Abstract
Lysophosphatidylcholine (LPC) modulates the inflammatory response and reduces mortality in animal models of sepsis. Here, we investigate the effects of LPC from synthetic (sLPC) and natural, soy bean derived LPC, (nLPC) sources on the organ injury/dysfunction caused by systemic administration of lipopolysaccharide (LPS) or peptidoglycan (PepG) and lipoteichoic acid (LTA).
Rats were subjected to (i) endotoxaemia (LPS 6 mg kg–1 i.v.) and treated with sLPC (1–100 mg kg−1), (ii) endotoxaemia and treated with nLPC (10 mg kg−1) or (iii) Gram-positive shock (PepG 10 mg kg–1 and LTA 3 mg kg–1 i.v.) and treated with sLPC (10 mg kg−1).
Endotoxaemia or Gram-positive shock for 6 h resulted in increases in serum makers of renal dysfunction and liver, pancreatic and neuromuscular injury.
Administration of sLPC, at 1 or 2 h after LPS, dose dependently (1–10 mg kg−1) reduced the organ injury/dysfunction. High doses of sLPC (30 and 100 mg kg−1) were shown to be detrimental in endotoxaemia. sLPC also afforded protection against the organ injury/dysfunction caused by Gram-positive shock. nLPC was found to be protective in endotoxaemic animals.
The beneficial effects of sLPC were associated with an attenuation in circulating levels of interleukin-1β (IL-1β).
In conclusion, LPC dose and time dependently reduces the organ injury and circulating IL-1β levels caused by Gram-negative or Gram-positive shock in the rat. Thus, we speculate that appropriate doses of LPC may be useful in reducing the degree of organ injury and dysfunction associated with shock of various aetiologies.
Keywords: Endotoxin, lipoteichoic acid, lysophosphatidylcholine, LPC, LPS, oxidised lipoprotein, peptidoglycan, sepsis, shock
Introduction
Lysophosphatidylcholine (LPC) is the major component of oxidised low-density lipoprotein and plays a role in the pathophysiology of atherosclerosis (Kume et al., 1992; Sugiyama et al., 1994; Rikitake et al., 2002). LPC is produced by the hydrolysis of phosphatidylcholine, mainly by the action of phospholipase A2. LPC has been shown to elicit many immunomodulatory functions. For instance, LPC (i) enhances the chemotaxis of macrophages, monocytes and T lymphocytes, (ii) activates macrophages and (iii) upregulates the expression of adhesion molecules (Kume et al., 1992; Yun et al., 2004). LPC is reportedly recognised by the G protein-coupled receptors, G2A (from G2 accumulation) (Kabarowski et al., 2001) and GPR199 (Soga et al., 2005).
Notably, LPC has also been shown to reduce tissue factor release induced by lipopolysaccharide (LPS) from monocytes (Engelmann et al., 1999) and to upregulate eNOS expression (Zembowicz et al., 1995), and either of these effects may be of benefit in conditions associated with organ injury and dysfunction associated with shock. Yan et al. (2004) have reported that treatment of mice with LPC reduces mortality in animal models of septic shock caused by either caecal ligation and puncture or intraperitoneal (i.p.) injection of Escherichia coli. This protective effect was attributed to the ability of LPC to (i) increase bacterial clearance, (ii) reduce neutrophil deactivation and (iii) reduce the levels of TNF-α and interleukin-1β (IL-1β). Chen et al. (2005) have recently reported that LPC reduces circulating high-mobility group box 1 (HMGB1) levels. In patients with sepsis, circulating levels of LPC have been shown to be reduced and to inversely correlate with patient outcome (Drobnik et al., 2003). Although LPC has been shown to be protective in animal models of sepsis (Yan et al., 2004; Chen et al., 2005), the effect of LPC in a model of multiple organ injury caused by wall fragments of Gram-positive bacteria has not been investigated. This is of particular clinical importance, as Gram-positive organisms account for over 50% of reported cases of sepsis in the US between 1979 and 2000 (Martin et al., 2003). Although Gram-positive bacteria do not contain endotoxin, there is good evidence that wall fragments of Gram-positive bacteria (and particularly Staphylococcus aureus) cause systemic inflammation, shock and multiple organ failure. Systemic administration of peptidoglycan (PepG) from S. aureus causes inflammation and liver injury in the rat (Wang et al., 2004), and PepG and lipoteichoic acid (LTA) from S. aureus synergise to cause shock, systemic inflammation and multiple organ failure (De Kimpe et al., 1995; Kengatharan et al., 1998). Thus, coadministration of LTA and PepG reproduces key features of the pathophysiology of Gram-positive shock.
This study was designed to establish if synthetic LPC (sLPC) affects the (i) haemodynamic alterations, (ii) renal dysfunction, (iii) liver injury, (iv) pancreatic injury, (v) neuromuscular injury and (vi) alteration in plasma IL-1β and IL-6 levels caused by systemic administration of either LPS (endotoxin, from the Gram-negative bacterium E. coli) or, importantly, by coadministration of PepG and LTA (from the Gram-positive bacterium S. aureus), in the anaesthetised rat.
Commercially available LPC is either (i) synthetically produced or (ii) produced by enzymatic action on naturally occurring sources of purified phosphatidylcholine and therefore might play a role on drug effectiveness. Thus, in an addition, we have compared the effects of sLPC with those of a soy bean derived, natural occurring LPC (natural, soy bean derived LPC (nLPC)) in a rat model of endotoxic shock.
Methods
Surgical procedure and quantification of organ injury and dysfunction
This study was carried out on 154 male Wistar rats (Charles River, Kent, U.K.) weighing 240–340 g, receiving a standard diet and water ad libitum. The investigation was performed in accordance with the Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act 1986, published by HMSO, London. All animals were anaesthetised with thiopentone sodium (Intraval®, 120 mg kg−1, i.p.), and anaesthesia was maintained by supplementary injections of thiopentone sodium (approximately 1–2 mg kg−1 h−1 i.v.) as required. The general surgical procedures were performed as previously described (Millar & Thiemermann, 2002). Upon completion of the surgical procedure, mean arterial pressure (MAP) and heart rate (HR) were allowed to stabilise for 20 min. At 6 h after administration of LPS, 1.5 ml of blood was collected into a serum gel S/1.3 tube (Sarstedt, Germany) from a catheter placed in the carotid artery, and serum values for indices of renal dysfunction, liver injury, pancreatic injury and neuromuscular injury were examined.
