Abstract
In flowering plants, the vegetative nucleus and the two sperm cells are proposed to form a functional assemblage, the male germ unit (MGU). Here, we describe the developmental pathway of MGU assembly in Arabidopsis and report two classes of mutations that affect the integrity and/or the positioning of the MGU in the mature pollen grain. In germ unit malformed (gum) mutants, the vegetative nucleus is positioned adjacent to the pollen grain wall, separate from the two sperm cells, whereas in MGU displaced (mud) mutants, the intact MGU is displaced to the pollen grain wall. mud and gum mutants correspond to male-specific gametophytic mutations that also reduce pollen fitness. Genetic mapping showed that the gum1 and gum2 mutations are genetically linked, possibly allelic, whereas the mud1 and mud2 mutations correspond to two unlinked loci mapping on different chromosomes. The hierarchical relationship between mud and gum mutations was investigated by phenotypic analysis of double mutants. gum1 appeared to act earlier than mud1 and mud2, affecting initial MGU assembly and its stability during pollen maturation. In contrast, mud1 and mud2 mutations appear to act only on MGU positioning during final maturation. From in planta analyses of pollen germination in mud and gum mutants, we conclude that the initial proximity and positioning of MGU components is not required for their entrance into the pollen tube, but the efficiency of MGU translocation is reduced.
Flowering plants produce mature dehydrated pollen grains competent for rapid germination and pollen tube growth. In 70% of species, the mature pollen grain contains a small generative cell that is completely enclosed within a large vegetative cell (bicellular pollen species). After germination, the generative cell undergoes a mitotic division to form the two sperm cells necessary for double fertilization. In tricellular pollen species, the generative cell undergoes the second mitotic division prior to anthesis. In the majority of tricellular pollen species that have been examined, the two compact, but elongated, sperm cells are in direct physical association with and partially surrounded by the vegetative cell nucleus (for review, see Mogensen, 1992; Dumas et al., 1998). The associated vegetative nucleus and sperm cells are proposed to form a functional assemblage, termed the male germ unit (MGU), with a potential role in the controlled transport and delivery of the sperm cells (Dumas et al., 1984a). Moreover, the sperm cell dimorphism observed in several species suggests that the MGU is a “polarized fertilization unit” in which the sperm cells are predetermined to fuse with the egg nucleus or the two polar nuclei. Preferential fertilization has been observed in at least two species (Roman, 1947, 1948; Russell, 1985).
Since its conception, the MGU aroused renewed interest in pollen organization and fertilization, and stimulated a number of descriptive studies leading to the ultrastructural characterization and computer-assisted reconstruction of the MGU (for review, see Mogensen, 1992). In the MGU, one sperm cell is connected to the vegetative nucleus via a “tail,” or cell extension, containing forked arrays of microtubules. This tail penetrates the highly convoluted vegetative nucleus (McConchie et al., 1985), whereas the two sperm cells are enclosed within a vegetative cell membrane-bound compartment, and are linked by a transverse cell wall and evaginations of their plasma membranes (Dumas et al., 1984a, 1984b; Charzinska et al., 1989). In mature bicellular pollen systems, and in tricellular pollen of monocotyledonous species such as maize (Zea mays), the MGU is not observed in mature pollen, but assembly occurs early during pollen tube growth (Hu and Yu, 1988; Rougier et al., 1991). Despite these apparent differences in the timing of MGU assembly, the existence of MGUs in angiosperm pollen and their presumptive importance in double fertilization are now largely accepted (for review, see Dumas et al., 1998).
To date, MGU assembly has only been described during pollen germination and tube growth in bicellular pollen species (Hu and Yu, 1988; Rougier et al., 1991). The ontogeny of MGU assembly in tricellular pollen species remains largely unknown. In particular, it is not known whether associations between the three components exist early during bicellular development, from the time of sperm formation, or whether the associations are established later. Moreover, the molecular and genetic basis of MGU assembly and positioning are still unknown.
Mutational analysis is proving to be an effective approach for identifying gametophytic genes involved in regulating cell division, polarity, and cell fate during pollen development and function (Chen and McCormick, 1996; Feldmann et al., 1997; Hulskamp et al., 1997; Bonhomme et al., 1998; Howden et al., 1998; Park et al., 1998; Grini et al., 1999; Johnson and McCormick, 2001; Procissi et al., 2001). Given the stereotypical organization and central location of the MGU in mature Arabidopsis pollen grains, we used the MGU as a structural marker in a screen to identify genes controlling MGU assembly and/or intracellular architecture. Here, we describe the process of MGU assembly during pollen development in Arabidopsis. We describe the identification and characterization of two distinct classes of pollen mutants, germ unit malformed (gum) and MGU displaced (mud), that act gametophytically to affect the assembly and positioning of the MGU, respectively. We provide cytological and genetic evidence that demonstrates genetic control of MGU assembly and positioning, and we suggest that correct MGU organization is required for efficient transmission of the male gametes through pollen.
RESULTS
Isolation of mud and gum Mutants
In mature Arabidopsis pollen grains, the vegetative nucleus and associated sperm cell pair (MGU) occupy a central position in the vegetative cell cytoplasm (Owen and Makaroff, 1995). Therefore, mutations affecting pollen intracellular architecture should disrupt this stereotypical organization. Mature 4,6-diamidino-2-phenylindole (DAPI)-stained pollen from approximately 10,000 M2 individuals from an ethylmethane sulfonate (EMS)-mutagenized Arabidopsis population were screened by epifluorescence microscopy. Two classes of mutants affecting the MGU were identified, two individuals termed gum and two individuals termed mud, all of which arose in different M1 parental groups.
Mutants were backcrossed as female to the wild-type (Nossen [No-O]) and the pollen phenotype of progeny was scored. Approximately 50% of the backcrossed progeny of each mutant showed a mutant phenotype, indicating that M2 plants were heterozygous, and that mutant phenotypes were expressed in heterozygotes. Homozygous plants showing approximately 100% mutant pollen were identified and homozygosity confirmed by analysis of self- and backcross progeny (data not shown). Mutant pollen was similar in size and appearance to the wild type. Vital staining for plasma membrane integrity using fluorescein diacetate (Heslop-Harrison and Heslop-Harrison, 1970) showed that more than 96% of mature pollen grains were viable in gum and mud homozygotes (>400 spores scored for each mutants). Furthermore, no obvious aberrant sporophytic phenotypes were observed in homozygous mutants, suggesting that these mutations act specifically in the male gametophyte to affect MGU organization.
