Abstract
3,4-Methylenedioxymethamphetamine (MDMA or ‘ecstasy') decreases the 5-HT concentration, [3H]-paroxetine binding and tryptophan hydroxylase activity in rat forebrain, which has been interpreted as indicating 5-HT neurodegeneration. This has been questioned, particularly the 5-HT loss, as MDMA can also inhibit tryptophan hydroxylase. We have now evaluated the validity of these parameters as a reflection of neurotoxicity.
Male DA rats were administered MDMA (12.5 mg kg−1, i.p.) and killed up to 32 weeks later. 5-HT content and [3H]-paroxetine binding were measured in the cortex, hippocampus and striatum. Parallel groups of treated animals were administered NSD-1015 for determination of in vivo tryptophan hydroxylase activity and 5-HT turnover rate constant.
Tissue 5-HT content and [3H]-paroxetine binding were reduced in the cortex (26–53%) and hippocampus (25–74%) at all time points (1, 2, 4, 8 and 32 weeks). Hydroxylase activity was similarly reduced up to 8 weeks, but had recovered at 32 weeks. The striatal 5-HT concentration and [3H]-paroxetine binding recovered by week 4 and hydroxylase activity after week 1. In all regions, the reduction in 5-HT concentration did not result in an altered 5-HT synthesis rate constant.
Administering MDMA to animals when housed at 4°C prevented the reduction in [3H]-paroxetine binding and hydroxylase activity observed in rats housed at 22°C, but not the reduction in 5-HT concentration.
These data indicate that MDMA produces long-term damage to serotoninergic neurones, but this does not produce a compensatory increase in 5-HT synthesis in remaining terminals. It also highlights the fact that measurement of tissue 5-HT concentration may overestimate neurotoxic damage.
Keywords: 3,4-Methylenedioxymethamphetamine (MDMA or “ecstasy”); serotoninergic neurotoxicity; 5-HT, [3H]-paroxetine binding; 5-hydroxytryptophan; tryptophan hydroxylase; 5-HT synthesis rate constant; hyperthermia; hypothermia; low ambient room temperature
Introduction
3,4-Methylenedioxymethamphetamine (MDMA, ‘ecstasy') is a recreational drug commonly used by young people, particularly at dance clubs. There is substantial evidence that the compound produces long-lasting neurotoxic changes in the brain of experimental animals. Immunohistochemical studies have reported an apparent loss of 5-HT nerve terminals (Commins et al., 1987; Jensen et al., 1993) and biochemical studies have reported on the loss of [3H]-paroxetine binding to the presynaptic 5-HT transporter (Battaglia et al., 1987; Hewitt & Green, 1994) and a decrease in tryptophan hydroxylase activity (Schmidt & Taylor, 1987; Stone et al., 1987). However, one conventional marker of neurotoxicity, namely the increased expression of glial fibrillary acidic protein (GFAP), is not seen following MDMA administration, which suggests that gliosis has not occurred (O'Callaghan & Miller, 1993; 2002; ORIO et al., 2004). Furthermore, expression of the serotonin transporter protein (SERT) has been reported to be unchanged after MDMA even though ligand binding to the transporter decreases (Wang et al., 2004; 2005), although these data have been recently challenged in another study that did find marked loss of SERT after a neurotoxic dose of MDMA (Xie et al., 2006). These findings can be contrasted with the clear evidence for an increase in GFAP expression and decrease in SERT protein observed after administration of the neurotoxin 5,7-dihydroxytryptamine (Wang et al., 2004).
MDMA administration also induces significant long-term loss of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in most regions of the forebrain (see Green et al., 2003), and this loss is often used as a simple index of neurotoxicity. However, a problem in interpreting this long-term loss of cerebral tissue 5-HT concentration as an index of neurodegeneration is that MDMA also inhibits tryptophan hydroxylase activity (Stone et al., 1986; 1987; 1989; Schmidt & Taylor, 1987), the rate-limiting enzyme of 5-HT synthesis (Green & Sawyer, 1966; Moir & Eccleston, 1968). Inhibition of this enzyme would, therefore, be expected to decrease the tissue concentration of 5-HT. As the half-life of this enzyme is around 2–3 days (Meek & Neff, 1972), it could be argued that any effect on 5-HT synthesis and tissue concentration would only be apparent for the first few days after administration. This view is supported by the observation that a non-neurotoxic dose of MDMA resulted in a major loss of hydroxylase activity for up to 2 weeks in several brain regions (Stone et al., 1987), whereas a neurotoxic dose decreased tryptophan hydroxylase activity for 110 days following MDMA administration, which was interpreted as a reflection of 5-HT terminal loss (Stone et al., 1987). However, the problem with all the long-term studies on tryptophan hydroxylase activity is that measurement of the activity of the enzyme was made ex vivo following removal of the tissue from MDMA-treated rats. Optimal conditions (with addition of cofactors and substrate) were therefore being employed. No long-term study has examined the ability of the enzyme to synthesize 5-HT in the brain in vivo following earlier administration of a neurotoxic dose of MDMA.