Experimental design
Study Ia: sLPC dose and time response in a model of Gram-negative shock
Animals were assigned to eight experimental groups. A dose of 10 mg kg−1 has previously been shown to protect against mortality in a mouse model of caecal ligation and puncture (Yan et al., 2004) and was therefore the included in this study; and in the dose–response studies we tested doses ranging from 1 to 10 mg kg−1:
Sham-Control. Rats were treated with 2% BSA in PBS (vehicle for sLPC, 1 ml kg−1 i.v.) and vehicle for LPS (1 ml kg−1 saline i.v.), but did not receive LPS (n=9).
Sham-sLPC. Rats were treated with sLPC (10 mg kg−1 i.v.) and vehicle for LPS (1 ml kg−1 saline i.v., n=9).
LPS-Control. Rats received E. coli LPS (6 mg kg−1 i.v. over 10 min) and 1 h later received the vehicle for sLPC (1 ml kg−1 of 2% BSA in PBS, n=12).
sLPC 1 mg kg−1 1 h post-LPS (sLPC 1 mg kg−1 1 h). Rats received E. coli LPS (6 mg kg−1 i.v. over 10 min) and 1 h later received sLPC (1 mg kg−1 i.v., n=5).
sLPC 3 mg kg−1 1 h post-LPS (sLPC 3 mg kg−1 1 h). Rats received E. coli LPS (6 mg kg−1 i.v. over 10 min) and 1 h later received sLPC (3 mg kg−1 i.v., n=5).
sLPC 10 mg kg−1 1 h post-LPS (sLPC 10 mg kg−1 1 h). Rats received E. coli LPS (6 mg kg−1 i.v. over 10 min) and 1 h later received sLPC (10 mg kg−1 i.v., n=10).
sLPC 10 mg kg−1 2 h post-LPS (sLPC 10 mg kg−1 2 h). Rats received E. coli LPS (6 mg kg−1 i.v. over 10 min) and 1 h later received sLPC (10 mg kg−1 i.v., n=6).
sLPC 10 mg kg−1 4 h post-LPS (sLPC 10 mg kg−1 4 h). Rats received E. coli LPS (6 mg kg−1 i.v. over 10 min) and 1 h later received sLPC (10 mg kg−1 i.v., n=4).
Study Ib: high-dose LPC in a model of Gram-negative shock
A separate study was designed to investigate the effects of higher doses of sLPC (30 or 100 mg kg−1) 1 h after LPS administration. As these larger amounts of sLPC were difficult to dissolve in the amount of vehicle used in Study Ia, we have carried out an additional study in which all groups of animals received 2 ml kg−1 of 2% BSA in PBS. Thus, the following five groups of animals were studied:
Sham-Control. Rats were treated with 2% BSA in PBS (vehicle for sLPC, 2 ml kg−1 i.v.) and vehicle for LPS (1 ml kg−1 saline i.v.), but did not receive LPS (n=4).
LPS-Control. Rats received E. coli LPS (6 mg kg−1 i.v. over 10 min) and 1 h later received the vehicle for sLPC (2 ml kg−1 of 2% BSA in PBS, n=8).
sLPC 10 mg kg−1 1 h post-LPS (sLPC 10 mg kg−1 1 h). Rats received E. coli LPS (6 mg kg−1 i.v. over 10 min) and 1 h later received sLPC (10 mg kg−1 i.v., n=6).
sLPC 30 mg kg−1 1 h post-LPS (sLPC 30 mg kg−1 1 h). Rats received E. coli LPS (6 mg kg−1 i.v. over 10 min) and 1 h later received sLPC (30 mg kg−1 i.v., n=6).
sLPC 100 mg kg−1 1 h post-LPS (sLPC 100 mg kg−1 1 h). Rats received E. coli LPS (6 mg kg−1 i.v. over 10 min) and 1 h later received sLPC (100 mg kg−1 i.v., n=3).
Study II: nLPC in a model of Gram-negative shock
A dose of 10 mg kg−1 nLPC 1 h after LPS administration was chosen based on the results of Study Ia. Four experimental groups were included:
Sham-Control. Rats were treated with 2% BSA in PBS (vehicle for nLPC, 1 ml kg−1 i.v.) and vehicle for LPS (1 ml kg−1 saline i.v.), but did not receive LPS (n=7).
Sham-nLPC. Rats were treated with nLPC (10 mg kg−1 i.v.) and vehicle for LPS (1 ml kg−1 saline i.v, n=6).
LPS-Control. Rats received E. coli LPS (6 mg kg−1 i.v. over 10 min) and 1 h later received the vehicle for nLPC (1 ml kg−1 of 2% BSA in PBS, n=10).
nLPC 10 mg kg−1 1 h post-LPS (nLPC 10 mg kg−1 1 h). Rats received E. coli LPS (6 mg kg−1 i.v. over 10 min) and 1 h later received nLPC (10 mg kg−1 i.v., n=7).
Study III: sLPC in a Gram-positive shock model
Animals were assigned to four experimental groups. The time and dose regimen of 10 mg kg−1 sLPC 1 h was chosen based on the results of Study Ia:
Sham-Control. Rats were treated with 2% BSA in PBS (vehicle for sLPC, 1 ml kg−1 i.v.) and vehicle for LPS (1 ml kg−1 saline i.v.), but did not receive LPS (n=10).
Sham-LPC. Rats were treated with sLPC (10 mg kg−1 i.v.) and vehicle for PepG/LTA (1 ml kg−1 saline i.v, n=7).