MGU Organization in Wild-Type, gum, and mud Pollen
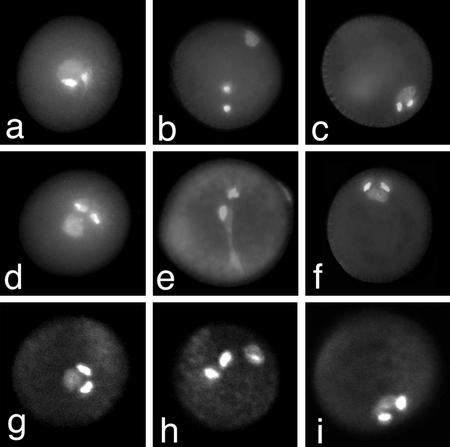
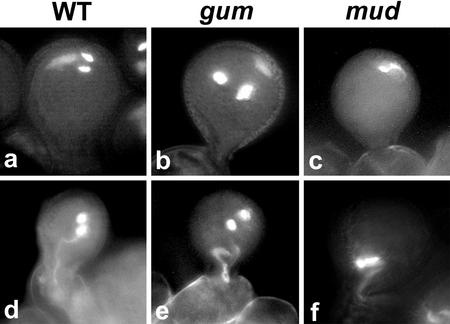
The mud1 and mud2 mutants showed a similar and striking pollen phenotype in that the associated sperm cells and vegetative nucleus were strongly displaced to periphery of the pollen grain as a single associated unit (Fig. 1, c, f, and i). The gum1 and gum2 mutants also showed a striking but distinct pollen phenotype wherein the vegetative nucleus was positioned against the pollen grain wall and was clearly separated from the sperm cell pair (Fig. 1, b and h). Optical sectioning of DAPI-stained pollen of gum1/gum1 plants by CLSM confirmed that the vegetative nucleus was always strongly displaced, adjacent to the pollen wall, whereas the sperm cells were located centrally or in the cortical cytoplasm (Fig. 1h). Despite their separation, the vegetative nucleus and the sperm cells were still sometimes observed to be physically associated via a long extension of the vegetative nucleus (Fig. 1e). CLSM analysis of homozygous mud1/mud1 and mud2/mud2 pollen confirmed that the intact MGU was always strongly displaced to the pollen wall (Fig. 1i). The MGU in both mud mutants characteristically showed a more compact structure than the wild type, with the sperm cells tightly associated with each other and with the vegetative nucleus.
Figure 1.
Pollen phenotype of wild type, gum1, and mud1. Mature pollen of wild type (a and d), gum1 (b and e), and mud1 (c and f) stained with DAPI. Optical confocal laser scanning microscopy (CLSM) sections of mature pollen from wild type (g), gum1 (h), and mud1 (i) stained with ethidium bromide.
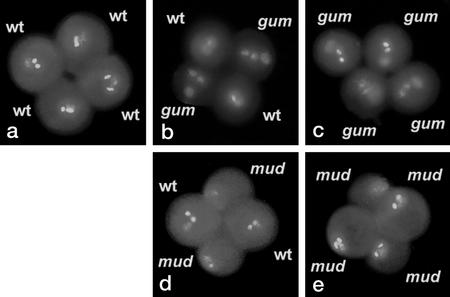
We investigated whether there was any preferential positioning of MGU components relative to the polar axis of the pollen grain (Park et al., 1998). Pollen grains were observed perpendicular to their polar axes and were divided into three transverse segments from the pole to the equator for scoring. No preferential association of MGU components with polar, intermediate, or equatorial segments was observed in homozygous gum and mud mutants (approximately 400 spores scored for each mutant). gum and mud plants were also crossed with the qrt1 mutant in which pollen is released as a tetrad with a fixed spatial orientation (Preuss et al., 1994). The position of MGU components in tetrads of qrt/qrt; gum/gum (Fig. 2c) and qrt/qrt; mud/mud (Fig. 2e) were scored (n > 200) as specified above. In a similar manner, the vegetative nucleus (in gum1 and gum2) or the intact MGU (in mud1 and mud2) appeared randomly distributed between segments along the polar axis of the pollen grain.
Figure 2.
Tetrad analysis of gum and mud mutations. Single mature tetrads of qrt/qrt; +/+ (a), qrt/qrt; +/gum1 (b), qrt/qrt; gum1/gum1 (c), qrt/qrt; +/mud1 (d), and qrt/qrt; mud1/mud1 (e).
gum and mud Are Male-Specific Gametophytic Mutations That Reduce Pollen Transmission
Wild-type plants showed less than 1% aberrant pollen with defects in the positioning or appearance of the MGU. In contrast, the proportion of aberrant pollen in +/mud or in +/gum heterozygotes was about 50%, and was greater than 90% in homozygotes (Table I), suggesting that these mutations act gametophytically and are highly penetrant. Confirmation of gametophytic expression was obtained by tetrad analysis using the qrt mutant, which revealed that two members of each tetrad showed a mutant phenotype in heterozygous +/gum1 and +/mud1 plants (Fig. 2, b and d).
Table I.
Frequency of pollen phenotypic classes in gum1, gum2, mud1, and mud2 mutants
| WT | gum | mud | Aborted | |
|---|---|---|---|---|
| Genotype |  |
 |
 |
 |
| +/+ | 98.2 | 0.4 | 0.8 | 0.6 |
| gum1/+ | 50.6 | 47.5 | 1.7 | 0.2 |
| gum1/gum1 | 4.8 | 93.8 | 1.2 | 0.2 |
| gum2/+ | 49.5 | 48.2 | 0.5 | 0.5 |
| gum2/gum2 | 5.8 | 92.2 | 1.6 | 0.4 |
| mud1/+ | 50.1 | 3 | 45.7 | 1.2 |
| mud1/mud1 | 7.6 | 1.5 | 89.5 | 1.4 |
| mud2/+ | 51.8 | 1.6 | 44.1 | 2.5 |
| mud2/mud2 | 6.6 | 1.5 | 90.1 | 1.8 |
The percentages of spores in each phenotypic classes are shown. Counts were made on heterozygous and homozygous individuals. +/+, No-O, parental ecotype; aborted, collapsed pollen with no visible nuclei. Data are derived from >1,000 spores counted from four backcross progeny.