The current study has therefore examined the effect of MDMA administration to rats on the cerebral concentration of 5-HT and 5-HIAA, the binding of [3H]-paroxetine to the presynaptic 5-HT transporter and the ability of the brain to hydroxylate tryptophan in vivo. This was performed by measuring the formation of 5-hydroxytryptophan (5-HTP) following injection of the L-aromatic amino-acid decarboxylase inhibitor NSD-1015 (3-hydroxybenzylhydrazine). This technique also allows the measurement of the 5-HT synthesis rate constant. Rats were given a dose of MDMA that is known to produce a long-term decrease in cerebral 5-HT concentration and the effects on the 5-HT parameters listed above measured in groups of rats 1, 2, 4, 8 and 32 weeks later. In addition, a study was performed in which MDMA was given to rats when housed in cool conditions to try and minimise MDMA-induced neurotoxicity (Colado et al., 1998; Malberg & Seiden, 1998) in order to determine whether measurement of [3H]-paroxetine binding and 5-HT concentration always indicated a similar degree of neurotoxic damage to 5-HT terminals.
Methods
Animals, drugs and reagents
Adult male Dark Agouti rats (Harlan Iberica, Barcelona, Spain) weighing 175–250 g were used. They were housed in groups of six in conditions of constant temperature (21±2°C) and a 12 h light/dark cycle (lights on: 07 : 00 h) and given free access to food and water. Rats were injected with a single dose of MDMA (12.5 mg kg−1, i.p.) or saline and killed 1, 2, 4, 8 and 32 weeks later. For the study on the effect of ambient temperature, the animals were kept at 4°C for 4 h before the injection of MDMA and maintained at this ambient temperature for 6 h after treatment.
One group of animals was examined for measurement of tissue 5-HT, 5-HIAA concentrations and [3H]-paroxetine binding and another group for measurement of the 5-HTP concentration following administration of NSD-1015.
MDMA and NSD-1015 were dissolved in 0.9% w v−1 NaCl (saline) and injected in a volume of 1 ml kg−1. Doses are quoted in terms of the base. Control animals were injected with saline. (±)-MDMA.HCl was obtained from Ultrafine Chemicals Ltd (Manchester, U.K.) and NSD-1015 from Sigma-Aldrich Quimica (Spain). [3H]-Paroxetine (Specific activity=19.1 Ci mmol−1) was obtained from PerkinElmer (Spain).
All experimental procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the Universidad Complutense (following EU Directive 86/609/EEC).
Tryptophan hydroxylase activity in vivo
Tryptophan hydroxylase activity was determined by measuring the concentration of 5-HTP 30 min following administration of the L-aromatic amino-acid decarboxylase inhibitor NSD-1015, given at a dose of 100 mg kg−1, i.p. (Carlsson et al., 1972). Measurement of the basal 5-HTP concentration was not made as this compound is not detectable in control animals in the absence of a decarboxylase inhibitor.
Results are reported as ng 5-HTP formed per gram tissue 30 min−1. As 5-HT synthesis involves a single compartment system, the rate of 5-HTP formation can, at steady state, be considered to equate to the rate of 5-HT synthesis (Neff et al., 1969). Consequently, conversion of these data to nmol g−1 h−1 together with conversion of amine concentration data to nmol g−1 allows an estimate to be made of both the rate of synthesis of 5-HT and also the rate constant for synthesis (the reciprocal of the time required to replace the amine pool) in the tissue examined. This rate constant is determined by dividing the synthesis rate (nmol g−1 h−1) by the tissue concentration of 5-HT (nmol g−1) and is reported in h−1 (see Carlsson et al., 1972).
Measurement of 5-HTP, 5-HT and 5-HIAA levels in cerebral tissue
Animals were killed either 1, 2, 4, 8 or 32 weeks after saline or MDMA (12.5 mg kg−1) administration by cervical dislocation and decapitation, the brains rapidly removed and cortex, hippocampus and striatum dissected out on ice. Tissue was homogenized and 5-HTP, 5-HT and 5-HIAA measured by HPLC. Briefly, for 5-HT and 5-HIAA, the mobile phase consisted of KH2PO4 (0.05 M), octanesulfonic acid (0.16 mM), EDTA (0.1 mM) and methanol (16%), and was adjusted to pH 3 with phosphoric acid, filtered and degassed. The flow rate was 1 ml min−1. For 5-HTP, a similar mobile phase (1 mM octanesulfonic acid) at a flow rate of 0.7 ml min−1 was used.
The HPLC system consisted of a pump (Waters 510) linked to an automatic sample injector (Loop 200 μl, Waters 717 plus Autosampler), a stainless-steel reversed-phase column (Spherisorb ODS2, 5 μm, 150 × 4.6 mm) with a precolumn and a coulometric detector (Coulochem II, Esa, U.S.A.). The working electrode potential was set at 400 mV with a gain of 500 nA. The current produced was monitored by using integration software (Unipoint, Gilson).
