Abstract
The potential cardioprotective effect of ACE inhibitors has been attributed to the inhibition of bradykinin degradation. Recent data in rats documented a kallidin-like peptide, which mimics the cardioprotective effect of ischaemic preconditioning. This study investigates in isolated Langendorff rat heart the effect of the ACE inhibitor captopril, the role of bradykinin, kallidin-like peptide, and nitric oxide (NO).
The bradykinin level in the effluent of the control group was 14.6 pg ml−1 and was not affected by captopril in the presence or absence of kinin B2-receptor antagonist, HOE140.
The kallidin-like peptide levels were approximately six-fold higher (89.8 pg ml−1) and increased significantly by treatment with captopril (144 pg ml−1), and simultaneous treatment with captopril and HOE140 (197 pg ml−1).
Following 30 min ischaemia in the control group, the creatine kinase activity increased from 0.4 to 53.4 U l−1. In the captopril group and in the captopril+L-NAME group, the creatine kinase activity was significantly lower (18.5 and 22.8 U l−1). This beneficial effect of captopril was completely abolished by the kinin B2-receptor antagonist, HOE140, as well as by the kallidin antiserum.
Perfusion of the hearts with kallidin before the 30 min ischaemia, but not with bradykinin, yielded an approximately 50% reduction in creatine kinase activity after reperfusion.
Pretreatment with L-NAME alone and simultaneously with captopril, and with kallidin, respectively, suggests a kinin-independent action of NO before the 30 min ischaemia on coronary flow and a kinin-dependent action after ischaemia.
These data show that captopril increases kallidin-like peptide in the effluent. Kallidin-like peptide via kinin B2 receptor seems to be the physiological mediator of cardioprotective actions of captopril against ischaemic reperfusion injury. HOE140 as well as the kallidin antiserum abolished the cardioprotective effects of captopril.
Keywords: Pharmacological preconditioning, bradykinin, kallidin-like peptide, icatibant, HOE140, captopril, NO synthase inhibitor, L-NAME, B2-receptor antagonist
Introduction
Angiotensin-converting enzyme (ACE) inhibitors have been shown to prolong the survival of patients after cardiac infarction (Pfeffer et al., 1992). There is mounting evidence to show that at least part of the beneficial effects of ACE inhibitors are owing to the reduced bradykinin degradation. Bradykinin has been shown to play a major role in preconditioning and protection of the ischaemic myocardium (for a review see, Baxter & Ebrahim, 2002). The numerous experimental data on the cardioprotective effect of ACE inhibitors in the isolated hearts of dogs (Zhang et al., 1999), guinea-pigs (Zahler et al., 1999), and rats (Varin et al., 2000) revealed that the kinin B2 receptor via activation of the endothelial nitric oxide (NO) synthase (Linz et al., 1996) is involved in the protection against ischaemic reperfusion injury and reperfusion-induced arrhythmia. Moreover, ACE inhibitors via an accumulation of bradykinin mediate a beneficial effect on the cardiac energy metabolism (Linz et al., 1997). In this context, an endogenous kallikrein–kinin system with tissue kallikrein gene (Xiong et al., 1990; Nolly et al., 1994) and low-molecular weight kininogen gene expression (Takano et al., 1997; Nagaoka et al., 1999) has been demonstrated in the heart.
It is generally accepted that in humans, as in other mammals, the kallikrein–kinin system consists of a plasma, and a tissue kallikrein–kinin system (for a review see, Bhoola et al., 1992). The plasma kallikrein–kinin system mediates its effects via bradykinin, which is released from high-molecular weight kininogen by plasma kallikrein. The tissue kallikrein–kinin system generates kallidin, which is released from low-molecular weight kininogen by tissue kallikrein. Both systems seem to be differently regulated in humans (Hilgenfeldt et al., 1998). Despite a clear discrimination between both systems, their importance in different physiological mechanisms has never been investigated. Moreover, bradykinin was believed to be the only kinin acting on the B2 receptors in rats. Recently, however, we have demonstrated in rats a kallidin-like peptide, which differs from kallidin by the exchange of an N-terminal lysine- vs an arginine residue (Hilgenfeldt et al., 2005).
In order to investigate the cardioprotective effect of the kallikrein–kinin system more precisely, we have analysed the release of bradykinin and kallidin-like peptide in the coronary effluent of the isolated Langendorff rat heart during ischaemic preconditioning. During the ischaemic preconditioning, the levels of kallidin-like peptide were significantly increased, whereas the bradykinin levels remained unchanged. Previously, it was shown that the ischaemic preconditioning-mediated cardioprotection could be abrogated by the administration of a specific kallidin antiserum and by the kinin B2-receptor antagonist HOE140 (Liu et al., 2005).
As already mentioned, the beneficial effects of kinins in the heart have been verified indirectly by the administration of ACE inhibitors, which could be abolished with kinin B2-receptor antagonists (Sato et al., 2000). The present study investigates the cardioprotective effect of captopril after 30 min of cardiac ischaemia on reperfusion arrhythmia and the importance of both peptides, bradykinin- and kallidin-like peptide, in this process. Furthermore, the role of NO in mediating the cardioprotective effect of kinin was examined. In order to mimic the effect of ischaemic preconditioning, different substances were administered in three 5-min cycles, separated by three 5-min cycles with the pure medium, during the preischaemic perfusion.
Methods
Animals
Male SPF Sprague–Dawley rats (Charles River, Germany), 260–320 g, were kept under standard conditions with free access to food and water. All experiments were performed in accordance with the FELASA guidelines for animal experimentation.