The reduced frequency of mutants in self-progeny of gum and mud heterozygotes (Table II) suggested that their male and/or female transmission might be affected. The transmission of each mutation was determined by performing reciprocal testcrosses of heterozygous mutants to wild type. The transmission efficiency of the mutant allele describes the fraction of mutant alleles that are transmitted to the progeny (Howden et al., 1998). There was no reduction in female transmission of gum and mud mutations, whereas male transmission was reduced in all four mutants (Table II). In gum1 and gum2 heterozygotes, only approximately 63% of pollen carrying the mutant allele succeeded in fertilization to produce viable progeny. In a converse manner, approximately 37% of gum pollen and from 49% to 71% of mud pollen failed in some aspect of the progamic phase. As no seed abortion was observed in self- or outcrosses through the male, these mutations do not appear to have any obvious postfertilization effects that could account for their reduced male transmission.
Table II.
Genetic transmission analysis of gum and mud mutations
| Mutation |
+/gum × +/gum (Self)
|
+/gum × +/+ (Female Transmission)
|
+/+ × +/gum (Male Transmission)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| gum | WT | Percentage of gum | gum | WT | TEf | gum | WT | TEm | |
| gum1 | 288 | 132 | 68.6 | 214 | 223 | 95.9ns | 163 | 259 | 62.9s |
| gum2 | 381 | 196 | 66 | 189 | 205 | 92.2ns | 204 | 304 | 67.1s |
|
+/mud × +/mud
|
+/mud × +/+
|
+/+ × +/mud
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| mud | WT | Percentage of mud | mud | WT | TEf | mud | WT | TEm | |
| mud1 | 425 | 237 | 64.2 | 288 | 299 | 96.3ns | 170 | 338 | 50.3s |
| mud2 | 379 | 275 | 58 | 264 | 284 | 92.9ns | 123 | 425 | 28.9s |
Nos. of progeny producing wild-type and mutant pollen are shown together with the calculated transmission efficiency (TE, no. of mutant/no. of wild-type progeny × 100) through male (TEm) and female (TEf) gametes. Self and reciprocal crosses between heterozygous mutants and wild type are depicted with the female partner shown first. s, TE values that differed significantly (P(x2 > X) = 0.001) from the expected value of 100%. ns, TE values that did not differ significantly (P(x2 > x) = 0.001) from the expected value of 100%. WT, Wild type.
mud1, mud2, gum1, and gum2 Define at Least Three Independent Loci
The determination of allelism for gametophytic mutants is laborious and involves the generation of tetraploid trans-heterozygous mutants that produce diploid gametophytes (Kamps et al., 1996; Grossniklaus et al., 1998). However, if the gametophytic mutations are transmitted to some extent, diploid trans-heterozygous mutants can be produced and their analysis could provide an indication of how closely linked two loci are. We crossed gum1/gum1 and gum2/gum2 homozygotes. If gum1 and gum2 define the same locus or are closely linked, F1 progeny will have the genotype gum1/gum2, and the percentage of pollen grains expressing the gum phenotype should be similar to that observed in gum1/gum1 and gum2/gum2 homozygotes. However, if these mutations define two unlinked genetic loci, F1 plants will have the genotype +/gum1; +/gum2. These plants will produce four different pollen genotypes: +; gum1, ±; gum2, gum1; gum2, and ±; ±. If double-mutant (gum1; gum2) pollen are viable, the percentage of pollen expressing the gum phenotype will be approximately 75%.
Approximately 90% of pollen grains of F1 progeny showed a gum phenotype (n = 560) with no other aberrant classes. The two gum mutations are genetically linked and may be allelic or located in two genes mapping with 5 to 10 cM of each other. Similar analysis was performed for F1 populations resulting from the cross mud1/mud1 × mud2/mud2. In this case, about 75% of pollen grains showed a mud phenotype (n = 973), indicating that mud1 and mud2 define two unlinked loci. No other aberrant or aborted phenotypes were observed.
All four mud and gum mutations were independently mapped. mud1 was mapped to chromosome III, less than 2 cM north from AthGAPAb (43.6 cM). mud2 was located on chromosome II, 2 cM north of nga168 (73.7 cM), between the bacterial artificial chromosome (BAC) clones F16 M14 and T19C21. gum1 and gum2 were mapped on chromosome IV between the markers M506-SSLP (21.9 cM) and nga8 (26.6 cM). These data confirm that mud1 and mud2 represent different genetic loci and that gum1 and gum2 may be allelic or may represent closely linked mutations in separate genes.
Genetic Interactions between gum and mud Mutations
To investigate the potential roles of GUM and MUD genes in MGU organization, we investigated genetic interactions between gum1, mud1, and mud2. For example, the double heterozygote +/gum1; +/mud1 should produce four pollen haplotypes: wild-type (+; +), mud (+; mud2), gum (gum1; +), and double mutant (gum1; mud1). If the expression of the gum1 phenotype does not depend on mud1 expression, these phenotypes are expected to be additive, and double-mutant pollen would show intermediate or novel phenotype. However, if they act sequentially during MGU assembly and positioning, the double-mutant class should reflect the phenotype of the earliest acting mutation.
Pollen populations of +/gum1; +/mud1 double heterozygotes were scored, and three phenotypic classes of pollen grains were observed (Table III): wild-type (22.4%), mud (25.4%), and gum (52.2%). Similar results were obtained in the analysis of +/gum1; +/mud2 double mutants (Table IV). Therefore, the combination of gum and mud mutations does not produce novel phenotypes, but results in the gum phenotype. We conclude that these mutations act sequentially and that gum1 acts earlier than mud1 and mud2.
Table III.
Distribution of pollen phenotypic classes in (+/gum1; +/mud1) double heterozygotes
| Genotype | (+/gum1; +/mud1) | |||
|---|---|---|---|---|
| Observed phenotype | 22.4% Wild Type (n = 125) | 25.4% mud (n = 142) | 52.2% gum (n = 292) | |
| Corresponding haplotype | (+; +) | (+; mud1) | (gum1; +) | (gum1; mud1) |
 |
 |
 |
||
| Pollen phenotypes | Wild type | mud | gum | |
Table IV.