[3H]-Paroxetine binding in tissue homogenates
[3H]-Paroxetine binding was measured by the method described in detail by Hewitt & Green (1994). The animals were killed, the brain was rapidly removed and dissected on ice within 2 min. Cortex, hippocampus and striatum from individual animals were homogenized in ice-cold Tris-HCl (50 mM; pH 7.4) containing NaCl (120 mM) and KCl (5 mM) using an Ultra-Turrax. The homogenate was centrifuged at 30,000 × g for 10 min at 4°C. The supernatant was discarded and the wash procedure repeated twice more. The pellet was finally resuspended in the Tris buffer at a concentration of 10 mg tissue ml−1. In order to obtain an estimate of the maximal density of [3H]-paroxetine-labelled 5-HT uptake sites, the assay solution (1 ml) contained a saturating concentration of [3H]-paroxetine (1 nM) and 800 μl tissue preparation with the addition of 5-HT (100 μM) for determination of nonspecific binding. Assay solutions were incubation for 90 min at room temperature. Assays were terminated by rapid filtration and counting of the radioactivity by scintillation spectrometry. Protein concentrations were measured by the method of Lowry et al. (1951).
Measurement of rectal temperature
Immediately before and up to 6 h after MDMA injection, temperature was measured by use of a digital readout thermocouple (BAT-12, Microprobe Thermometer, Physitemp Instruments, Inc, NJ, U.S.A.) with a resolution of 0.1°C and accuracy of ±0.1°C attached to a RET- 2 rat rectal probe which was inserted 2.5 cm into the rectum of the rat, the animal being lightly restrained by holding in the hand. A steady readout was obtained within 10 s of probe insertion.
Statistics
All neurochemical data were analysed by one-way ANOVA followed by Newman–Keuls test (GraphPad Prism). Analysis of the temperature data was by use of the statistical computer package BMDP/386 Dynamic (BDMP Statistical Solutions, Cork, Ireland).
Results
Effect of MDMA on the 5-HT and 5-HIAA levels and [3H]-paroxetine binding
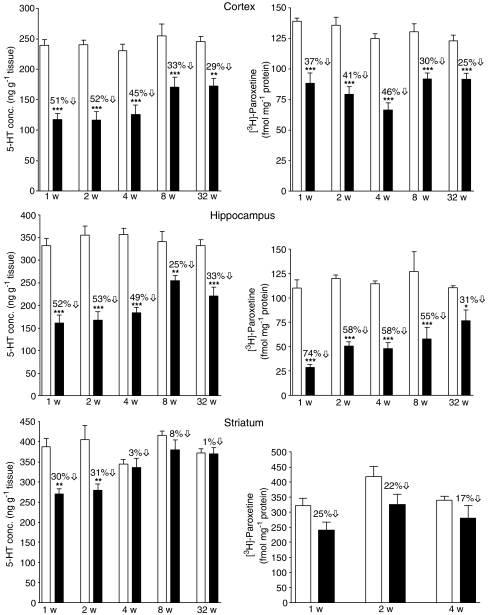
The concentration of 5-HT in the cortex, hippocampus and striatum in the control groups given saline 1–32 weeks earlier was remarkably consistent, particularly given the different periods of the year over which the study was conducted (Figure 1). Rats given MDMA (12.5 mg kg−1) in place of saline had a major decrease in the concentration of 5-HT compared with the control group in both the cortex and hippocampus over the whole 32-week period (Figure 1). The decrease in 5-HT in the striatum of rats given MDMA was relatively transient, normal concentrations being found at 4 weeks and beyond (Figure 1). In general, the MDMA-treated rats showed a similar parallel loss of 5-HIAA to the 5-HT content in all three regions (data not shown).
Figure 1.
Changes in the concentration of 5-HT and density of [3H]-paroxetine labelled 5-HT uptake sites in the cerebral cortex, hippocampus and striatum of rats 1, 2, 4, 8 and 32 weeks after MDMA 12.5 mg kg−1, i.p. administration at a room temperature of 22°C. Open bars represent saline-treated animals and filled bars represent MDMA-treated animals. Results shown as mean±s.e.m., n=5–14. Different from the corresponding saline group: * P<0.05, **P<0.01, ***P<0.001. One-way ANOVA followed by Newman–Keuls test.
Binding of [3H]-paroxetine to the 5-HT uptake site in the tissues was decreased in the MDMA-treated rats to a similar degree and with the same time course as the 5-HT changes seen in all three regions (Figure 1).
Tryptophan hydroxylase activity in vivo following MDMA
The accumulation of 5-HTP following NSD-1015 administration was similar within the control groups of each region at all times following saline injection (Table 1). MDMA administration resulted in a substantial decrease in accumulation (synthesis) of 5-HTP up to 8 weeks later in the cortex and hippocampus but only at week 1 in the striatum (Table 1). This decrease mirrored the decrease in tissue 5-HT concentration and [3H]-paroxetine binding (Figure 2).