Langendorff heart perfusion
Rats were anaesthetised with pentobarbital sodium, intraperitoneally, 60 mg kg−1 body weight and injected 500 U heparin intravenously to prevent blood clotting. After opening of the chest, we rapidly chilled the hearts with ice-cold saline to stop the contraction and to reduce oxygen consumption. We cannulated the ascending aorta and immediately perfused the coronary arteries with prechilled medium. Then, we quickly excised the hearts and mounted them on the thermostated Langendorff apparatus (whole procedure within 1 min). Spontaneous beating started within a few seconds of retrograde perfusion with non-recirculating modified Krebs–Henseleit buffer containing (mM): 118 NaCl; 24 NaHCO3; 4 KCl; 1.2 KH2PO4; 1 MgSO4; 5 D-glucose; 2 pyruvate sodium; and 2.5 CaCl2. The medium was prepared with a 95% O2 + 5% CO2 mixture at 37°C (pH 7.4) and passed through a 3 μm filter before entering the heart in order to remove any particular contamination.
The experiments were conducted under conditions of constant perfusate pressure (coronary perfusion pressure) of 70 mmHg. Coronary flow was measured volumetrically. During the initial equilibrium, the left ventricular end-diastolic pressure (LVEDP) was adjusted to 0–5 mmHg. Left ventricular developed pressure (LVDP) and heart rate were monitored using the latex balloon catheter placed in the left ventricle and connected to a pressure transducer and bridge amplifier (WPI, Germany). All data of cardiac function parameters (LVDP, LVEDP) were recorded and analysed using the software developed by Shandong Pharmacy Institute (China).
Experimental protocol
The experiments started after an equilibration time of 20 min. A 30-min treatment period was followed by 30 min of global ischaemia (no-flow). Then, we reperfused the hearts for another 30 min.
Treatment periods
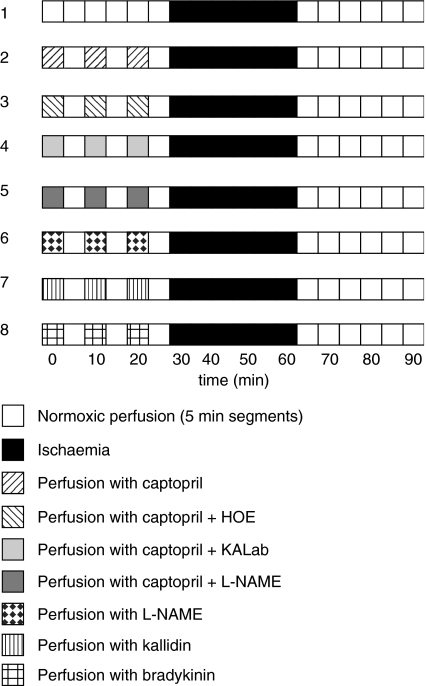
As shown in Figure 1, the 30-min alternative experimental procedure before the 30 min ischaemia included:
Normoxic perfusion (control group).
Perfusion with three cycles of 5 min duration with medium containing captopril, 10 μM, separated by three perfusion cycles of 5 min duration without captopril (Cap group).
Perfusion with three cycles of 5 min duration with medium containing captopril, 10 μM, plus HOE140, 100 nM, separated by three perfusion cycles of 5 min duration without both drugs (Cap+HOE group).
Perfusion with three cycles of 5 min duration with medium containing captopril, 10 μM plus kallidin antiserum at a dilution of 1 : 1000 separated by three perfusion cycles of 5 min duration without captopril+kallidin antiserum (Cap+KALab group).
Perfusion with three cycles of 5 min duration with medium containing captopril, 10 μM, plus L-NAME, 10 μM, separated by three cycles of 5 min duration without both drugs (Cap+L-NAME group).
Perfusion with three cycles of 5 min duration with medium containing L-NAME, 10 μmol, separated by three cycles of 5 min duration without L-NAME (L-NAME group).
Perfusion with three cycles of 5 min duration with medium containing kallidin, 10 nM, separated by three perfusion cycles of 5 min duration without kallidin (kallidin group).
Perfusion with three cycles of 5 min duration with medium containing bradykinin, 10 nM, separated by three perfusion cycles of 5 min duration without bradykinin (bradykinin group). The applied bradykinin concentration was taken according to the paper of Linz et al. (1997).
Figure 1.
Schematic of eight different perfusion protocols.
Exclusion criteria
During equilibration, we excluded hearts if they met one of the following criteria: (1) unstable contractile function, (2) coronary flow outside the range of 9–15 ml min−1, (3) heart rate below 240 beats min−1 or appearance of severe arrhythmia, (4) left ventricular pressure below 70 mmHg, and (5) creatine kinase (CK) activities in the effluent >1 mU ml−1 at the end of equilibration.
In order to measure kinins and CK released by the heart, the coronary effluent from the pulmonary artery was continuously collected.
Chemicals
Bradykinin and kallidin have been purchased from Bachem, Heidelberg, Germany. Kallidin-like peptide was kindly provided by Professor Metzler-Nolte, IPMB, Heidelberg, Germany. All buffer substances were purchased from Merck AG, Darmstadt, Germany. Captopril was purchased from Sigma-Aldrich, Taufkirchen, Germany. The kinin B2-receptor antagonist, HOE140 (Icatibant), was a kind gift from Dr Wirth, Sanofi-Aventis, Frankfurt, Germany.
Kinins immunoassay
Bradykinin- and kallidin-like peptides were measured with two specific and selective antisera (Hilgenfeldt et al., 1995) by radioimmunoassay as described recently (Liu et al., 2005).
Characteristics of the kallidin antiserum
In a previous experiment, a bradykinin tracer was incubated with a kallidin-like peptide standard curve in order to analyse bradykinin binding to the kallidin antiserum (lot no. 4H79.6). No binding of the bradykinin tracer to the kallidin antiserum was observed, indicating that the serum does not interfere with the cardiac bradykinin (Liu et al., 2005).