PepG/LTA-Control. Rats received PepG/LTA (10 mg kg−1 PepG and 3 mg kg−1 LTA i.v. over 10 min) and 1 h later received the vehicle for sLPC (1 ml kg−1 of 2% BSA in PBS, n=10).
sLPC 10 mg kg−1 1 h post PepG/LTA (sLPC 10 mg kg−1 1 h). Rats received PepG/LTA (10 mg kg−1 PepG and 3 mg kg−1 LTA i.v. over 10 min) and 1 h later received sLPC (10 mg kg−1 i.v., n=10).
Measurements of IL-1β and IL-6 plasma levels
Carotid blood was drawn 6 h after administration of LPS or coadministration of PepG/LTA to heparinised tubes, centrifuged at 6000 × g and plasma was collected. A Quantikine® Rat Immunoassay from R&D Systems (Minneapolis, MN, U.S.A.) was performed to determine the serum levels of IL-1β and IL-6 following the manufactures protocol. Samples were analysed using a microplate reader set to 450 nm and corrected at a wavelength of 540 nm.
Purification of PepG
PepG was isolated from S. aureus as previously described (Foster, 1992). Covalently attached proteins were removed by treatment with pronase at 2 mg ml−1 for 1 h at 60°C. Anionic polymers were removed from the PepG by the treatment of purified cell walls (10 mg (dry weight) ml−1) with hydrofluoric acid (48%, v v−1) for 24 h at 4°C. The insoluble PepG was then washed by centrifugation (14,000 × g, 5 min) and resuspension once in 100 ml of Tris-HCl (pH 8.0) and five times in distilled water until the pH was neutral. The PepG was then recovered by centrifugation as described above and resuspended in saline (0.9%, w v−1) before sterilisation by autoclaving and storage at −20°C. PepG extract was subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis with no evidence of any protein whatsoever. PepG was also enzymatically digested, and it gave the expected reversed-phase high-pressure liquid chromatography muropeptide profile with no spurious products.
Materials
sLPC (L-A-LPC stearoyl, C18:0), nLPC (L-A-LPC from soy beans), E. coli LPS (serotype 0127:B8), LTA from S. aureus and BSA were obtained from Sigma-Aldrich Company Ltd (Dorset, U.K.). Thiopentone sodium (Intraval Sodium®) was purchased from Rhône Mérieux Ltd (Essex, U.K.). Nonpyrogenic saline (0.9% NaCl) was acquired from Baxter Healthcare Ltd (Norfolk, U.K.). PBS was attained from Invitrogen (Paisley, U.K.). LPC suspensions were prepared in PBS containing 2% BSA and ultrasonnicated for 45 s (15 bursts of 3 s).
Statistical evaluation
All data are presented as mean±s.e.m. of n observations, where n represents the number of animals or blood samples studied. For repeated haemodynamic measurements a two-way analysis of variance (ANOVA) was performed followed by Bonferroni post-test. Data without repeated measurements were analysed by one-way ANOVA, followed by a Dunnett's post hoc test for multiple comparisons. A P-value of less than 0.05 was considered statistically significant.
Results
Study I: sLPC does not attenuate the circulatory failure associated with endotoxaemia
Prior to LPS administration, mean baseline values for MAP and HR were similar in all experimental groups (P>0.05, Table 1). Administration of LPS (6 mg kg−1) resulted in a significant, biphasic fall in MAP when compared to Sham-Controls (P<0.05). Therapeutic administration of sLPC did not affect the fall in MAP caused by LPS at any time point (P>0.05). Endotoxaemia for 6 h resulted in a significant increase in HR compared to Sham-Controls (P<0.05). This tachycardia was not affected by sLPC (P>0.05, Table 1). Similarly, in Study Ib, therapeutic administration of sLPC (10 or 30 mg kg−1 1 h) did not affect the hypotension or tachycardia associated with endotoxaemia (P>0.05, data not shown). Rats, which had received the highest dose of sLPC studied (100 mg kg−1) died within 5 min of the administration of sLPC and, hence, no haemodynamic data were collected (n=3).
Table 1.
Study Ia: alterations in (a) MAP and (b) HR
| (a) | |||||||
|---|---|---|---|---|---|---|---|
|
Group |
Mean MAP (mmHg)± s.e.m. |
||||||
| Baseline | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | |
| Sham-Control |
119±5 |
114±3a |
113±3 |
111±5 |
105±5 |
102±4 |
99±3a |
| Sham sLPC |
131±3 |
119±3a |
122±5 |
113±3 |
105±4 |
103±3 |
101±3a |
| LPS-Control |
126±3 |
98±2b |
108±2 |
108±2 |
99±4 |
89±3 |
79±5b |
| sLPC 1 mg kg−1 1 h |
125±5 |
87±5b |
101±4 |
105±4 |
95±3 |
88±5 |
80±6b |
| sLPC 3 mg kg−1 1 h |
126±2 |
91±6b |
109±4 |
111±5 |
102±4 |
96±3 |
79±4b |
| sLPC 10 mg kg−1 1 h |
128±5 |
96±4b |
108±4 |
108±4 |
101±4 |
93±4 |
81±4b |
| sLPC 10 mg kg−1 2 h |
123±6 |
93±4b |
105±2 |
104±4 |
99±5 |
91±4 |
80±4b |
| sLPC 10 mg kg−1 4 h |
132±4 |
87±5b |
100±4 |
102±5 |
92±6 |
89±7 |
79±4b |
| (b) | |||||||
|---|---|---|---|---|---|---|---|
|
Group |
Mean HR (b.p.m.)± s.e.m. |
||||||
| Baseline | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | |
| Sham-Control |
419±13 |
398±18a |
414±16a |
417±16a |
408±12a |
405±17a |
404±12a |
| Sham sLPC |
414±10 |
415±11a |
424±11a |
425±11a |
419±9a |
427±7a |
414±11a |
| LPS-Control |
416±9 |
453±9b |
466±10b |
477±9b |
475±8b |
485±8b |
471±10b |
| sLPC 1 mg kg−1 1 h |
410±10 |
429±7 |
478±11b |
472±8b |
480±9b |
479±16b |
471±19b |
| sLPC 3 mg kg−1 1 h |
423±11 |
447±11 |
476±10b |
469±10b |
468±16b |
474±15b |
477±12b |
| sLPC 10 mg kg−1 1 h |
414±6 |
437±9 |
452±18 |
473±14b |
485±10b |
487±11b |
484±12b |
| sLPC 10 mg kg−1 2 h |
400±7 |
436±11 |
465±14b |
465±18b |
498±16b |
488±20b |
479±15b |
| sLPC 10 mg kg−1 4 h | 415±4 | 419±13 | 456±10 | 485±9b | 462±8b | 475±10b | 479±14b |
P<0.05 when compared to LPS-Control.