Distribution of pollen phenotypic classes in (+/gum1; +/mud2) double heterozygotes
| Genotype | (+/gum1; +/mud2) | |||
|---|---|---|---|---|
| Observed phenotype | 24.9% Wild Type (n = 185) | 21.5% mud (n = 160) | 53.6% gum (n = 399) | |
| Corresponding haplotype | (+; +) | (+; mud2) | (gum1; +) | (gum1; mud2) |
 |
 |
 |
||
| Pollen phenotypes | Wild type | mud | gum | |
MGU Assembly in Arabidopsis
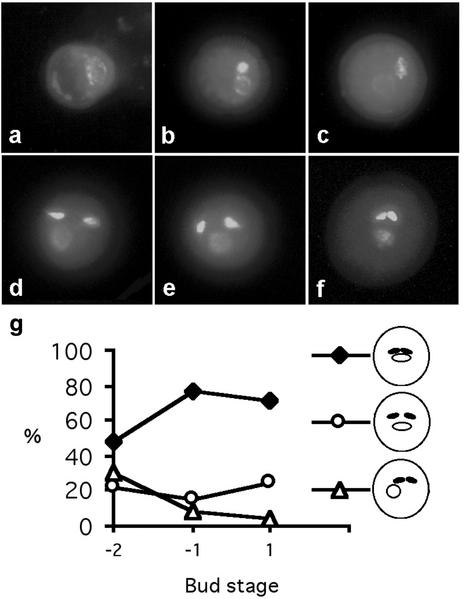
To understand the origins of the mud and gum pollen phenotypes, we first determined the pattern of MGU assembly in wild-type Arabidopsis by examining DAPI-stained pollen released from single anthers at different developmental stages. Immediately after microspore division (pollen mitosis I [PMI]×), the generative cell is located against the pollen grain wall (Fig. 3a). In early bicellular pollen (Fig. 3b), the generative cell subsequently becomes detached, migrates inward, and the generative cell and vegetative nucleus are observed together. After vacuole fission (late bicellular stage), the generative cell remains close to the vegetative nucleus in 99% of pollen grains (Fig. 3c). Immediately after PMII, the two sperm cells were observed close to the vegetative nucleus in approximately 95% of pollen grains (Fig. 3, d and e). In early tricellular pollen, three phenotypic classes were observed. The progressive changes in the percentage of these phenotypic classes (Fig. 3g) during pollen maturation allowed us to propose a sequence of events.
Figure 3.
Developmental analysis of MGU assembly and positioning in wild-type pollen. a, Polarized microspore; b, early bicellular pollen; c, late bicellular stage; d and e, early tricellular pollen; f, mature pollen. g, Graph showing the frequency of typical wild-type phenotypes (illustrated) at the early tricellular (−2), late tricellular (−1), and mature pollen (+1) stages. More than 400 spores were counted at each stage.
Following PMII, one sperm cell remains close to the cortical vegetative nucleus, whereas the second one moves away. MGU components are subsequently observed together in the center of the pollen grain but show different levels of compaction. In mature pollen immediately before dehydration (−1 stage), the MGU appears relatively compact and centrally located in the majority of pollen grains (71%), whereas the MGU components appear more separated in 25% of mature pollen grains. In the mature dehydrated pollen (+1 stage), the MGU appears even more compact.
In mature wild-type pollen stained with DAPI in which the vegetative nucleus was sufficiently separated from the sperm cells, we observed linkage between one sperm cell and the vegetative nucleus. Only one of the sperm cell pair was linked to a DAPI-staining extension of the vegetative nucleus. In a similar manner, in mutant gum pollen where the sperm cell pair was clearly separated from the vegetative nucleus, the vegetative nucleus occasionally showed a long tail or extension in contact with one sperm cell (Fig. 1e). These observations provide evidence for a physical connection between the vegetative nucleus and one of the sperm cell pair in Arabidopsis.
MGU Assembly in gum1 and mud1 Mutants
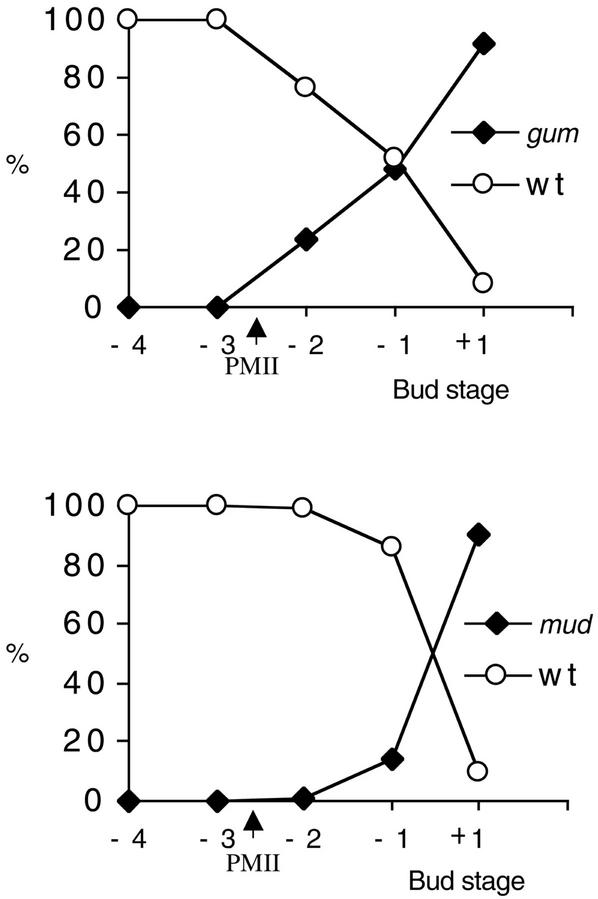
MGU assembly and positioning was observed in developing pollen of homozygote gum and mud mutants. No significant difference in the position of the vegetative nucleus and generative cell could be observed between wild-type and gum1 pollen prior to PMII. However, at −2 stage (early tricellular), 24% of pollen showed a clear gum1 phenotype with the sperm cells separated from the vegetative nucleus and the vegetative nucleus displaced to the pollen wall (Fig. 4). The displacement of the vegetative nucleus against the wall, which was not observed in wild type, is a characteristic marker for the gum phenotype. The percentage of pollen showing this typical gum1 phenotype increased during pollen maturation to 92% in mature pollen (+1 stage). Similar results were obtained for the analysis of gum2 mutant (data not shown). We conclude that both gum mutations disturb the efficiency of initial MGU assembly and subsequently appear to reduce its stability during maturation.
Figure 4.
Frequency of wild-type and mutant phenotypes during pollen development in gum1 and mud1. The frequencies of wild-type and mutant phenotypes at the bicellular (−4), bicellular/PMII (−3), early tricellular (−2), late tricellular (−1), and mature pollen (+1) stages are presented for gum1 and mud1. More than 400 spores were counted at each stage.