CK assay
CK activity was measured in the effluent of the perfused hearts with a colorimetric enzyme assay kit provided by Sigma-Aldrich, Taufkirchen, Germany, as described previously (Liu et al., 2005).
Statistical analysis
The data are expressed as mean values with standard deviation (s.d.) or standard error of the mean (s.e.m.) as indicated; n=number of hearts in every group (at least eight). Statistical significance between the groups was verified with one-way analysis of variance with subsequent Student–Newman–Keuls post hoc tests. Differences between mean responses comparing two groups were determined by t-test. Values of P<0.05 (two-tailed test) were regarded as statistically significant.
Results
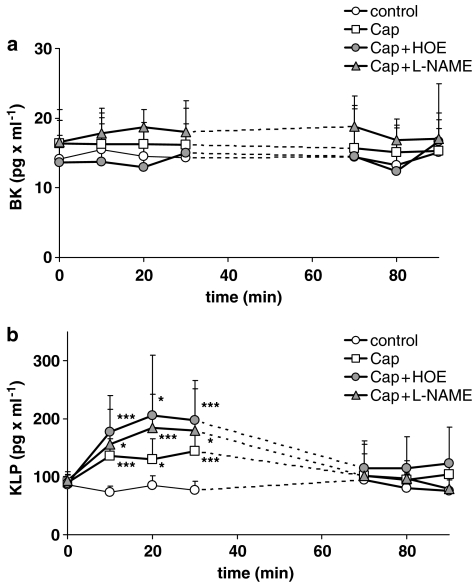
In the control group, the mean bradykinin level released into the effluent of the isolated rat Langendorff hearts before 30 min ischaemia was 14.6±6.57 pg ml−1 (mean s.d. of individual values). Before the 30-min no-flow ischaemia period, the bradykinin levels did not significantly change after treatment with captopril, captopril+HOE, and captopril+L-NAME. Even after 30 min ischaemia, the bradykinin levels in the effluent of the control group remained constant (14.3±3.15 pg ml−1) and did not display statistically significant differences vs the other groups (Figure 2a).
Figure 2.
(a) Bradykinin level (BK) in the coronary effluent and (b) kallidin-like peptide level (KLP) in the coronary effluent of the control group (open circles), of the captopril group (open squares), of the captopril+HOE group (Cap+HOE) (grey circles), and of the captopril+L-NAME group (Cap+L-NAME) (grey triangles) before and after 30 min ischaemia. Owing to different duration of reperfusion arrhythmia after the end of the 30 min ischaemia, the collection of the samples was stared 10 min later. Mean values with s.e.m., n=10, *P<0.05, **P<0.01, ***P<0.005 vs control group.
At the beginning of the experiment (time 0), the mean kallidin-like peptide levels in the effluent of all groups were 89.8±15.9 pg ml−1 and thus approximately six-fold higher than the bradykinin levels. The kallidin-like peptide levels significantly increased during the first 5-min perfusion cycle in all three groups treated with captopril. In the captopril+HOE group, the kallidin-like peptide levels were highest in the effluent during the second treatment period (206±102 pg ml−1), but did not reach statistical significance vs the captopril group (130±44 pg ml−1) (Figure 2b). In the captopril+L-NAME group, the kallidin-like peptide levels did not differ from those of the captopril group, although a significant decline in the coronary flow was observed during this period of perfusion (Table 1). After the 30-min no-flow ischaemia in the control group, the mean kallidin-like peptide concentration (83.9±25.6 pg ml−1) was neither significantly different to the level before the 30 min ischaemia nor to those of all three captopril groups.
Table 1.
Coronary flow (ml min−1) of the isolated perfused rat hearts
|
Group |
Time (min) |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 70 | 80 | 90 | |
| Control |
11.2±0.99 |
11.3±1.01 |
11.3±1.01 |
11.1±1.03 |
6.4±0.97 |
6.5±1.23 |
6.3±1.33 |
| Captopril |
11.3±1.14 |
12.1±1.55 |
11.9±1.63 |
11.4±1.53 |
8.51±1.92** |
8.21±1.73* |
7.79±1.52* |
| Captopril+HOE140 |
10.5±1.01 |
12.0±1.89 |
11.9±1.68 |
11.7±1.65 |
7.5±1.96 |
7.75±1.69 |
7.60±1.07* |
| Captopril+KALab |
11.0±0.82 |
10.6±1.11 |
10.3±0.96 |
10.1±0.85 |
6.38±1.25 |
6.38±0.48 |
6.38±0.95 |
| Captopril+L-NAME |
11.0±1.32 |
10.2±1.48* |
8.94±1.38** |
8.11±1.52*** |
6.91±1.11 |
6.31±1.25 |
6.06±1.25 |
|
L-NAME |
10.7±0.58 |
11.0±1.0 |
8.67±1.53** |
7.83±1.26*** |
6.33±0.58 |
6.33±0.58 |
6.33±0.58 |
| Kallidin |
10.1±0.37 |
11.5±0.89 |
11.4±0.49 |
10.9±0.71 |
7.65±0.97*** |
7.18±1.21*** |
7.07±1.25*** |
| Bradykinin | 10.3±1.06 | 10.7±1.31 | 10.7±1.32 | 10.5±1.30 | 4.95±1.11 | 5.22±0.80 | 5.89±0.69 |
P<0.05;
P<0.005;
P<0.0005 vs control group.