P<0.05 when compared to Sham-Control by ANOVA followed by Bonferroni post-test.
Study I: sLPC dose and time dependently attenuates the organ injury and dysfunction caused by LPS
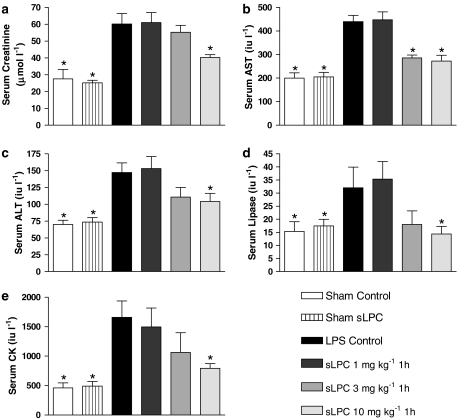
When compared to sham-operated controls, endotoxaemia for 6 h resulted in significant increases in serum levels of creatinine (renal dysfunction), aspartate transaminase (AST) and alanine transaminase (ALT) (liver injury), lipase (pancreatic injury) and creatine kinase (CK) (neuromuscular injury) (P<0.05) (Figure 1).
Figure 1.
Alterations in the serum levels of (a) creatinine, (b) AST, (c) ALT, (d) lipase and (e) CK in rats subjected to the surgical procedure alone or subjected to endotoxaemia. Animals were treated with various sLPC doses (1, 3 or 10 mg kg−1) 1 h after LPS administration. *P<0.05 when compared with LPS-Control by ANOVA followed by Dunnett's post hoc test.
Treatment of animals with sLPC (10 mg kg−1) at 1 h after the administration of LPS attenuated the LPS-induced rise in the serum levels of creatinine, AST, ALT, lipase and CK (P<0.05) (Figure 1). Administration of a lower dose of sLPC (3 mg kg−1) attenuated the rise in the serum levels of AST (P<0.05, Figure 1b), but not any of the other parameters of organ injury/dysfunction measured. When compared to LPS-Controls (P>0.05), a low dose of sLPC (1 mg kg−1) did not significantly alter the serum levels of any of the markers of organ injury/dysfunction measured.
Administration of sLPC (10 mg kg−1) 2 h after LPS also attenuated the organ injury caused by LPS, although the observed effects were less pronounced than those seen with administration of sLPC 1 h after LPS. Specifically, in rats treated with sLPC at 2 h after LPS, the serum levels of AST and CK were significantly lower than in the LPS-Control group (P<0.05, Table 2). All of the observed beneficial effects of sLPC (10 mg kg−1) were lost when this dose of sLPC was given at 4 h after injection of LPS (P>0.05, Table 2).
Table 2.
Study Ia: the organ protective effects of sLPC in endotoxaemia are dependent on the time of sLPC administration
|
Group |
Organ injury or dysfunction parameter (mean±s.e.m.) |
||||
|---|---|---|---|---|---|
| Creatinine (μmol l−1) | AST (IU l−1) | ALT (IU l−1) | Lipase (IU l−1) | CK (IU l−1) | |
| Sham-Control |
28±6* |
199±23* |
70±6* |
15±3* |
462±86* |
| LPS-Control |
60±6 |
440±26 |
147±15 |
32±6 |
1659±277 |
| sLPC 10 mg kg−1 1 h |
40±2* |
272±25* |
104±12* |
14±3* |
794±80* |
| sLPC 10 mg kg−1 2 h |
52±10 |
312±29* |
114±7 |
18±3 |
882±168* |
| sLPC 10 mg kg−1 4 h | 63±4 | 366±51 | 132±29 | 31±5 | 1108±183 |
P<0.05 compared to LPS-Control by ANOVA followed by Dunnett's post hoc test.
Administration of sLPC (30 mg kg−1) 1 h after administration of LPS did not attenuate the endotoxin-induced rise in serum levels of creatinine, AST, ALT, lipase and CK (Table 3). Interestingly, the serum ALT levels were significantly higher in animals treated with 30 mg kg−1 of sLPC than in the respective LPS-Control animals (P<0.05). Rats, which had received the highest dose of sLPC studied (100 mg kg−1) died within 5 min of the administration of sLPC and, hence, no plasma samples for the determination of organ injury were collected (n=3).
Table 3.
Study Ib: high-dose sLPC is not organ protective in endotoxaemia
|
Group |
Organ injury or dysfunction parameter (mean±s.e.m.) |
||||
|---|---|---|---|---|---|
| Creatinine (μmol l−1) | AST (IU l−1) | ALT (IU l−1) | Lipase (IU l−1) | CK (IU l−1) | |
| Sham-Control |
20±1* |
183±32* |
57±7* |
13±2* |
294±59* |
| LPS-Control |
65±6 |
471±67 |
174±22 |
42±6 |
1519±201 |
| sLPC 10 mg kg−1 1 h |
41±6* |
253±31* |
100±10* |
16±4* |
703±139* |
| sLPC 30 mg kg−1 1 h | 58±8 | 527±79 | 246±24* | 40±9 | 1272±326 |
P<0.05 compared to LPS-Control by ANOVA followed by Dunnett's post hoc test.