Table 1.
Synthesis of 5-HTP (ng g−1 per 30 min) in brain regions at various times following administration of MDMA 12.5 mg kg−1 at a room temperature of 22°C
|
Time |
Cortex |
Hippocampus |
Striatum |
|||
|---|---|---|---|---|---|---|
| (weeks) | Saline | MDMA | Saline | MDMA | Saline | MDMA |
| 1 |
124±7 |
71±6** |
160±10 |
73±6*** |
131±9 |
84±8** |
| 2 |
129±8 |
64±11*** |
154±7 |
99±12f |
141±13 |
121±10 |
| 4 |
136±13 |
82±14* |
179±7 |
116±10** |
145±13 |
141±13 |
| 8 |
121±8 |
75±4* |
160±14 |
110±15* |
139±6 |
135±10 |
| 32 | 126±7 | 118±11 | 155±8 | 125±12 | 149±2 | 142±7 |
Animals were treated with MDMA (12.5 mg kg−1, i.p.) and given NSD-1015 (100 mg kg−1, i.p.) 30 min before killing for determination of tissue 5-HTP concentration. Results shown as mean±s.e. mean, n=3–7. Different from the corresponding saline group:
P<0.05,
P<0.01,
P<0.001,
P=0.052. One-way ANOVA followed by Newman–Keuls test.
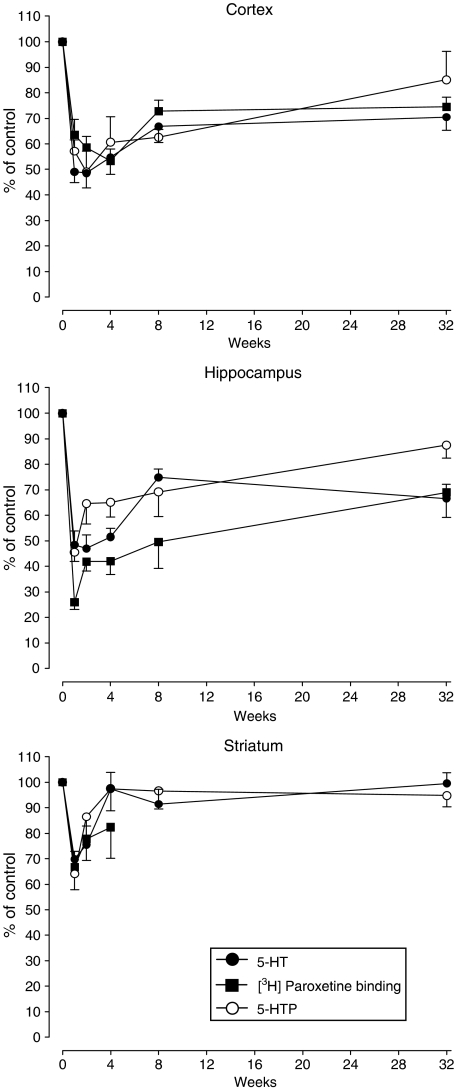
Figure 2.
Changes in the concentration of 5-HT and 5-hydroxytryptophan (5-HTP) and density of [3H]-paroxetine labelled 5-HT uptake sites in cerebral cortex, hippocampus and striatum of rats 1, 2, 4, 8 and 32 weeks after MDMA 12.5 mg kg−1, i.p. administration at a room temperature of 22°C. Data are taken from Figure 1 and Table 1 and expressed as % of corresponding control. Results shown as mean±s.e.m., n=3–14.
5-HT synthesis rate constant in MDMA-treated rats
The parallel decrease in the ability of tryptophan hydroxylase to synthesize 5-HTP and the tissue 5-HT concentration (Figure 2) suggested that the decrease in the tissue amine concentration occurred either because the hydroxylase activity was lowered by MDMA or that there were fewer functioning 5-HT terminals in which 5-HT was being synthesised as data are calculated per g tissue. We, therefore, examined the synthesis rate constant for 5-HT (see Methods).
The synthesis rate constants were similar in the control groups within each region (Table 2). The rate in the MDMA-treated groups was similar to that seen in the saline-injected animals at each time point, being within 10–30% of the control value at each time point with no trend over time (Table 2).
Table 2.