The maintenance of the coronary flow was influenced by NO. The NO synthase inhibitor, L-NAME, significantly reduced the coronary flow when administered in the second and third perfusion cycle before the 30 min ischaemia (Table 1). Kinins seem to be of little, if any, importance for the acute effect of NO, as in the presence of captopril neither the kinin B2-receptor antagonist HOE140 nor the kallidin antiserum caused any change in coronary flow before the 30-min no-flow ischaemia. The perfusion of the isolated heart with kallidin and bradykinin, respectively, did not cause any acute change in coronary flow. Ten minutes after 30 min ischaemia in the captopril group and in the kallidin group, but not in the bradykinin group, the coronary flow was significantly higher, compared to all other groups. This postischaemic effect is mediated by NO, as it was completely abrogated in the presence of NO synthase inhibitor, L-NAME (captopril+L-NAME group).
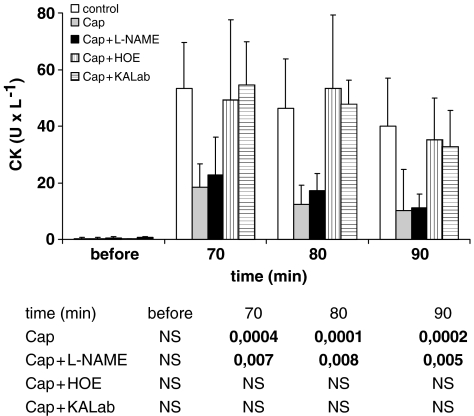
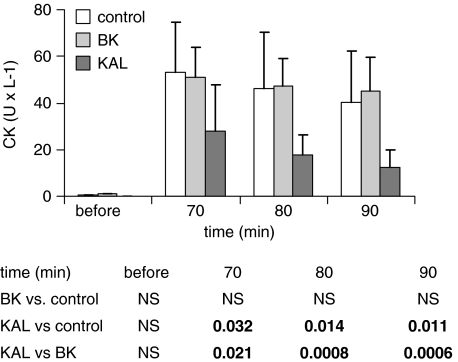
Before the 30-min no-flow ischaemia in all groups, the CK activity is within the range of 0.2–0.6 U l−1. Ten minutes after the 30 min ischaemia in the control group, the CK levels in the perfusate increased to 53.4±16.1 U l−1. The CK levels in the captopril group were significantly lower (18.5±8.26 U min−1 at t: 70 min) (Figure 3). This beneficial effect was not affected by the NO synthase inhibitor, L-NAME. The low CK activity in the captopril group was completely abolished if captopril was applied to the medium together with the kinin B2-receptor antagonist HOE140 and with the kallidin antiserum before the 30 min ischaemia. Perfusion of the isolated hearts with kallidin before the 30-min no-flow ischaemia yielded a highly significant reduction in CK release 10 min after the start of reperfusion (27.5±19.8 U l−1). In contrast, we could not obtain a similar effect with an equal concentration of bradykinin. In the bradykinin group, the CK activity did not show any significant difference vs the control group (Figure 4).
Figure 3.
CK activity in the coronary effluent of control group (white columns), of the captopril group (Cap) (light grey columns), of the captopril+L-NAME group (Cap+L-NAME) (black columns), of the captopril+HOE group (Cap+HOE) (striated columns), and of the captopril+kallidin antiserum (Cap+KALab) group (fasciated column), before and after 30 min ischaemia (upper panel). Mean values with s.e.m. The lower panel displays statistical significant differences (P-values) vs the control group. NS, nonsignificant.
Figure 4.
CK activity in the coronary effluent of control group (white columns), of the bradykinin group (BK) (light grey columns), and of the kallidin group (KAL) (black columns), before and after 30 min ischaemia (upper panel). Mean values with s.e.m. The lower panel displays statistical significant differences. NS, nonsignificant.
The LVDP before the 30-min no-flow ischaemia period was not changed in any of the groups. After the 30-min no-flow ischaemia, captopril- and captopril+L-NAME-treated hearts showed a significant improvement in LVDP. The beneficial effect of captopril was completely abrogated by the kinin B2-receptor antagonist, HOE140, as well as by the kallidin antiserum. LVDP was also significantly improved after kallidin but not after bradykinin administration (Table 2).
Table 2.
LVDP (mmHg) of the isolated perfused rat hearts
|
Group |
Time (min) |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 70 | 80 | 90 | |
| Control |
85.2±14.5 |
86.8±16.5 |
85.8±15.5 |
84.5±16.5 |
33.1±14.6 |
44.3±16.4 |
51.4±15.1 |
| Captopril |
78.1±11.6 |
80.0±11.0 |
80.0±12.3 |
76.5±11.6 |
56.6±15.8*** |
69.3±15.1** |
68.5±17.1* |
| Captopril+HOE140 |
84.6±9.8 |
89.5±10.5 |
85.5±10.8 |
82.8±10.3 |
42.0±23.3 |
53.4±20.0 |
60.7±24.7 |
| Captopril+ KALab |
84.0±8.45 |
81.8±9.6 |
85.3±6.50 |
84.5±7.05 |
55.5±17.7 |
45.3±11.9 |
57.7±14.7 |
| Captopril+L-NAME |
87.7±10.9 |
82.6±14.9 |
72.0±22.1 |
68.3±19.6 |
65.8±10.3*** |
72.0±7.2** |
74.7±3.9** |
|
L-NAME |
86.3±8.1 |
77.7±17.8 |
69.0±15.6 |
— |
— |
56.3±8.1 |
66.0±7.8 |
| Kallidin |
86,4±5.6 |
90.2±6.8 |
89.7±10.7 |
88.8±12.3 |
58.3±5.56*** |
70.1±8.92** |
70.0±10.3** |
| Bradykinin | 84.7±9.3 | 85.3±9.76 | 86.9±14.3 | 90.7±10.2 | 29.7±12.0 | 48.4±13.4 | 50.1±13.0 |
P<0.05;
P<0.005;
P<0.0005 vs control group.