Study II: nLPC does not attenuate the circulatory failure associated with endotoxaemia
Before LPS administration, baseline MAP and HR were similar in all experimental groups, ranging from 129±4 to 122±5 mmHg and 418±12 and 398±11 beats per minute (b.p.m.), respectively (P>0.05, data not shown). Similarly to Study Ia, administration of LPS caused a significant, biphasic fall in MAP and increase in HR when compared to Sham-Controls (P<0.05). Specifically, 6 h after LPS administration, MAP and HR were 78±3 mmHg and 490±15 b.p.m. compared to 105±4 mmHg and 410 b.p.m., respectively, in sham-operated controls. Therapeutic administration of nLPC did not affect the fall in MAP or rise in HR caused by LPS at any time point (P>0.05, data not shown).
Study II: nLPC attenuates the organ injury and dysfunction caused by LPS
When compared to sham-operated controls, endotoxaemia for 6 h resulted in significant increases in serum levels of creatinine (renal dysfunction), AST and ALT (liver injury), lipase (pancreatic injury) and CK (neuromuscular injury) (P<0.05, Table 4).
Table 4.
Study II: nLPC protects against organ injury and dysfunction associated with endotoxaemia
|
Group |
Organ injury or dysfunction parameter (mean±s.e.m.) |
||||
|---|---|---|---|---|---|
| Creatinine (μmol l−1) | AST (IU l−1) | ALT (IU l−1) | Lipase (IU l−1) | CK (IU l−1) | |
| Sham-Control |
29±6* |
135±27* |
69±10* |
15±3* |
457±76* |
| Sham nLPC |
23±2* |
115±18* |
71±16* |
11±2* |
420±38* |
| LPS-Control |
65±6 |
439±26 |
147±14 |
32±8 |
1659±276 |
| nLPC 10 mg kg−1 1 h | 45±3* | 216±14* | 84±8* | 14±6* | 550±71* |
P<0.05 compared to LPS-Control by ANOVA followed by Dunnett's post hoc test.
The LPS-induced increase in serum parameters of organ injury and dysfunction were significantly attenuated by the administration of 10 mg kg−1 nLPC (Table 4). Specifically, nLPC attenuated the increases in serum levels of creatinine, AST, ALT, lipase and CK when compared to animals receiving LPS alone (P<0.05).
Study III: sLPC does not attenuate the circulatory failure associated with Gram-positive shock
At baseline (i.e. before administration of PepG/LTA), MAP and HR were similar in all experimental groups, ranging from 130±4 to 125±7 mmHg and 410±7 and 397±5 b.p.m, respectively (P>0.05). Six hours following administration of PepG/LTA, animals exhibited a significant (P<0.05) fall in MAP and increase in HR (84±1 mmHg and 471±11 b.p.m, respectively) compared to sham-operated animals (106± 2 mmHg and 399±6 b.p.m, respectively). sLPC did not attenuate the hypotension or tachycardia associated with the administration of Gram-positive cell wall fragments at anytime point (P>0.05, data not shown).
Study III: sLPC attenuates the organ injury and dysfunction caused by Gram-positive shock
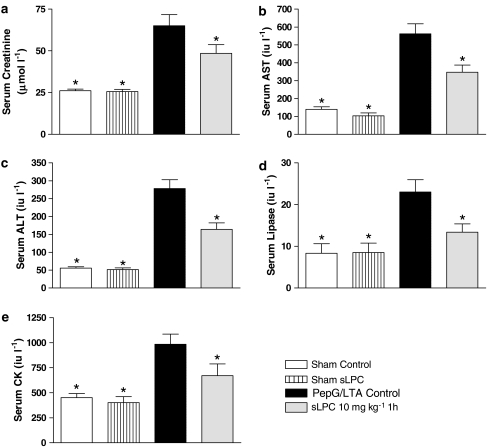
When compared to animals receiving saline alone, administration of PepG/LTA caused a significant increase in serum markers of organ injury and dysfunction. Specifically, PepG/LTA caused an increase in the circulating levels of creatinine, indicative of renal dysfunction, AST and ALT, indicative of liver injury, lipase, indicating pancreatic injury and, CK, indicating neuromuscular injury (P<0.05, Figure 2).
Figure 2.
Alterations in the serum levels of (a) creatinine, (b) AST, (c) ALT, (d) lipase and (e) CK in rats subjected to the surgical procedure alone or subjected to Gram-positive shock. Animals were treated with sLPC 10 mg kg−1 1 h after PepG/LTA administration. *P<0.05 when compared with PepG/LTA-Control by ANOVA followed by Dunnett's post hoc test.
Administration of sLPC (10 mg kg−1) 1 h after LPS attenuated the rises in the serum levels of creatinine, AST, ALT, lipase and CK caused by coadministration of PepG/LTA, compared to animals administered with PepG/LTA and treated with sLPC vehicle alone (P<0.05, Figure 2).
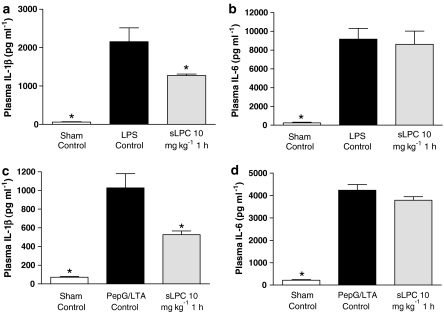
sLPC attenuates the increase in plasma IL-1β caused by LPS or PepG/LTA
Endotoxaemia for 6 h resulted in significant rises in the plasma levels of IL-1β and IL-6 (P<0.05, Figure 3a and b). Administration of sLPC (10 mg kg−1) 1 after LPS significantly reduced the rise in the plasma levels of IL-1β (P<0.05), but not in IL-6, when compared to LPS-Control. Similarly, coadministration of PepG/LTA resulted in significant rises in the plasma levels of IL-1β and IL-6 (P<0.05, Figure 3c and d) compared to Sham-operated animals. Administration of sLPC (10 mg kg−1) at 1 h after PepG/LTA significantly reduced the rise in the plasma levels of IL-1β, but not IL-6, when compared to the PepG/LTA-Control (P<0.05).