In contrast, mud1 did not show any obvious difference in vegetative nucleus or sperm cell positioning immediately after PMII. At −2 stage, 53% of mud1 pollen grains showed a compact and central MGU similar to wild type (Fig. 4). The mud1 phenotype was first observed at late tricellular (−1 stage), where 14% of pollen grains showed the distinctive mud phenotype. This percentage increased to 90% in mature pollen (+1 stage). Similar results were obtained for the analysis of mud2 mutant (data not shown). We conclude that mud1 and mud2 do not affect MGU assembly, but act on its positioning during pollen maturation. Furthermore, mutant phenotypes of mud1 and mud2 were observed later during pollen development than in the gum1 and gum2 mutants, suggesting that GUM1 acts earlier than MUD1 and MUD2.
MGU Stability during Pollen Development
As the mud1 and gum1 mutations appear to act on the integrity and cytoplasmic stability of the MGU, we attempted to physically induce mud and gum phenotypes in wild-type pollen at different developmental stages by centrifugation. Centrifugation has previously been used to alter nuclear positioning in developing microspores to investigate division polarity (Terasaka and Niitsu, 1990). Following centrifugation of −4 stage buds, the vegetative nucleus and generative cell were strongly displaced together in 88% of pollen grains (n = 400). After PMII and during pollen maturation, centrifugation produced a gum-like phenotype (86%, n = 900) with a cortical vegetative nucleus and more centrally located sperm cells. In mature pollen, the vegetative nucleus and sperm cells were displaced together, producing a mud-like phenotype in 30% of pollen grains (n = 900). It is surprising that MGU position was not affected in 70% of mature pollen grains. These results suggest that sperm cell position may be stabilized earlier during pollen maturation, whereas the stabilization of vegetative nucleus position, and of the assembled MGU, may occur only during final maturation, perhaps during dehydration.
MGU Movement during in Vivo Germination
To determine if the entrance of the three MGU components into the pollen tube occurs randomly or follows a well-defined scheme, we performed in vivo pollinations and monitored MGU entrance into the pollen tube. The first pollen tubes were observed within 15 min of pollination. At this time, the MGU was still located inside the pollen grain, but was displaced toward the wall opposite the site of germination (Fig. 5a). The first MGUs were observed to move into the pollen tube 48 to 53 min after pollination. We observed more than 50 entrances of the MGU into the pollen tube, and in all cases, the elongated vegetative nucleus preceded the sperm cells (Fig. 5d). We conclude that the exit and translocation of the MGU during in vivo pollen germination follows a regular order in Arabidopsis.
Figure 5.
MGU translocation during in vivo pollen germination in wild type, gum1, and mud1. Wild-type (a), gum1 (b), and mud1 (c) pollen 20 min after pollination. Typical appearance of the MGU during entrance into the pollen tube 50 min after germination in wild type (d), gum1 (e), and mud1 (f).
To determine if a different pattern or efficiency of MGU translocation could partially explain the observed reduced transmission efficiency, MGU entrances into the pollen tube were scored for gum and mud homozygotes. In gum, the first pollen tubes were observed within 15 min of pollination as in wild type. However, only the vegetative nucleus was observed displaced toward the wall opposite the site of germination (Fig. 5b). Although the initial cell reorganization was different between gum and wild type, this did not affect the order of translocation of MGU components: As in wild type, the elongated vegetative nucleus preceded the sperm cells (n = 40). However, after 2 h of germination, 49% and 46% of pollen grains (n > 200) still contained a MGU in gum1 and gum2, respectively. In contrast, only 21% (n > 200) of wild-type pollen still contained a MGU 2 h after germination; therefore, both gum mutations appear to reduce the efficiency of MGU translocation.
In mud, the first pollen tubes were observed within 15 min of pollination. As in wild type, the MGU was displaced toward the wall opposite the site of germination (Fig. 5a). We observed more than 40 entrances of the MGU into the pollen tube, and in all cases, the elongated vegetative nucleus preceded the sperm cells as in wild type (Fig. 5f). However, after 2 h of germination, 49.1% and 53% of pollen grains (n > 200) still contained a MGU in mud1 and mud2, respectively. We conclude that both mud mutations do not affect the pattern of MGU movement, but do reduce the efficiency of translocation.
DISCUSSION
Isolation of Four Male-Specific Gametophytic Mutations That Disrupt MGU Organization
Morphological screening of physically and chemically mutagenized populations has proven effective in identifying gametophytic mutants affecting pollen cell division, polarity, and cell fate (Chen and McCormick, 1996; Park et al., 1998; Johnson and McCormick, 2001; Twell et al., 1998; Twell, 2002). We have used this strategy to identify two novel classes of gametophytic mutations that affect the structural integrity and/or positioning of the MGU. To avoid mutant phenotypes resulting from weak alleles or the combination of several mutations, we focused our work on highly penetrant mutations and selected four independent mutants, gum1, gum2, mud1, and mud2.
Alternative strategies for identifying gametophytic mutations affecting pollen development have been based upon marker segregation ratio distortion in populations mutagenized by T-DNA insertion (Feldmann et al., 1997; Moore et al., 1997; Bonhomme et al., 1998; Howden et al., 1998), or EMS (Grini et al., 1999). Although these screens have also been successful, mutations without severe effects on male transmission, such as gum and mud, would not be selected.
MGU Assembly and Positioning in Arabidopsis
Our observations provide evidence for an initial assembly of the Arabidopsis MGU at or soon after PMII and that is completed only at the end of pollen maturation. The observed asymmetric association of sperm cells with the vegetative nucleus, shortly after PMII in Arabidopsis, suggests that only one sperm cell is directly associated with the vegetative nucleus. This pattern conforms to descriptions of the spatial association of MGU components proposed for Chinese cabbage (Brassica campestris) and cauliflower (B. oleracea; Dumas et al., 1985; McConchie et al., 1987). Furthermore, the observation of a long extension of the vegetative nucleus connecting only one sperm cell in some mutant gum pollen grains provides additional evidence for the regular organization of the MGU in Arabidopsis.