No significant differences between the groups could be found before the 30 min ischaemia. Thereafter, the LVEDP was significantly lower following captopril treatment with or without L-NAME and after kallidin treatment. This reduction in postischaemic LVEDP was completely abrogated when captopril was administered together with HOE140 or the specific kallidin antiserum (Table 3).
Table 3.
LVEDP (mmHg) of the isolated perfused rat hearts
|
Group |
Time (min) |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 70 | 80 | 90 | |
| Control |
1.10±1.60 |
1.20±1.87 |
1.20±1.87 |
1.00±2.00 |
56.8±12.2 |
43.9±10.2 |
40.6±10.6 |
| Captopril |
2.08±2.39 |
1.67±2.50 |
1.67±2.75 |
1.50±3.00 |
32.1±8.58*** |
22.2±6.93** |
19.7±5.73*** |
| Captopril+HOE |
2.50±3.38 |
2.50±3.07 |
2.63±3.02 |
2.38±3.16 |
53.8±16.6 |
36.3±14.4 |
29.9±11.4 |
| Captopril+KALab |
1.25±2.06 |
1.50±2.38 |
1.25±1.89 |
1.50±1.73 |
48.8±17.3 |
37.3±8.66 |
32.0±10.2 |
| Captopril+L-NAME |
1.29±2.14 |
1.29±1.98 |
1.30±1.41 |
1.30±1.71 |
37.6±13.1** |
27.7±7.95** |
24.9±8.11** |
|
L-NAME |
0.67±1.15 |
0.33±1.53 |
1.33±1.53 |
1.00±2.65 |
54.0±8.89 |
30.7±2.89 |
25.7±5.51* |
| Kallidin |
1.80±0.84 |
1.40±0.55 |
1.60±0.55 |
1.51±0.84 |
30.2±5.81*** |
20.4±8.20** |
19.4±7.73** |
| Bradykinin | 1.43±2.37 | 1.57±2.51 | 1.14±2.27 | 1.0±2.58 | 62.6±12.3 | 46.0±17.2 | 43.6±17.3 |
P<0.05 vs control;
P<0.005 vs control group;
P<0.0001 vs control.
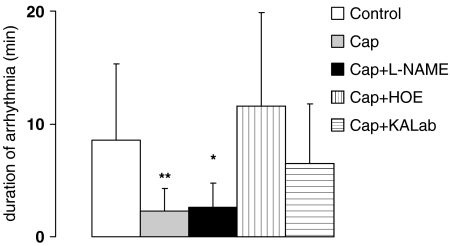
After the 30-min no-flow ischaemia during the early reperfusion period, all hearts developed tachycardia or fibrillation. The duration of arrhythmia is lowest after treatment with captopril in the presence and absence of L-NAME (Figure 5). This effect was completely abolished by the kinin B2-receptor antagonist and the kallidin antiserum.
Figure 5.
Duration of arrhythmia (min) after postischaemic reperfusion of control group (white columns), of captopril group (Cap) (light grey columns), of captopril+L-NAME group (Cap+L-NAME) (dark grey columns), of captopril+HOE group (Cap+HOE) (vertically stripped columns), and of captopril+kallidin antiserum group (Cap+KALab) (horizontally stripped). Mean values with s.e.m., *P<0.05, **P<0.01 vs control group.
Discussion
The release of kinins from the heart following myocardial ischaemia was first discussed in connection with angina pectoris and myocardial pain (Sicuteri et al., 1967). A significant increase in bradykinin release following occlusion of the left anterior descending coronary artery suggested its contribution to the generation of cardiac pain following ischaemia (Kimura et al., 1973). These data were confirmed in dogs by using a highly sensitive radioimmunoassay for kinins (Matsuki et al., 1987). Recently, we have demonstrated that kallidin-like peptide (Arg1-kallidin) is released into the coronary effluent of the isolated perfused rat heart (Liu et al., 2005). This opened the alternative of the role of kallidin-like peptide rather than bradykinin in the cardioprotection of ischaemic preconditioning.
ACE inhibitors prolonged the survival of patients after cardiac infarction (Pfeffer et al., 1992). Despite a recent report excluding the role of kinins in mediating cardioprotection of ACE inhibitors (Campbell et al., 1999), there is mounting evidence that at least part of the beneficial effect of the ACE inhibition can be attributed to the bradykinin accumulation (Baumgarten et al., 1993). In this context, it is generally accepted that ACE inhibitors are able to reduce the degradation of both bradykinin and kallidin. In addition to ACE, neutral endopeptidase (NEP) is supposed to be important in the degradation of bradykinin in the heart (Kokkonen et al., 1999; Raut et al., 1999).
The actual study was designed in order to mimic the cardioprotective effect obtained by ischaemic preconditioning (Liu et al., 2005). Instead of three 5-min ischaemic cycles, three 5-min treatment cycles with captopril were performed (pharmacological preconditioning) before the 30 min no-flow ischaemia. In this study, we clearly show that the ACE inhibitor, captopril, does not change the bradykinin levels in the effluent of the heart. These findings are in agreement with the recent data of Kokkonen et al. (1999), Raut et al. (1999), and Campbell et al. (1999), in human atrial tissue, showing that ACE plays only a minor role in the metabolism of cardiac bradykinin.