Figure 3.
Alterations in plasma concentrations of IL-1β and IL-6 in rats subjected to endotoxaemia (a and b) or Gram-positive shock (c and d, respectively) and treated with sLPC (10 mg kg−1 1 h) or vehicle. *P<0.05 when compared with control by ANOVA followed by Dunnett's post hoc test.
Discussion
Here, we demonstrate that the systemic administration of wall fragments from Gram-negative (endotoxin) or Gram-positive (PepG/LTA) bacteria results in substantial increases in the serum concentrations of creatinine, indicating the development of acute renal dysfunction. Administration of either LPS or PepG/LTA also caused increases in the serum levels of the transaminases AST and ALT, indicating hepatocellular injury, and increased serum levels of lipase and CK, indicative of pancreatic and neuromuscular injury, respectively. Furthermore, development of circulatory failure (hypotension and tachycardia) was observed, indicative of a systemic inflammatory response.
LPC is a potent immunomodulator in vitro, which has recently been reported to reduce mortality in septic mice (Yan et al., 2004). Here, we demonstrate for the first time that treatment with sLPC reduces the multiple organ injury and dysfunction caused by endotoxaemia in the rat. Notably, the protective effects of LPC were observed after a therapeutic administration regime. When given 1 h after the onset of endotoxaemia, sLPC (10 mg kg−1) reduced the renal dysfunction and the hepatic, pancreatic, and neuromuscular injury. This attenuation was dependent on the dose given. While 1 mg kg−1 had no effect on organ injury/dysfunction, 3 mg kg−1 caused a small beneficial effect (serum AST levels), while 10 mg kg−1 of sLPC reduced the renal dysfunction and the liver, pancreatic and neuromuscular injury caused by LPS. Even when administered as late as 2 h after LPS, sLPC was still able to reduce the liver and neuromuscular injury caused by LPS. This may well be of clinical importance, as it has been suggested that many therapeutic approaches for septic shock have failed in clinical trials, as in experimental settings they have been given prophylactically (before the onset of shock) rather than therapeutically (after the onset of shock and inflammation). Thus, our findings, that LPC given after the induction of acute severe endotoxaemia protects the organs against the associated injury and dysfunction, suggests a broader window for the therapeutic use of LPC against the systemic inflammatory response.
We also show for the first time that the source of the LPC does not affect its ability to attenuate the organ injury associated with endotoxaemia. This may be important, as natural phosphatidylcholine derivatives (i.e. from soy bean and egg yolk) have been used conventionally in therapeutic phospholipid emulsions (Chono et al., 2005). However, we have shown that sLPC, that is not from a natural source, markedly attenuated the multiple organ injury and dysfunction caused by acute severe endotoxaemia in the rat. Thus, sLPC or nLPC may be of therapeutic value in phospholipid rich therapeutics, especially in conditions involving systemic or local inflammation.
The most novel finding of this study is our discovery that the therapeutic administration of sLPC also attenuated the organ injury and dysfunction in a model of Gram-positive shock (caused by coadministration of PepG/LTA). Specifically, a 10 mg kg−1 dose 1 h after LPS administration resulted in a reduction in the serum markers of organ injury/dysfunction associated with administration of PepG/LTA. This is of particular significance, as Gram-positive organisms account for over 50% of reported sepsis cases in the US between 1979 and 2000 (Martin et al., 2003). Furthermore, as the diagnosis of the causative agent(s) causing septic shock requires time and is often inconclusive; and in many cases septic shock is caused by mixed bacterial infections. Thus, the search for potential therapies, which are effective in Gram-negative as well as Gram-positive shock are of high priority. Here, we demonstrate that sLPC protects against the organ injury/dysfunction caused by wall-fragments of Gram-negative (LPS) and Gram-positive bacteria (PepG/LTA).
Interestingly, the fall in blood pressure caused by either LPS or PepG/LTA was not prevented by LPC. This is not entirely surprising, as many agents which do not affect the fall in blood pressure in these models do reduce organ injury (Collin et al., 2005), while others which do attenuate the fall in blood pressure may not (Wray et al., 1998). Thus, there is no strict correlation between haemodynamic effects and outcome in this model. Furthermore, we have recently reported that the co-administration of LPS and PepG, which does not cause a fall in MAP does cause a significant degree of organ injury/dysfunction as well as systemic inflammation (Dugo et al., 2005). Also, there is clinical evidence that an improvement in blood pressure alone does not necessarily prevent organ dysfunction and injury. For instance, raising MAP from 65 to 85 mmHg in septic patients using fluid and norepinephrine does not improve the impairment in either metabolic variables (oxygen delivery, oxygen consumption or arterial lactate) or renal function (urine flow, serum creatinine and creatinine clearance) (Bourgoin et al., 2005). Similarly, LeDoux et al. (2000) have shown that increasing MAP from 65 to 85 mmHg with norepinephrine does not significantly affect systemic oxygen metabolism, skin microcirculatory blood flow, urine output or splanchnic perfusion in patients with septic shock. Thus, we believe that the observed beneficial effects of LPC are not due to a haemodynamic effect.
What then is the mechanism(s) by which LPC attenuates the inflammatory response and consequently the multiple organ injury and dysfunction? It is well established that the production of proinflammatory cytokines is greatly enhanced by LPS. We, therefore, investigated whether LPC affects the plasma levels of proinflammatory cytokines IL-1β and IL-6. We found that both severe endotoxaemia and Gram-positive shock resulted in significant increases in the levels of these proinflammatory mediators in the plasma. We report that the increased production of IL-1β in both models was reduced by the therapeutic treatment with sLPC. This is in accordance with previous studies, in which LPC reduced the increased circulating levels of TNF-α and IL-1β caused by caecal ligation and puncture in the mouse (Yan et al., 2004). However, establishing if sLPC directly reduces circulating levels of IL-1β, or if this reduction is a consequence of other inflammatory effects remains to be established. Interestingly, sLPC did not affect the rise in the plasma levels of IL-6 caused by administration of either endotoxaemia or Gram-positive shock, thus indicting that sLPC is not acting by reducing the inflammatory response as a whole. Although LPC does reduce the mortality caused by caecal ligation and puncture, this was, similarly, not associated with a reduction in the circulating levels of IL-6 (Yan et al., 2004).