5-Hydroxytryptamine synthesis rate constant (h−1) in brain regions at various times following administration of MDMA (12.5 mg kg−1) at a room temperature of 22°C
|
Time (weeks) |
Cortex |
Hippocampus |
Striatum |
|||
|---|---|---|---|---|---|---|
| Saline | MDMA | Saline | MDMA | Saline | MDMA | |
| 1 |
0.83 |
0.97 |
0.77 |
0.72 |
0.54 |
0.50 |
| 2 |
0.86 |
0.81 |
0.69 |
0.95 |
0.55 |
0.69 |
| 4 |
0.94 |
1.04 |
0.80 |
0.93 |
0.67 |
0.67 |
| 8 |
0.76 |
0.71 |
0.75 |
0.69 |
0.54 |
0.57 |
| 32 |
0.82 |
1.10 |
0.75 |
0.90 |
0.64 |
0.61 |
| |
|
|
|
|
|
|
| Mean±s.e.m | 0.84±0.03 | 0.92±0.07 | 0.75±0.02 | 0.83±0.05 | 0.59±0.03 | 0.61±0.03 |
The amine synthesis rate constant (the reciprocal of the time required to replace amine pool) was determined by dividing the synthesis rate measured in Table 1 by the tissue concentration of 5-HT and is reported as time (h−1); see Methods for full details.
Effect of ambient temperature on the MDMA-induced increase in rectal temperature
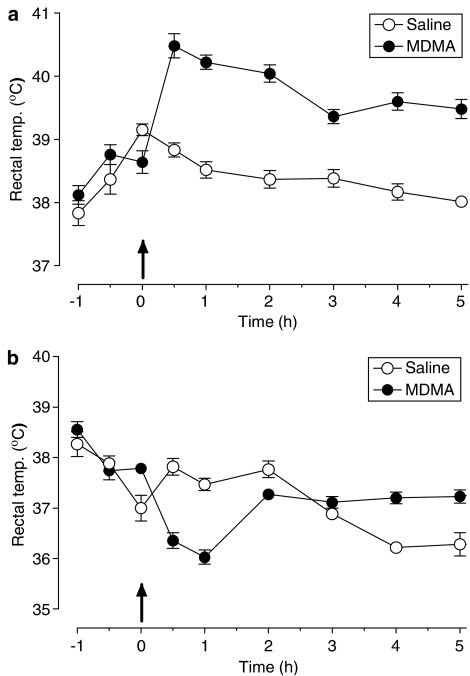
MDMA, given at an ambient temperature of 22°C, produced a sharp increase in rectal temperature, which reached a peak of 1.5°C above the temperature of saline-treated animals 30 min after injection and which remained elevated for at least 5 h (Figure 3a).
Figure 3.
Effect of MDMA administered at different ambient temperatures on rectal temperature. Rats were maintained at (a) 22 or (b) 4°C for 4 h before and 6 h after receiving MDMA (12.5 mg kg−1, i.p.). MDMA administered at standard room temperature produced an acute increase in rectal temperature (F(1,9)=150.42, P<0.001). Administration of the drug at low room temperature produced an initial hypothermia (F(1,11)=63.01, P<0.001) followed by a slight rise in rectal temperature (F(1,11)=27.06, P<0.001). Results shown as mean±s.e.m., n=5–7. Two-way ANOVA.
When MDMA was administered to animals at an ambient temperature of 4°C, a biphasic pattern was observed. Initially, MDMA produced a decrease in rectal temperature compared with saline-treated controls, which lasted for 60 min after administration but which reverted at 120 min and in fact produced a slight hyperthermic response from 180 to 300 min (Figure 3b).
Effect of ambient temperature on cortical 5-HT concentration, [3H]-paroxetine binding and tryptophan hydroxylase activity following MDMA administration
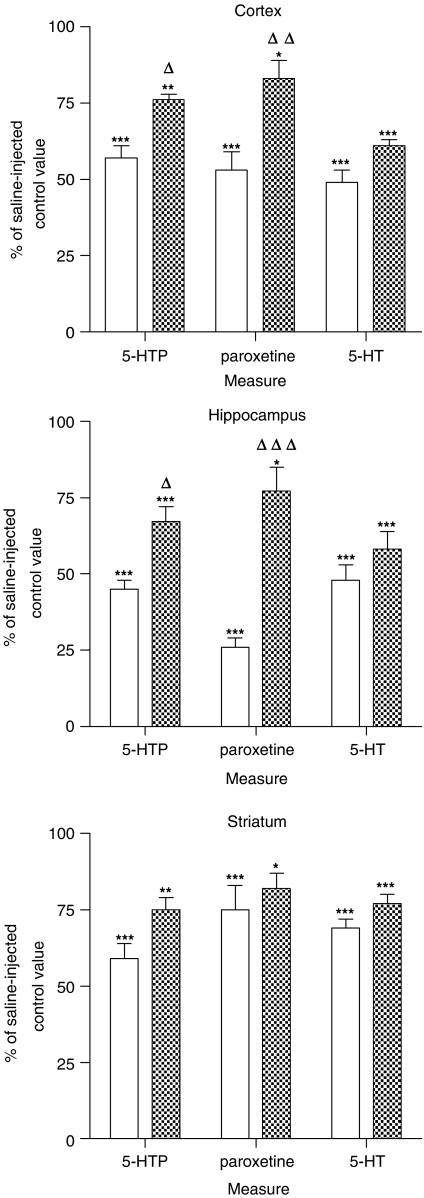
Seven days after the administration of MDMA to rats when housed at an ambient temperature of 22°C, there was a substantial loss of [3H]-paroxetine binding in the cerebral cortex (Figure 4; data taken from results presented in Figure 1). This loss was significantly lessened by administering the drug to animals housed at 4°C (Figure 4). A similar attenuation in the tryptophan hydroxylase activity (%-HTP accumulation after NSD-1015) was seen in animals housed at 4°C during MDMA administration compared to those housed at 22°C (Figure 4). In contrast, the 5-HT concentration loss in animals housed at 22°C was only mildly lessened by housing animals at 4°C during drug administration (Figure 4).