The effect of centrifugation on positioning of MGU components during development suggests that sperm cell position may be stabilized in the cytoplasm early during pollen maturation, whereas the stabilization of the vegetative nucleus and the complete MGU occur only during final maturation. In this regard, cytoskeleton reorganizations are associated with the morphological changes that occur during pollen maturation (Van Lammeren et al., 1985; Hause et al., 1992; Gervais et al., 1994). The localization of F-actin at the periphery of the generative cell, but not initially around the vegetative nucleus (Hause et al., 1992; Gervais et al., 1994), provides a potential structural mechanism to initially anchor the sperm cells independent of the vegetative nucleus. Independent stabilization is consistent with the greater sensitivity of the vegetative nucleus to centrifugal displacement in early tricellular pollen. In mature pollen, the vegetative nucleus is surrounded by microfilaments, whereas the sperm cells remain unlabeled (Hause et al., 1992; Gervais et al., 1994). gum mutations could disturb the progressive anchoring of the vegetative nucleus, whereas mud mutations, expressed later, could act on the cytoskeletal components anchoring the mature vegetative nucleus.
gum and mud Act Sequentially on MGU Assembly and Positioning
Because the association of MGU components in wild-type pollen is clearly observed first at the early tricellular stage, two main questions concerning MGU assembly in gum and mud pollen were formulated. First, do MGUs form and dissociate, or does initial MGU assembly fail completely in gum pollen? And second, when is the MGU displaced to the periphery in mud pollen? A typical gum phenotype was first observed only after PMII in gum homozygotes. At this stage, 28% of pollen grains possessed a centrally located MGU, therefore gum mutations reduce, but do not completely block, initial MGU assembly. During maturation, gum also appears to act on the stability of MGUs that are initially assembled, because less than 6% of mature gum pollen possess an assembled MGU. Developmental analysis of mud1 and mud2 suggest that both mud mutations act later to destabilize components required for MGU positioning at late tricellular to mature (dehydrated) stages. Moreover, it is likely that the dramatic dehydration of pollen during final maturation to a water content of 10% to 15% (w/v) demands stabilization of cytoplasmic components including the MGU. Therefore, MUD gene products could play a role in maintaining cytoplasmic organization during pollen dehydration.
gum and mud Mutations Reduce MGU Translocation Efficiency
Our observations show that gum and mud pollen germinate on the stigma with a similar efficiency to wild type, but both classes of mutations reduce male transmission. This could suggest that MGU positioning or organization in mature pollen may influence the efficiency of delivery of the male gametes. In planta analyses showed that the efficiency of MGU translocation into the pollen tube was reduced in gum and mud mutants, which could account for their reduced transmission. In gum mutants, the initial separation of the vegetative nucleus and sperm cell pair may account for the delayed entrance of the MGU into the pollen tube. However, it remains possible for both mutants that subsequent steps in MGU transport and delivery are also affected.
The MGU is proposed to have a potential role in the controlled transport and delivery of the sperm cells (Dumas et al., 1984a). The translocation of MGU components into the pollen tube follows a regular order, with the vegetative nucleus leading in the majority of species that have been examined (Russell and Cas, 1981; Heslop-Harrison et al., 1986; Rougier et al., 1991; Heslop-Harrison and Heslop-Harrison, 1996). However, in Secale cereale (Heslop-Harrison and Heslop-Harrison, 1987) and Alopecurus pratensis (Heslop-Harrison and Heslop-Harrison, 1988), no specific order is observed, suggesting species differences in the mechanism of MGU translocation. Our observations in Arabidopsis show that in planta, the vegetative nucleus precedes the sperm cells during entrance into the pollen tube.
Despite their initial separation, translocation of the vegetative nucleus and sperm cell pair still occur in gum, and they enter the pollen tube in the correct order. Therefore, initial movement of MGU components appears to occur independently in gum without affecting the order. In this regard, there is evidence to support the independent movement of myosin-coated sperm cells along cortical F-actin bundles (Heslop-Harrison and Heslop-Harrison, 1989; Zhang and Russell, 1999). Moreover, MGU transport is also known to be dependent upon a microtubule network (Åström et al., 1995) that can determine the order of translocation in some species (Heslop-Harrison and Heslop-Harrison, 1996). The strict order of MGU entrance in gum suggests that such a system still operates, wherein movement of the sperm cells is dependent upon the prior translocation of the vegetative nucleus. In any case, a deeper understanding of how these mutations affect MGU organization and movement may be achieved through the localization of cytoskeletal components during pollen maturation and tube growth. This should be facilitated by the use of transgenic lines expressing green fluorescent protein fusion proteins that decorate F-actin and microtubule arrays (Kost et al., 1998; Marc et al., 1998; Hasezawa et al., 2000).
GUM and MUD genes could potentially encode proteins linking MGU components to each other and/or to cortical locations respectively. The distinctive phenotypes of mud and gum mutations, together with their strong penetrance, will facilitate cloning of their respective genes to uncover their roles in the organization of this unique reproductive assemblage.
MATERIALS AND METHODS
Mutant Screen and Growth Conditions
Seeds were sown in 3:1 compost:sand mix. Plants were grown under greenhouse conditions with supplementary lighting (16 h of light at 22°C). An EMS-mutagenized No-O population was generated and screened using a DAPI staining solution (0.1 m sodium phosphate, pH 7, 1 mm EDTA, 0.1% [w/v] Triton X-100, and 0.4 mg mL−1 DAPI; high grade, Sigma, St. Louis) as described in Park et al. (1998). Pollen from approximately 10,000 M2 individuals derived from each of 10 M1 parental groups screened for aberrant organization or positioning of the MGU. Selected EMS mutants were backcrossed twice before detailed morphological analysis.
Cytological and Phenotypic Analysis of Pollen
The relationship between pollen and flower developmental stages was determined as follows: +1 stage, first open flower (mature tricellular pollen); −1 and −2 stages, first and second unopened flower buds (immature tricellular pollen); −3 stage, third unopened bud (pollen mitosis II and bicellular pollen); −4 stage, fourth unopened buds (bicellular pollen). Mature pollen or spores from earlier stages were incubated in DAPI staining solution and were observed using light and epifluorescence microscopy as described in Park et al. (1998). To analyze the entrance of the vegetative nucleus and sperm cells in vivo, pollinations of wild-type No-O pistils were performed using wild-type pollen or pollen from mud and gum homozygotes. At different times after pollination, pistils were transferred onto a microscope slide, quickly fixed with two drops of ethanol:acetic acid (3:1, v/v), treated with DAPI staining solution, and directly analyzed under a coverslip. For CLSM, mature pollen was incubated in a ethidium bromide solution (0.1 m sodium phosphate, pH 7, 1 mm EDTA, 0.1% [w/v] Triton X-100, and 0.5 μg mL−1 ethidium bromide) and viewed using a CLS microscope (TCS4D; Leica, Wetzlar, Germany) with an excitation wavelength of 568 nm and emission filter at 630 nm.