Could LPC block LPS receptor activation? Interestingly, components of oxidised low density lipoprotein have been reported to attenuate the LPS-induced expression of proinflammatory genes by inhibiting the binding of LPS to cell surface receptor complex on monocytes and endothelial cells, thus preventing the inflammatory response (Walton et al., 2003). In our model of severe endotoxaemia, however, maximal serum concentrations of TNF-α (and therefore the initial inflammatory response to LPS) are observed between 60 and 90 min after LPS administration (McDonald et al., 2003). Notably, here we show that sLPC is protective when given 1 h after the administration of LPS. Thus, the beneficial effects of LPC are unlikely to be solely due to the reduced binding of LPS to the cell-surface receptor complex, suggesting that LPC may possess several different protective mechanisms downstream of LPS-receptor stimulation. This hypothesis is further supported by our novel observation that therapeutic administration of sLPC is also protective in a model of Gram-positive shock, in which LPS is not directly involved.
A diverse range of other protective mechanisms have been suggested using experimental models of inflammation, including increased bacterial killing by neutrophils and increased i.p. bacterial clearance in vitro (Yan et al., 2004), decreased circulating levels of HMGB1 in septic mice (Chen et al., 2005), reduced expression of tissue factor by monocytes/macrophages (Engelmann et al., 1999) and increased activation of eNOS by upregulating transcription and/or decreasing mRNA degradation (Zembowicz et al., 1995; Cieslik et al., 1998). Further investigation of the protective mechanism(s) of LPC is warranted.
Limitations of the study: we have carried out a full dose–response curve to gain a better understanding of the effects and potential side effects of sLPC. When doing this, we discovered that a higher dose of sLPC (30 mg kg−1) was not able to reduce the organ injury/dysfunction caused by LPS, and even significantly increased the liver injury (increase in serum levels of ALT) caused by LPS. Most notably, three rats treated with an even higher dose of sLPC (100 mg kg−1) died within 5 min of administration of sLPC. The mechanism(s) underlying these detrimental effect(s) of sLPC are not clear. Nevertheless, these findings clearly indicate that higher doses of sLPC cause adverse effects and also that the therapeutic window for the use of sLPC in vivo is fairly narrow.
In conclusion, we report that LPC dose and time dependently attenuates the multiple organ injury and dysfunction caused by either LPS (model of Gram-negative shock) or PepG and LTA (model of Gram-positive shock). This beneficial effect of LPC is not dependent on the source of LPC, as the (beneficial) effects of both synthetic and natural LPC (from soybean) are very similar. Although the therapeutic window for sLPC was narrow (in LPS-shock), we speculate that appropriate doses of LPC may be useful in reducing the degree of organ injury and dysfunction associated with shock of various aetiologies.
Acknowledgments
This study was supported by grants provided by the Medical Research Council, William Harvey Research Foundation, U.K. and Helsingin Sanomat Centennial Foundation, Finland.
Abbreviations
- ALT
alanine transaminase
- AST
aspartate transaminase
- CK
creatine kinase
- HMGB1
high-mobility group box 1
- HR
heart rate
- IL
interleukin
- LPC
lysophosphatidylcholine
- LPS
lipopolysaccharide, endotoxin
- LTA
lipoteichoic acid
- MAP
mean arterial pressure
- nLPC
natural, soy bean derived LPC
- PepG
peptidoglycan
- sLPC
synthetic LPC
References
- BOURGOIN A., LEONE M., DELMAS A., GARNIER F., ALBANESE J., MARTIN C. Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit. Care Med. 2005;33:780–786. doi: 10.1097/01.ccm.0000157788.20591.23. [DOI] [PubMed] [Google Scholar]
- CHEN G., LI J., QIANG X., CZURA C.J., OCHANI M., OCHANI K., ULLOA L., YANG H., TRACEY K.J., WANG P., SAMA A.E., WANG H. Suppression of HMGB1 release by stearoyl lysophosphatidylcholine: an additional mechanism for its therapeutic effects in experimental sepsis. J. Lipid Res. 2005;46:623–627. doi: 10.1194/jlr.C400018-JLR200. [DOI] [PubMed] [Google Scholar]
- CHONO S., TAUCHI Y., DEGUCHI Y., MORIMOTO K. Efficient drug delivery to atherosclerotic lesions and the antiatherosclerotic effect by dexamethasone incorporated into liposomes in atherogenic mice. J. Drug Target. 2005;13:267–276. doi: 10.1080/10611860500159030. [DOI] [PubMed] [Google Scholar]
- CIESLIK K., ZEMBOWICZ A., TANG J.L., WU K.K. Transcriptional regulation of endothelial nitric-oxide synthase by lysophosphatidylcholine. J. Biol. Chem. 1998;273:14885–14890. doi: 10.1074/jbc.273.24.14885. [DOI] [PubMed] [Google Scholar]
- COLLIN M., ANUAR F.B., MURCH O., BHATIA M., MOORE P.K., THIEMERMANN C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br. J Pharmacol. 2005;146:498–505. doi: 10.1038/sj.bjp.0706367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE KIMPE S.J., KENGATHARAN M., THIEMERMANN C., VANE J.R. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DROBNIK W., LIEBISCH G., AUDEBERT F.X., FROHLICH D., GLUCK T., VOGEL P., ROTHE G., SCHMITZ G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J. Lipid Res. 2003;44:754–761. doi: 10.1194/jlr.M200401-JLR200. [DOI] [PubMed] [Google Scholar]