Figure 4.
Effect of ambient temperature on the levels of 5-HT, 5-hydroxytryptophan (5-HTP) and density of [3H]-paroxetine labelled 5-HT uptake sites in cerebral cortex, hippocampus and striatum of rats kept at 22 (open bars) or 4°C (filled bars) for 4 h before and 6 h after MDMA 12.5 mg kg−1, i.p. administration. Animals were killed 7 days after treatment. For the quantification of 5-HTP, rats were treated with NSD-1015 (100 mg kg−1, i.p.) 30 min before killing. Results shown as mean±s.e.m., n=5–7. Different from the corresponding saline group: *P<0.05, **P<0.01, ***P<0.001. Different from MDMA-treated animals at 22°C: ΔP<0.05, ΔΔP<0.01, ΔΔΔP<0.001. One-way ANOVA followed by Newman–Keuls test. Levels in saline-treated rats at 22 and 4°C were: for cortex 5-HT (239±9 and 189±17 ng g−1 tissue, respectively), 5-HTP (270±16 and 272±14 ng g−1 tissue, respectively) and 5-HT uptake sites (139±2 and 131±6 fmol mg−1 protein, respectively); for hippocampus 5-HT (332±15 and 411±20 ng g−1 tissue, respectively), 5-HTP (308±19 and 316±10 ng g−1 tissue, respectively) and 5-HT uptake sites (110±8 and 119±9 fmol mg−1 protein, respectively); for striatum 5-HT (388±20 and 464±12 ng g−1 tissue, respectively), 5-HTP (525±34 and 572±31 ng g−1 tissue, respectively) and 5-HT uptake sites (322±25 and 334±7 fmol mg−1 protein, respectively).
Measurements of these biochemical markers in the hippocampus of rats injected with MDMA in ambient temperatures of either 22 or 4°C revealed an almost identical pattern of change to those seen in the cortex, again the MDMA-induced 5-HT loss in animals housed at 22°C being similar to that seen in animals housed at 4°C, whereas both the loss of [3H]-paroxetine binding and loss of hydroxylase activity was smaller in animals given the drug at a 4°C ambient temperature (Figure 4). Such a pattern was not clearly observed in the striatum, but the loss of all three markers was much smaller than in the other two regions examined (Figure 4).
Discussion
Following administration of an acute dose of MDMA to rats, the plasma and brain concentrations of the drug rise rapidly with a peak concentration around 90 min later in both tissues. The concentration then drops rapidly thereafter, the tissue concentration at 6 h being approximately 10% of the peak value (Chu et al., 1996). The long-term neurotoxic consequences are however long-lasting and profound. In the current study, the loss of 5-HT biochemical markers in the brain of MDMA-treated rats, namely loss of tissue 5-HT and 5-HIAA content and [3H]-paroxetine binding, was observed in the cortex up to 32 weeks after drug administration. This confirmed the findings of several other groups (Battaglia et al., 1988; Scanzello et al., 1993; Sabol et al., 1996). The fact that recovery of 5-HT levels in the striatum is more rapid than the cortex agrees with Sabol et al. (1996) and supports some of our recent work, where we observed the striatum to be more resistant to MDMA-induced 5-HT loss than the hippocampus and cortex (Sanchez et al., 2004). It has been argued that enhanced dopamine release plays a key role in MDMA-induced 5-HT neurotoxicity (Stone et al., 1988; Shankaran et al., 1999), but this hypothesis is weakened by the apparent resistance to damage of the striatum, a dopamine-rich region. Other work involving administration of the dopamine precursor L-dopa with MDMA has also failed to provide data supporting the hypothesis (Colado et al., 1999; Yuan et al., 2002). A recent study (Breier et al., 2006) has gone some way to resolving these apparently conflicting observations by demonstrating that MDMA administration markedly enhances the tissue concentration of tyrosine. Tyrosine, in turn, is hydroxylated (possibly non-enzymatically) to L-dopa, which is then decarboxylated to dopamine in 5-HT-containing terminals (Breier et al., 2006), thereby contributing to oxidant stress and neurodegeneration (see Colado & Green, 1995; Colado et al., 1997).