Centrifugation Experiments
Single open flower buds were placed in a 1.5-mL microfuge tube containing 500 μL of 15% (w/v) Suc solution prepared using distilled water and were centrifuged at 13,000g for 10 min. After centrifugation, the pollen pellet was transferred to a microscope slide, immediately permeabilized by fixation in a drop of ethanol:acetic acid (3:1, v/v), and stained in DAPI solution. Closed flower buds were centrifuged as above and the anthers were dissected out and permeabilized by quick fixation in ethanol:acetic acid (3:1, v/v). Fixed anthers were disrupted on microscope slides by gentle squashing in DAPI solution under a coverslip.
Genetic Analysis and Mapping
Genetic transmission of mutations through the male and female gametes was determined by carrying out reciprocal test crosses in which heterozygous mutants were crossed to wild type (No-O). For tetrad analysis, homozygous qrt1/qrt1 mutants (Preuss et al., 1994) in Columbia (Col-O) ecotype were first backcrossed three times to No-O wild type and then crossed to heterozygous +/mud1 or +/gum1 mutants. Heterozygous +/qrt1 F1 plants showing mud or gum phenotype were selected. qrt1/qrt1::+/mud1 and qrt1/qrt1::+/gum1 individuals and qrt1/qrt1::mud1/mud1 and qrt1/qrt1::gum1/gum1 double homozygotes were identified in the F2 population by screening for plants showing quartet and mud or gum phenotypes.
All four mud and gum mutations were independently mapped using PCR-based molecular markers in F2 populations following outcrossing with the polymorphic Col-O ecotype. Wild-type, heterozygous, and homozygous F2 plants were identified by screening DAPI-stained pollen. DNA preparation and PCR-based mapping were performed as described in Park et al. (1998). Three additional simple-sequence length polymorphism (SSLP) markers polymorphic for No-O and Col-O were generated: 1) CRTW1, chromosome II, BAC F16 M14 (5′-CAGCGGCCA- CGGTATG-3′, 5′-CACCTTC CAAATCTCCCC-3′; Co-O, 205 bp; No-O, 280 bp; 2) T19C21–19, chromosome II, BAC T19C21 (5′-CCTATATCTAGCTAAGCCTAGAAC-3′, 5′-CTGACGAACCAACTTTGGAGTTGC-3′; Co-O, 495 bp; No-O, 505 bp; and 3) M506-SSLP, chromosome IV, BAC F4H6 (5′-GGTGATGAAACCCCATGGTTTGG-3′; 5′-GGTTCAACTATAAATTAAGTGCGG-3′; Co-O, 141 bp, No-O, 155 bp.
Footnotes
E.L. was funded by a Marie Curie Research Fellowship (no. ERBFMBICT972310) under the European Economic Community Training and Mobility of Researchers program. The initial screening work was funded by the Biotechnology and Biological Science Research Council (research grant no. CAD04305 to D.T.) under the Cell Commitment and Determination Initiative.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.003301.
LITERATURE CITED
- Åström H, Sorri O, Raudaskoski M. Role of microtubules in the movement of the vegetative nucleus and generative cell in tobacco pollen tubes. Sex Plant Reprod. 1995;8:61–69. [Google Scholar]
- Bonhomme S, Horlow C, Vezon D, de Laissardiere S, Guyon A, Ferault M, Marchand M, Bechtold N, Pelletier G. T-DNA mediated disruption of essential gametophytic genes in Arabidopsis is unexpectedly rare and cannot be inferred from segregation distortion alone. Mol Gen Genet. 1998;260:444–452. doi: 10.1007/s004380050915. [DOI] [PubMed] [Google Scholar]
- Charzinska M, Murgia M, Milanesi C, Cresti M. Origin of sperm cell association in the “male germ unit” of Brassica pollen. Protoplasma. 1989;149:1–4. [Google Scholar]
- Chen YC, McCormick S. Sidecar pollen, an Arabidopsis thaliana male gametophytic mutant with aberrant cell divisions during pollen development. Development. 1996;122:3243–3253. doi: 10.1242/dev.122.10.3243. [DOI] [PubMed] [Google Scholar]
- Dumas C, Berger F, Faure JE, Matthys-Rochon E. Gametes, fertilization and early embryogenesis in flowering Plants. Adv Bot Res. 1998;28:231–261. [Google Scholar]
- Dumas C, Knox RB, Gaude T. Emerging physiological concepts in fertilization. Plant Physiol. 1984a;15:17–20. [Google Scholar]
- Dumas C, Knox RB, Gaude T. Pollen-pistil interactions: new concepts from electron microscopy and cytochemistry. Int Rev Cytol. 1984b;90:239–272. [Google Scholar]
- Dumas C, Knox RB, Gaude T. The spatial association of the sperm cells and vegetative nucleus in the pollen grain of Brassica. Protoplasma. 1985;124:168–174. [Google Scholar]
- Feldmann KA, Coury DA, Christianson ML. Exceptional segregation of a selectable marker (KanR) in Arabidopsis identifies genes important for gametophytic growth and development. Genetics. 1997;147:1411–1422. doi: 10.1093/genetics/147.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais C, Simmonds DH, Newcomb W. Actin microfilament organization during pollen development of Brassica napus cv. Topas Protoplasma. 1994;183:67–76. [Google Scholar]
- Grini PE, Schnittger A, Schwarz H, Zimmermann I, Schwab B, Jurgens G, Hulskamp M. Isolation of ethyl methanesulfonate-induced gametophytic mutants in Arabidopsis thaliana by a segregation distortion assay using the multimarker chromosome 1. Genetics. 1999;151:849–863. doi: 10.1093/genetics/151.2.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus U, Vielle-Calzada JP, Hoeppner MA, Gagliano WB. Maternal control of embryogenesis by MEDEA, a polycomb group gene in Arabidopsis. Science. 1998;280:446–450. doi: 10.1126/science.280.5362.446. [DOI] [PubMed] [Google Scholar]
- Hasezawa S, Ueda K, Kumagai F. Time-sequence observations of microtubule dynamics throughout mitosis in living cell suspensions of stable transgenic Arabidopsis: direct evidence for the origin of cortical microtubules at M/G1 interface. Plant Cell Physiol. 2000;41:244–250. doi: 10.1093/pcp/41.2.244. [DOI] [PubMed] [Google Scholar]