The possibility that kallidin rather than bradykinin might be the endogenous cardioprotective peptide stimulated by ACE inhibitors has never been considered. One can assume that human kallidin and rat kallidin-like peptide are equivalent regarding their degradation by ACE (Hilgenfeldt et al., 2005). The data of Liu et al. (2005) suggest that during ischaemic preconditioning of the isolated perfused rat heart, kallidin-like peptide is generated. In the effluent of the perfused rat heart, treatment with the ACE inhibitor significantly increases the levels of kallidin-like peptide but not the levels of bradykinin. This contradicts the data of Campbell et al. (1999), who found no change in cardiac kallidin levels induced by ACE inhibitor in human atrial tissue. There is an obvious lack of reliable and specific kallidin data in humans, which are needed to underline its physiological importance. This obvious controversy in the kinin levels may be linked to the methodological differences in the analytical methods used (Hilgenfeldt et al., 2005).
The cardioprotective effect of captopril was estimated by the CK activity, measured in the coronary effluent after the 30 min ischaemic period. After the 30-min no-flow ischaemia, the CK levels were significantly lower in the effluent of the captopril-treated hearts. The kinin B2-receptor antagonist, HOE140, as well as the specific kallidin antiserum were able to completely abolish this effect. Kallidin but not bradykinin pretreatment caused an effect similar to captopril pretreatment. In this context, it is interesting to note that the cardioprotective effect of kallidin might be far more effective than that of bradykinin, which can be assumed by the significant difference in the CK levels after 30-min no-flow ischaemia and pretreatment of kallidin and bradykinin, respectively, as shown in Figure 4. Moreover, the combined treatment with both captopril and the kinin B2-receptor antagonist, HOE140, did not change the bradykinin level, but caused a further increase in the kallidin-like peptide levels, suggesting a receptor displacement. Thus, in the isolated rat heart kallidin-like peptide is supposed to be the major physiological substrate of ACE and mediates the cardioprotective effect of ACE inhibitors. These data also suggest that bradykinin may be a secondary product most likely generated from kallidin, possibly by aminopeptidase M (Ward et al., 1991).
The inhibition of angiotensin II formation may be of minor importance for the cardioprotective effect of ACE inhibitor as in accordance with other reports (Parratt, 1994 and Wiemer et al., 1994) the ischaemic reperfusion injury can be blocked by kinin receptor antagonists but not by AT1-receptor blocker, losartan. Recently, in a myocardial infarction model, a cardioprotective effect with the combined ACE/NEP inhibitor was shown (Rastegar et al., 2000), which was abrogated by the kinin B2-receptor antagonist HOE140 (Ebrahim et al., 2004).
Kinins are potential stimulators of NO release from the endothelium either directly or via prostaglandins (Dreyer et al., 1991). Although NO has been pointed as a trigger in delayed preconditioning, its role in early phase of ischaemic preconditioning is controversial. The role of NO in ischaemic preconditioning and myocardial ischaemia–reperfusion injury has recently been reviewed in detail by Ferdinandy & Schulz (2003). The kinin action was reduced or blocked by inhibiting the synthesis of prostaglandins and NO (Massoudy et al., 1995; Liu et al., 1996). Our data contradict these findings and are in agreement with the findings of Goto et al. (1995). We found no significant differences in CK activity after no-flow ischaemia in the captopril group in the presence and absence of NO synthase inhibitor, L-NAME. NO is involved in the maintenance of the coronary flow as the coronary flow was significantly reduced in the presence of L-NAME before the 30-min no-flow ischaemia. In the captopril group, NO is improving the coronary flow after no-flow ischaemia, indicating a kinin-independent action of NO before the 30 min ischaemia on coronary flow and a kinin-dependent action after ischaemia. Thus, kallidin is stimulating NO synthase, which is responsible for the coronary flow. However, NO seems not to be involved in the cardioprotective mechanism of kallidin.
A further indication of the low importance of NO in cardioprotection is shown in the data of LVDP and LVEDP. Following 30-min no-flow ischaemia, a significant improvement in LVDP is abolished by the kinin B2-receptor antagonist and by the kallidin antiserum, but not by L-NAME. This finding confirms previous reports (Jin & Chen, 2000). Moreover, it seems that the inhibition of NO synthase by L-NAME is increasing the improvement of LVDP and LVEDP after captopril treatment. Additionally, pretreatment with L-NAME alone before the 30-min no-flow ischaemia caused a significant improvement in LVEDP 30 min after reperfusion.
In summary, our data indicate that in the isolated perfused rat heart, captopril mediates its cardioprotective effects by inhibiting the degradation of a kallidin-like peptide. The effect is predominantly linked to the kinin B2 receptor, and can be abolished by the specific kallidin antiserum and the kinin B2-receptor antagonist HOE140. Direct application of bradykinin and kallidin, respectively, supports a dominant cardioprotective role of kallidin. NO is responsible for the maintenance of the coronary flow, but does not account for major cardioprotective effects as judged by a reduction in the postischaemic CK release and the improvement in LVDP and LVEDP.
Acknowledgments
This work was supported by a grant of the Deutsche Forschungsgemeinschaft Hi 308/5-1, and by a grant of the Ketterer Foundation. The paper has partly been published as a dissertation of Xiuxin Liu in the Medical Faculty of the Ruprecht-Karls University, Heidelberg, Germany.