- DUGO L., COLLIN M., ALLEN D.A., PATEL N.S., BAUER I., MERVAALA E.M., LOUHELAINEN M., FOSTER S.J., YAQOOB M.M., THIEMERMANN C. GSK-3beta inhibitors attenuate the organ injury/dysfunction caused by endotoxemia in the rat. Crit. Care Med. 2005;33:1903–1912. doi: 10.1097/01.ccm.0000178350.21839.44. [DOI] [PubMed] [Google Scholar]
- ENGELMANN B., ZIESENISS S., BRAND K., PAGE S., LENTSCHAT A., ULMER A.J., GERLACH E. Tissue factor expression of human monocytes is suppressed by lysophosphatidylcholine. Arterioscler. Thromb. Vasc. Biol. 1999;19:47–53. doi: 10.1161/01.atv.19.1.47. [DOI] [PubMed] [Google Scholar]
- FOSTER S.J. Analysis of the autolysins of Bacillus subtilis 168 during vegetative growth and differentiation by using renaturing polyacrylamide gel electrophoresis. J. Bacteriol. 1992;174:464–470. doi: 10.1128/jb.174.2.464-470.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KABAROWSKI J.H., ZHU K., LE L.Q., WITTE O.N., XU Y. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science. 2001;293:702–705. doi: 10.1126/science.1061781. [DOI] [PubMed] [Google Scholar]
- KENGATHARAN K.M., DE K.S., ROBSON C., FOSTER S.J., THIEMERMANN C. Mechanism of Gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J. Exp. Med. 1998;188:305–315. doi: 10.1084/jem.188.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUME N., CYBULSKY M.I., GIMBRONE M.A., JR Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J. Clin. Invest. 1992;90:1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDOUX D., ASTIZ M.E., CARPATI C.M., RACKOW E.C. Effects of perfusion pressure on tissue perfusion in septic shock. Crit. Care Med. 2000;28:2729–2732. doi: 10.1097/00003246-200008000-00007. [DOI] [PubMed] [Google Scholar]
- MARTIN G.S., MANNINO D.M., EATON S., MOSS M. The Epidemiology of Sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- MCDONALD M.C., DHADLY P., COCKERILL G.W., CUZZOCREA S., MOTA-FILIPE H., HINDS C.J., MILLER N.E., THIEMERMANN C. Reconstituted high-density lipoprotein attenuates organ injury and adhesion molecule expression in a rodent model of endotoxic shock. Shock. 2003;20:551–557. doi: 10.1097/01.shk.0000097249.97298.a3. [DOI] [PubMed] [Google Scholar]
- MILLAR C.G., THIEMERMANN C. Carboxy-PTIO, a scavenger of nitric oxide, selectively inhibits the increase in medullary perfusion and improves renal function in endotoxemia. Shock. 2002;18:64–68. doi: 10.1097/00024382-200207000-00012. [DOI] [PubMed] [Google Scholar]
- RIKITAKE Y., HIRATA K., YAMASHITA T., IWAI K., KOBAYASHI S., ITOH H., OZAKI M., EJIRI J., SHIOMI M., INOUE N., KAWASHIMA S., YOKOYAMA M. Expression of G2A, a receptor for lysophosphatidylcholine, by macrophages in murine, rabbit, and human atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2002;22:2049–2053. doi: 10.1161/01.atv.0000040598.18570.54. [DOI] [PubMed] [Google Scholar]
- SOGA T., OHISHI T., MATSUI T., SAITO T., MATSUMOTO M., TAKASAKI J., MATSUMOTO S.I., KAMOHARA M., HIYAMA H., YOSHIDA S. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2005;326:744–751. doi: 10.1016/j.bbrc.2004.11.120. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA S., KUGIYAMA K., OHGUSHI M., FUJIMOTO K., YASUE H. Lysophosphatidylcholine in oxidized low-density lipoprotein increases endothelial susceptibility to polymorphonuclear leukocyte-induced endothelial dysfunction in porcine coronary arteries. Role of protein kinase C. Circ. Res. 1994;74:565–575. doi: 10.1161/01.res.74.4.565. [DOI] [PubMed] [Google Scholar]
- WALTON K.A., COLE A.L., YEH M., SUBBANAGOUNDER G., KRUTZIK S.R., MODLIN R.L., LUCAS R.M., NAKAI J., SMART E.J., VORA D.K., BERLINER J.A. Specific phospholipid oxidation products inhibit ligand activation of toll-like receptors 4 and 2. Arterioscler. Thromb. Vasc. Biol. 2003;23:1197–1203. doi: 10.1161/01.ATV.0000079340.80744.B8. [DOI] [PubMed] [Google Scholar]
- WANG J.E., DAHLE M.K., YNDESTAD A., BAUER I., MCDONALD M.C., AUKRUST P., FOSTER S.J., BAUER M., AASEN A.O., THIEMERMANN C. Peptidoglycan of staphylococcus aureus causes inflammation and organ injury in the rat. Crit. Care Med. 2004;32:546–552. doi: 10.1097/01.CCM.0000109775.22138.8F. [DOI] [PubMed] [Google Scholar]
- WRAY G.M., MILLAR C.G., HINDS C.J., THIEMERMANN C. Selective inhibition of the activity of inducible nitric oxide synthase prevents the circulatory failure, but not the organ injury/dysfunction, caused by endotoxin. Shock. 1998;9:329–335. doi: 10.1097/00024382-199805000-00003. [DOI] [PubMed] [Google Scholar]
- YAN J.J., JUNG J.S., LEE J.E., LEE J., HUH S.O., KIM H.S., JUNG K.C., CHO J.Y., NAM J.S., SUH H.W., KIM Y.H., SONG D.K. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat. Med. 2004;10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- YUN M.R., OKAJIMA F., IM D.S. The action mode of lysophosphatidylcholine in human monocytes. J. Pharmacol. Sci. 2004;94:45–50. doi: 10.1254/jphs.94.45. [DOI] [PubMed] [Google Scholar]
- ZEMBOWICZ A., TANG J.L., WU K.K. Transcriptional induction of endothelial nitric oxide synthase type III by lysophosphatidylcholine. J. Biol. Chem. 1995;270:17006–17010. doi: 10.1074/jbc.270.28.17006. [DOI] [PubMed] [Google Scholar]