- Hause G, Hause B, van Lammeren AAM. Microtubular and actin-filament configurations during microspore and pollen development in Brassica napus L. cv. Topas Can J Bot. 1992;70:1369–1376. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison JS, Heslop-Harrison Y. The comportment of the vegetative nucleus and generative cell in the pollen and pollen tubes of Helleborus foetidus L. Ann Bot. 1986;58:1–12. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. Evaluation of pollen viability by enzymatically induced fluorescence: intracellular hydrolysis of fluorescein diacetate. Stain Technol. 1970;45:115–120. doi: 10.3109/10520297009085351. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. An analysis of gamete and organelle movement in the pollen tube of Secale cereale L. Plant Sci. 1987;51:203–213. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. Tubulin and male-gamete interconnections in the pollen tubes of the grass Alopecurus pratensis. Ann Bot. 1988;61:249–254. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. Formation and movement of the vegetative nucleus of the angiosperm pollen tube: association with the actin cytoskeleton. J Cell Sci. 1989;93:299–308. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. Microtubules and the positioning of the vegetative nucleus and generative cell in the angiosperm pollen tube: a quantitative study. Proc Roy Soc Lond B. 1996;263:1299–1304. [Google Scholar]
- Howden R, Park SK, Moore JM, Orme J, Grossniklaus U, Twell D. Selection of T-DNA-tagged male and female gametophytic mutants by segregation distortion in Arabidopsis. Genetics. 1998;149:621–631. doi: 10.1093/genetics/149.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Yu HS. Preliminary observations on the formation of the male germ unit in pollen tubes of Cyphomandra betacea Sendt. Protoplasma. 1988;147:55–63. [Google Scholar]
- Hülskamp M, Parekh NS, Grini P, Scheitz K, Zimmermann I, Lolle SJ, Pruitt RE. The STUD gene is required for male-specific cytokinesis after telophase II of meiosis in Arabidopsis thaliana. Dev Biol. 1997;187:114–124. doi: 10.1006/dbio.1997.8554. [DOI] [PubMed] [Google Scholar]
- Johnson SA, McCormick S. Pollen germinates precociously in the anthers of raring-to-go, an Arabidopsis gametophytic mutant. Plant Physiol. 2001;126:685–695. doi: 10.1104/pp.126.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps TL, McCarty DR, Chase CD. Gametophyte genetics in Zea mays L.: dominance of a restoration-of-fertility allele (Rf3) in diploid pollen. Genetics. 1996;142:1001–1007. doi: 10.1093/genetics/142.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B, Spielhofer P, Chua NH. A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 1998;16:393–401. doi: 10.1046/j.1365-313x.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- Marc J, Granger CL, Brincat J, Fisher DD, Kao TH, McCubbin AG, Cyr RJ. A GFP-MAP4 reporter gene for visualizing cortical microtubule rearrangements in living epidermal cells. Plant Cell. 1998;10:1927–1939. doi: 10.1105/tpc.10.11.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConchie CA, Jobson S, Knox RB. Computer-assisted reconstruction of the male germ unit in pollen of Brassica campestris. Protoplasma. 1985;127:57–63. [Google Scholar]
- McConchie CA, Russell SD, Dumas C, Touhy M, Knox RB. Quantitative cytology of the sperm cells of Brassica campestris and Brassica oleracea. Planta. 1987;170:446–452. doi: 10.1007/BF00402978. [DOI] [PubMed] [Google Scholar]
- Mogensen HL. The male germ unit: concept, composition, and significance. Int Rev Cytol. 1992;140:129–147. [Google Scholar]
- Moore JM, Calzada JP, Gagliano W, Grossniklaus U. Genetic characterization of hadad, a mutant disrupting female gametogenesis in Arabidopsis thaliana. Cold Spring Harb Symp Quant Biol. 1997;62:35–47. [PubMed] [Google Scholar]
- Owen HA, Makaroff CA. Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. ecotype Wassilewskija (Brassicaceae) Protoplasma. 1995;185:7–21. [Google Scholar]
- Park SK, Howden R, Twell D. The Arabidopsis thaliana gametophytic mutation gemini pollen disrupts microspore polarity, division asymmetry and pollen cell fate. Development. 1998;125:3789–3799. doi: 10.1242/dev.125.19.3789. [DOI] [PubMed] [Google Scholar]
- Preuss D, Rhee SY, Davis RW. Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science. 1994;264:1458–1460. doi: 10.1126/science.8197459. [DOI] [PubMed] [Google Scholar]
- Procissi A, de Laissardiere S, Ferault M, Vezon D, Pelletier G, Bonhomme S. Five gametophytic mutations affecting pollen development and pollen tube growth in Arabidopsis thaliana. Genetics. 2001;158:1773–1783. doi: 10.1093/genetics/158.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman H. Mitotic nondisjunction in the case of interchanges involving the B-type chromosome in maize. Genetics. 1947;32:391–409. doi: 10.1093/genetics/32.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman H. Directed fertilization in maize. Proc Natl Acad Sci USA. 1948;34:46–52. doi: 10.1073/pnas.34.2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier M, Jnoud N, Said C, Russell S, Dumas C. Male gametophyte development and formation of the male germ unit in Populus deltoids following compatible pollination. Protoplasma. 1991;162:140–150. [Google Scholar]
- Russell SD. Preferential fertilization in Plumbago: ultrastructural evidence for gamete-level recognition in an angiosperm. Proc Acad Natl Sci USA. 1985;85:6129–6132. doi: 10.1073/pnas.82.18.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SD, Cas DD. Ultrastructure of the sperm of Plumbago zeleynica: cytology and association with the vegetative nucleus. Protoplasma. 1981;107:85–107. [Google Scholar]
- Terasaka O, Niitsu T. Unequal cell division and chromatin differentiation in pollen grain cells: microtubule dynamics associated with the unequal cell division. Bot Mag Tokyo. 1990;103:133–142. [Google Scholar]
- Twell D. Pollen developmental biology. In: O'Neill SD, Roberts JA, editors. Plant Reproduction: Annual Plant Reviews. Vol. 6. Sheffield, United Kingdom: Sheffield Academic Press; 2002. pp. 86–153. [Google Scholar]
- Twell D, Park SK, Lalanne E. Asymmetric division and cell fate determination in developing pollen. Trends Plant Sci. 1998;3:305–310. [Google Scholar]
- Van Lammeren AAM, Bednara J, Willemse MTM. Organization of the actin cytoskeleton during pollen development in Gasteria verrucosa (Mill.) H. Duval visualized with rhodamine-phalloidin. Planta. 1985;178:531–539. doi: 10.1007/BF00963823. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Russell SD. Sperm cell surface characteristics of Plumbago zeylanica L. in relation to transport into the embryo sac. Planta. 1999;208:539–544. [Google Scholar]