Abbreviations
- ACE
angiotensin-converting enzyme
- CK
creatine kinase
- LVDP
left ventricular developed pressure
- LVEDP
left ventricular end-diastolic pressure
- NEP
neutral endopeptidase
- NO
nitric oxide
References
- BAUMGARTEN C.R., LINZ W., KUNKEL G., SCHÖLKENS B.A., WIEMER G. Ramiprilat increases bradykinin outflow from isolated hearts of rat. Br. J. Pharmacol. 1993;108:293–295. doi: 10.1111/j.1476-5381.1993.tb12797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAXTER G.F., EBRAHIM Z. Role of bradykinin in preconditioning and protection of the ischaemic myocardium. Br. J. Pharmacol. 2002;135:843–854. doi: 10.1038/sj.bjp.0704548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHOOLA K.D., FIGUEROA C.D., WORTHY K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol. Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- CAMPBELL D.J., DUNCAN A.M, KLADIS A. Angiotensin-converting enzyme inhibition modifies angiotensin but not kallidin peptide levels in human atrial tissue. Hypertension. 1999;34:171–175. doi: 10.1161/01.hyp.34.2.171. [DOI] [PubMed] [Google Scholar]
- DREYER W.J., MICHAEL L.M., WEST S.M., SMITH C.W., ROTHLEIN R., ROSEN R.D., ANDERSON D.C., ENTMAN M.L. Neutrophil accumulation in ischaemic canine myocardium: insight into time course, distribution, and mechanism of localization during early perfusion. Circulation. 1991;84:400–411. doi: 10.1161/01.cir.84.1.400. [DOI] [PubMed] [Google Scholar]
- EBRAHIM Z., BAXTER G.F., YELLON D.M. Omapatrilat limits infarct size and lowers the threshold for induction of myocardial preconditioning through a bradykinin receptor-mediated mechanism. Cardiovasc. Drug Ther. 2004;18:127–134. doi: 10.1023/B:CARD.0000029030.49492.5a. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., SCHULZ R. Nitricoxide, superoxide, and peroxynitrite in myocardial ischaemia–reperfusion injury and preconditioning. Br. J. Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTO M., LIU Y., YANG X.M., ARDELL J.L., COHEN M.V., DOWNEY J.M. Role of bradykinin in protection of ischaemic preconditioning in rabbit hearts. Circulation Res. 1995;77:611–621. doi: 10.1161/01.res.77.3.611. [DOI] [PubMed] [Google Scholar]
- HILGENFELDT U., LINKE R., RIESTER U., KÖNIG W., BREIPOHL G. Strategy of measuring bradykinin and kallidin and their concentration in plasma and urine. Anal. Biochem. 1995;228:35–41. doi: 10.1006/abio.1995.1311. [DOI] [PubMed] [Google Scholar]
- HILGENFELDT U., PUSCHNER T., RIESTER U., FINSTERLE J., HILGENFELDT J., RITZ E. Low-salt downregulates plasma but not tissue kallikrein–kinin system. Am. J. Physiol. 1998;44:F88–F93. doi: 10.1152/ajprenal.1998.275.1.F88. [DOI] [PubMed] [Google Scholar]
- HILGENFELDT U., STANNEK C., LUKASOVA M., SCHNÖLZER M., LEWICKA S. Rat tissue kallikrein releases a kallidin-like peptide from rat low-molecular-weight kininogen. Br. J. Pharmacol. 2005;146:958–963. doi: 10.1038/sj.bjp.0706409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN Z.Q., CHEN X. Pretreatment with ramiprilat induces cardioprotection against free radical injury in guinea-pig isolated heart: involvement of bradykinin, protein kinase C and prostaglandins. Clin. Exp. Pharmacol. Physiol. 2000;27:257–262. doi: 10.1046/j.1440-1681.2000.03233.x. [DOI] [PubMed] [Google Scholar]
- KIMURA E., HASHIMOTO K., FURUKAWA S., HAYAKAWA H. Changes in bradykinin levels in coronary sinus blood after the experimental occlusion of a coronary artery. Am. Heart J. 1973;85:635–647. doi: 10.1016/0002-8703(73)90169-5. [DOI] [PubMed] [Google Scholar]
- KOKKONEN J.O., KUOPPALA A., SAARINEN J., LINDSTEDT K.A., KOVANEN P.T. Kallidin and bradykinin-degrading pathways in human heart: degradation of kallidin by aminopeptidase M-like activity and bradykinin by neutral endopeptidase. Circulation. 1999;99:1984–1990. doi: 10.1161/01.cir.99.15.1984. [DOI] [PubMed] [Google Scholar]
- LINZ W., WIEMER G., SCHÖLKENS B. The role of kinins in the pathophysiology of myocardial ischaemia: In vivo and in vitro studies. Diabetes. 1996;45 (Suppl 1):S51–S58. doi: 10.2337/diab.45.1.s51. [DOI] [PubMed] [Google Scholar]
- LINZ W., WIEMER G., SCHÖLKENS B.Beneficial effect of bradykinin on myocardial energy metabolism and infarct size Am. J. Cardiol. 199780118A–123A.3A [DOI] [PubMed] [Google Scholar]
- LIU X., LUKASOVA M., ZUBAKOVA R., LEWICKA S., HILGENFELDT U. A kallidin-like peptide is a protective cardiac kinin, released by ischaemic preconditioning of rat heart. Br. J. Pharmacol. 2005;146:952–957. doi: 10.1038/sj.bjp.0706402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y.H., YANG X.P., SHAROV V.G., SIGMON D.H., SABBATH H.N., CARRETERO O.A. Paracrine systems in the cardioprotective effect of angiotensin-converting enzyme inhibitors on myocardial ischaemia/reperfusion injury in rats. Hypertension. 1996;27:7–13. doi: 10.1161/01.hyp.27.1.7. [DOI] [PubMed] [Google Scholar]
