrd722. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.Agonists: the measurement of affinity and efficacy in functional assays A Pharmacology Primer: Theory, Application and Methods 2003aAmsterdam: Elsevier Academic Press; 73–92.ed. Kenakin, T. pp [Google Scholar]
- KENAKIN T.How different tissues process drug responses A Pharmacology Primer: Theory, Application and Methods 2003bAmsterdam: Elsevier Academic Press; 17–36.ed. Kenakin, T. pp [Google Scholar]
- KENAKIN T. Predicting therapeutic value in the lead optimization phase of drug discovery. Nat. Rev. 2003c;2:429–438. doi: 10.1038/nrd1110. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.P., BEEK D. Is prenalterol (H133/80) really a selective beta 1 adrenoceptor agonist? Tissue selectivity resulting from differences in stimulus–response relationships. J Pharmacol. Exp. Ther. 1980;213:406–413. [PubMed] [Google Scholar]
- KOMPA A.R., THOMAS W.G., SEE F., TZANIDIS A., HANNAN R.D., KRUM H. Cardiovascular role of urotensin II: effect of chronic infusion in the rat. Peptides. 2004;25:1783–1788. doi: 10.1016/j.peptides.2004.03.029. [DOI] [PubMed] [Google Scholar]
- LIM M., HONISETT S., SPARKES C.D., KOMESAROFF P., KOMPA A., KRUM H. Differential effect of urotensin II on vascular tone in normal subjects and patients with chronic heart failure. Circulation. 2004;109:1212–1214. doi: 10.1161/01.CIR.0000121326.69153.98. [DOI] [PubMed] [Google Scholar]
- LIU Q., PONG S.S., ZENG Z., ZHANG Q., HOWARD A.D., WILLIAMS D.L., JR, DAVIDOFF M., WANG R., AUSTIN C.P., MCDONALD T.P., BAI C., GEORGE S.R., EVANS J.F., CASKEY C.T. Identification of urotensin II as the endogenous ligand for the orphan G-protein-coupled receptor GPR14. Biochem. Biophys. Res. Commun. 1999;266:174–178. doi: 10.1006/bbrc.1999.1796. [DOI] [PubMed] [Google Scholar]
- MAGUIRE J.J., KUC R.E., WILEY K.E., KLEINZ M.J., DAVENPORT A.P. Cellular distribution of immunoreactive urotensin-II in human tissues with evidence of increased expression in atherosclerosis and a greater constrictor response of small compared to large coronary arteries. Peptides. 2004;25:1767–1774. doi: 10.1016/j.peptides.2004.01.028. [DOI] [PubMed] [Google Scholar]
- MCDONALD J., BARNES T.A., OKAWA H., WILLIAMS J., CALO' G., ROWBOTHAM D.J., LAMBERT D.G. Partial agonist behaviour depends upon the level of nociceptin/orphanin FQ receptor expression: studies using the ecdysone-inducible mammalian expression system. Br. J. Pharmacol. 2003;140:61–70. doi: 10.1038/sj.bjp.0705401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATACCHINI R., SANTICIOLI P., GIULIANI S., GRIECO P., NOVELLINO E., ROVERO P., MAGGI C.A. Urantide: an ultrapotent urotensin II antagonist peptide in the rat aorta. Br. J. Pharmacol. 2003;140:1155–1158. doi: 10.1038/sj.bjp.0705555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QI J.-S., MINOR L.K., SMITH C., HU B., YANG Y., ANDRADE-GORDON P., DAMIANO B. Characterization of functional urotensin II receptors in human skeletal muscle myoblasts: comparison with angiotensin II receptors. Peptides. 2005;26:683–690. doi: 10.1016/j.peptides.2004.11.018. [DOI] [PubMed] [Google Scholar]
- RICHARDS A.M., CHARLES C. Urotensin II in the cardiovascular system. Peptides. 2004;25:1795–1802. doi: 10.1016/j.peptides.2004.04.017. [DOI] [PubMed] [Google Scholar]
- RUSSELL F.D. Emerging roles of urotensin-II in cardiovascular disease. Pharmacol. Ther. 2004;103:223–243. doi: 10.1016/j.pharmthera.2004.07.004. [DOI] [PubMed] [Google Scholar]
- RUSSELL F.D., MEYERS D., GALBRAITH A.J., BETT N., TOTH I., KEARNS P., MOLENAAR P. Elevated plasma levels of human urotensin-II immunoreactivity in congestive heart failure. Am. J. Physiol. 2003;285:H1576–H1581. doi: 10.1152/ajpheart.00217.2003. [DOI] [PubMed] [Google Scholar]
- SONDERMEIJER B., KOMPA A., KOMESAROFF P., KRUM H. Effect of exogenous urotensin-II on vascular tone in skin microcirculation of patients with essential hypertension. Am. J. Hypertens. 2005;18:1195–1199. doi: 10.1016/j.amjhyper.2005.03.748. [DOI] [PubMed] [Google Scholar]
- SONG W., ASHTON N, BALMENT R.J. Effects of single bolus urotensin II injection in anaesthetised rats. J. Physiol. 2003;552P:P107. [Google Scholar]
- TZANIDIS A., HANNAN R.D., THOMAS W.G., ONAN D., AUTELITANO D.J., SEE F., KELLY D.J., GILBERT R.E., KRUM H. Direct actions of urotensin II on the heart: implications for cardiac fibrosis and hypertrophy. Circ. Res. 2003;93:246–253. doi: 10.1161/01.RES.0000084382.64418.BC. [DOI] [PubMed] [Google Scholar]
- ZHU Y.Z., WANG Z.J., ZHU Y.C., ZHANG L., OAKLEY R.M., CHUNG C.W., LIM K.W., LEE H.S., OZOUX M.L., LINZ W., BOHM M., KOSTENIS E. Urotensin II causes fatal circulatory collapse in anesthesized monkeys in vivo: a ‘vasoconstrictor' with a unique hemodynamic profile. Am. J. Physiol. 2004;286:H830–H836. doi: 10.1152/ajpheart.00406.2003. [DOI] [PubMed] [Google Scholar]
- ZOU Y., NAGAI R., YAMAZAKI T. Urotensin II induces hypertrophic responses in cultured cardiomyocytes from neonatal rats. FEBS Lett. 2001;508:57–60. doi: 10.1016/s0014-5793(01)03015-0. [DOI] [PubMed] [Google Scholar]