The loss of tryptophan hydroxylase activity from weeks 1 to 8 in both the cortex and hippocampus probably reflects the loss of the 5-HT terminals rather than loss of enzyme activity. Activity is measured by activity g−1 tissue and thus indicates loss of enzyme activity in functional 5-HT terminals g−1 tissue. Using these data and the concentration of 5-HT to calculate the amine synthesis rate constant, it was found that the tissue in all three regions was synthesizing 5-HT to replace the amine neuronal pool in intact and MDMA-treated rats at the same rate. The apparent decrease in hydroxylase activity therefore reflects 5-HT terminal neurotoxicity even 1 week after MDMA administration. These data also highlight another point; the long-term loss of tissue 5-HT is not accompanied by a compensatory increase in 5-HT synthesis.
A decrease in [3H]-paroxetine binding in the cortex for over 6 months following MDMA administration was reported by Battaglia et al. (1988) and has been confirmed in the current study, which also found a similar change in the hippocampus (but not striatum). The value of [3H]-paroxetine binding as an index of long-term neurotoxicity has been questioned (Sumnall et al., 2004); however, when taken with other indirect evidence (amine content and tryptophan hydroxylase activity) which showed essentially parallel changes (Figure 2), it is hard to deny the conclusion that serotonergic nerve terminal damage is present more than 6 months after administration of a single dose of MDMA of 12.5 mg kg−1.
Hypothermia, induced either by housing the animal in a cool environment when administering MDMA or giving a drug which prevents the normal MDMA-induced hyperthermia, markedly lessens the subsequent neurotoxicity (Malberg & Seiden, 1998; Colado et al., 1998; Green et al., 2003), although some neurotoxic loss of 5-HT can still occur in animals not displaying a hyperthermic response (O'Shea et al., 1998). The current study demonstrated the protective effect of preventing the normal MDMA-induced hyperthermic response by administering the drug to rats while they were housed at low ambient temperature. The loss of [3H]-paroxetine binding and loss of tryptophan hydroxylase activity were much smaller in the hippocampus and cortex of the mildly hypothermic animals than rats that showed a hyperthermic response following MDMA. However, the hypothermia-induced attenuation was much less apparent when 5-HT content was measured. If we assume that [3H]-paroxetine binding is a good index of 5-HT terminal integrity, and the hydroxylase activity measurement would support that contention, it therefore appears that measurement of 5-HT tissue content loss can, in some cases, overestimate neurotoxic damage. The pattern of change in normothermic and hypothermic animals was less clear in the striatum but the damage in this region was much smaller and thus it becomes difficult to detect any partial protection.
The question arises as to why the 5-HT loss in the hypothermic animals is so much greater than that of [3H]-paroxetine binding and we have no clear explanation for this. Nevertheless, these data do suggest that even allowing for the evidence of a good correlation between tissue 5-HT content and [3H]-paroxetine binding observed in the main part of this study, any claim of MDMA-induced neurotoxicity should always be supported (at least initially) by measurement of [3H]-paroxetine binding rather than merely relying on measurement of 5-HT tissue loss.
Acknowledgments
M.I.C. thanks Plan Nacional sobre Drogas (Ministerio de Sanidad), Ministerio de Educacion y Ciencia (Grant SAF2004-02603), and Ministerio de Sanidad (Grant G03/005) and E.O.S. thanks Ministerio de Educacion y Ciencia (SAF2003-05180) and Plan Nacional sobre Drogas (Ministerio de Sanidad) for financial support.
Abbreviations
- GFAP
glial fibrillary acidic protein
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HTP
5-hydroxytryptophan
- MDMA
3,4-Methylenedioxymethamphetamine
- NSD-1015
3-hydroxybenzylhydrazine
- SERT
serotonin transporter protein
References
- BATTAGLIA G., YEH S.Y., O'HEARN E., MOLLIVER M.E., KUHAR M.J., DE SOUZA E.B. 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H]paroxetine-labeled serotonin uptake sites. J Pharmacol Exp Ther. 1987;242:911–916. [PubMed] [Google Scholar]
- BATTAGLIA G., YEH S.E., DE SOUZA E.B. MDMA-induced neurotoxicity parameters of degeneration and recovery of brain serotonin systems. Pharmacol. Biochem. Behav. 1988;29:269–274. doi: 10.1016/0091-3057(88)90155-4. [DOI] [PubMed] [Google Scholar]