- MASSOUDY P., BECKER B.F., GERLACH E. Nitric oxide accounts for postischaemic protection resulting from angiotensin-converting enzyme inhibition: indirect evidence for a radical scavenger effect in isolated guinea pig heart. J. Cardiovasc. Pharmacol. 1995;25:440–447. doi: 10.1097/00005344-199503000-00014. [DOI] [PubMed] [Google Scholar]
- MATSUKI T., SHOJI T., YOSHIDA S., KUDOH Y., MOTOE M., INOUE M., NAKATA T., HOSODA S., SHIMAMOTO K., YELLON D., IMURA O. Sympathetically induced myocardial ischaemia causes the heart to release plasma kinin. Circulation Res. 1987;21:428–432. doi: 10.1093/cvr/21.6.428. [DOI] [PubMed] [Google Scholar]
- NAGAOKA M., YAYAMA K., TAKANO M., OKAMOTO H. Expression of kininogen genes in rat cardiomyocytes. Immunopharmacology. 1999;44:81–85. doi: 10.1016/s0162-3109(99)00113-7. [DOI] [PubMed] [Google Scholar]
- NOLLY H., CARBINI L.A., SCICLI G., CARRETERO O.A., SCICLI A.G. A local kallikrein–kinin system is present in rat hearts. Hypertension. 1994;23:919–923. doi: 10.1161/01.hyp.23.6.919. [DOI] [PubMed] [Google Scholar]
- PARRATT J.R. Cardioprotection by angiotensin converting enzyme inhibitors: the experimental evidence. Cardiovasc. Res. 1994;28:183–189. doi: 10.1093/cvr/28.2.183. [DOI] [PubMed] [Google Scholar]
- PFEFFER M.A., BRAUNWALD E., MOY L.A., BASTA L., BROWN E.J., CUDDY T.E., DAVIS B.R., GELTMAN E.M., GOLDMAN S., FLAKER G.C., KLEIN M., LAMAS G.A., PARKER M., ROULEAU J., ROULEAU J.L., RUTHERFORD J.R., WERTHEIMER J.H., HAWKINS C.M. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement study. N. Engl. J. Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- RASTEGAR M.A., MARCHINI F., MORAZZONI G., V(GH A., PAPP J.G., PARRATT J.R. The effect of Z13752A, a combined ACE/NEP inhibitor, on responses to coronary artery occlusion; a primary protective role for bradykinin. Br. J. Pharmacol. 2000;129:671–680. doi: 10.1038/sj.bjp.0703109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAUT R., ROULEAU J.L., BLAIS C.J.r., GOSSELIN H., MOLINARO G., SIROIS M.G., LEPAGE Y., CRINE P., ADAM A. Bradykinin metabolism in the postinfarcted rat heart: role of ACE and neutral endopeptidase 24.11. Am. J. Physiol. 1999;276:H1769–H1779. doi: 10.1152/ajpheart.1999.276.5.H1769. [DOI] [PubMed] [Google Scholar]
- SATO M., ENGELMANN R.M., OTANI H., MAULIK N., FLACK J.E., DEATON D.W., DAS D.K. Myocardial protection by preconditioning of heart with lorsatan, an angiotensin II type 1-receptor blocker: implication of bradykinin-dependent and bradykinin-independent mechanisms. Circulation. 2000;19 (Suppl 3):III346–III351. doi: 10.1161/01.cir.102.suppl_3.iii-346. [DOI] [PubMed] [Google Scholar]
- SICUTERI F., FRANCHI G., DELBIANCO P.L., DELBENE E. A contribution to the interpretation of shock and pain in myocardial infarction. Mallattie Cardiovasc. 1967;8:343–362. [PubMed] [Google Scholar]
- TAKANO M., KONDO J., YAYAMA K., OTANI M., SATO K., OKAMOTO H. Molecular cloning of cDNAs for mouse low-molecular-weight and high-molecular weight prekininogen. Biochim. Biophys. Acta. 1997;1352:222–230. doi: 10.1016/s0167-4781(97)00018-3. [DOI] [PubMed] [Google Scholar]
- VARIN R., MULDER P., TAMION F., RICHARD V., HENRY J.P., LALLEMAND F., LEREBOURS G., THUILLEZ C. Improvement of endothelial function by chronic angiotensin-converting enzyme inhibition in heart failure. Role of nitric oxide, prostanoids, oxidant stress, and bradykinin. Circulation. 2000;102:351–356. doi: 10.1161/01.cir.102.3.351. [DOI] [PubMed] [Google Scholar]
- WARD P.E., CHOW W.A., DRAPEAU G. Metabolism of bradykinin agonists and antagonists by plasma aminopeptidase P. Biochem. Pharmacol. 1991;25:721–727. doi: 10.1016/0006-2952(91)90028-4. [DOI] [PubMed] [Google Scholar]
- WIEMER G., SCHOELKENS B.A., LINZ W. Endothelial protection by converting enzyme inhibitors. Cardiovasc. Res. 1994;28:166–172. doi: 10.1093/cvr/28.2.166. [DOI] [PubMed] [Google Scholar]
- XIONG W., CHEN L.M., WOODLEY-MILLER C., SIMSON J.A., CHAO J. Identification, purification, and localization of tissue kallikrein in rat heart. Biochem. J. 1990;267:639–646. doi: 10.1042/bj2670639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAHLER S., KUPATT C., BECKER B.F. ACE-inhibition attenuates cardiac cell damage and preserves release of NO in the postischaemic heart. Immunopharmacology. 1999;44:27–33. doi: 10.1016/s0162-3109(99)00108-3. [DOI] [PubMed] [Google Scholar]
- ZHANG X., RECCHIA F.A., BERNSTEIN R., XU X., NASJLETTI A., HINTZE T.H. Kinin-mediated coronary nitric oxide production contributes to the therapeutic action of angiotensin-converting enzyme and neutral endopeptidase inhibitors and amlodipine in the treatment in heart failure. J. Pharmacol. Exp. Therap. 1999;288:742–751. [PubMed] [Google Scholar]