- BREIER J.M., BANKSON M.G., YAMAMOTO B.K. L-Tyrosine contributes to (+)-3,4-methylenedioxymethamphetamine-induced serotonin depletions. J. Neurosci. 2006;26:290–299. doi: 10.1523/JNEUROSCI.3353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSSON A., DAVIS J.N., KEHR W., LINDQVIST M., ATACK C.V. Simultaneous measurement of tyrosine and tryptophan hydroxylase activities in brain in vivo using an inhibitor of the aromatic amino acid decarboxylase. Nauyn-Schmied. Arch. Pharmacol. 1972;275:153–168. doi: 10.1007/BF00508904. [DOI] [PubMed] [Google Scholar]
- CHU T., KUMAGAI Y., DISTEFANO E.W., CHO A.K. Disposition of methylenedioxymethamphetamine and three metabolites in the brains of different rat strains and their possible roles in acute serotonin depletion. Biochem. Pharmacol. 1996;51:789–796. doi: 10.1016/0006-2952(95)02397-6. [DOI] [PubMed] [Google Scholar]
- COLADO M.I., GREEN A.R. The spin trap reagent a-phenyl-N-tert butyl nitrone (PBN) prevents ‘Ecstasy'-induced neurodegeneration of 5-HT neurones. Eur. J. Pharmacol. 1995;280:343–346. doi: 10.1016/0014-2999(95)00298-y. [DOI] [PubMed] [Google Scholar]
- COLADO M.I., O'SHEA E., GRANADOS R., MURRAY T.K., GREEN A.R. In vivo evidence for free radical involvement in the degeneration of rat brain 5-HT neurons which follows administration of MDMA (‘ecstasy') but not the degeneration which follows fenfluramine. Br. J. Pharmacol. 1997;121:889–900. doi: 10.1038/sj.bjp.0701213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLADO M.I., GRANADOS R., O'SHEA E., ESTEBAN B., GREEN A.R. Role of hyperthermia in the protective action of chlomethiazole against MDMA (‘ecstasy')-induced neurodegeneration, comparison with the novel NMDA channel blocker AR-R15896AR. Br. J. Pharmacol. 1998;124:479–484. doi: 10.1038/sj.bjp.0701859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLADO M.I., O'SHEA E., GRANADOS R., ESTEBAN B., MARTIN A.B., GREEN A.R. Studies on the role of dopamine in the degeneration of 5-HT nerve endings in the brain of Dark Agouti rats following 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”) administration. Br. J. Pharmacol. 1999;126:911–924. doi: 10.1038/sj.bjp.0702373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMMINS D.L., VOSMER G., VIRUS R.M., WOOLVERTON W.L., SCHUSTER C.R., SEIDEN L.S. Biochemical and histological evidence that methylenedioxymethamphetamine (MDMA) is toxic to neurons in the rat brain. J. Pharmacol. Exp. Ther. 1987;241:338–345. [PubMed] [Google Scholar]
- GREEN A.R., MECHAN A.O., ELLIOTT J.M., O'SHEA E., COLADO M.I. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy) Pharmacol. Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- GREEN H., SAWYER J.L. Demonstration, characterization, and assay procedure of tryptophan hydroxylase in rat brain. Anal. Biochem. 1966;15:53–64. doi: 10.1016/0003-2697(66)90247-8. [DOI] [PubMed] [Google Scholar]
- HEWITT K.E., GREEN A.R. Chlormethiazole, dizocilpine and haloperidol prevent the degeneration of serotonergic nerve terminals induced by administration of MDMA (‘Ecstasy') Neuropharmacology. 1994;33:1589–1595. doi: 10.1016/0028-3908(94)90134-1. [DOI] [PubMed] [Google Scholar]
- JENSEN K.F., OLIN J., HAYKAL-COATS N., O'CALLAGHAN J., MILLER D.B. Mapping toxicant-induced nervous system damage with a cupric silver stain: a quantitative analysis of neural degeneration induced by 3,4-methylenedioxymethamphetamine. NIDA Res. Monogr. 1993;136:133–149. doi: 10.1037/e495922006-008. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MALBERG J.E., SEIDEN L.S. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J. Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEEK J.L., NEFF N.H. Tryptophan 5-hydroxylase: approximation of half-life and rate of axonal transport. J. Neurochem. 1972;19:1519–1525. doi: 10.1111/j.1471-4159.1972.tb05096.x. [DOI] [PubMed] [Google Scholar]
- MOIR A.T., ECCLESTON D. The effects of precursor loading in the cerebral metabolism of 5-hydroxyindoles. J. Neurochem. 1968;15:1093–1108. doi: 10.1111/j.1471-4159.1968.tb06827.x. [DOI] [PubMed] [Google Scholar]
- NEFF N.H., LIN R.C., NGAI S.J., COSTA E. Turnover rate measurements of brain serotonin in unanesthetized rats. Adv. Biochem. Psychopharmacol. 1969;1:91–109. [PubMed] [Google Scholar]
- O'CALLAGHAN J.P., MILLER D.B.Neurotoxic effects of substituted amphetamines in rats and mice: challenges to current dogma Handbook of Neurotoxicology 2002Totowa, NJ: Humana Press; 269–301.ed. Massaro, E.J. pp [Google Scholar]
- O'CALLAGHAN J.P., MILLER D.B. Quantification of reactive gliosis as approach to neurotoxicity assessment. NIDA Res. Monogr. 1993;136:188–212. [PubMed] [Google Scholar]
- ORIO L., O'SHEA E., SANCHEZ V., PRADILLO J.M., ESCOBEDO I., CAMARERO J., MORO M.A., GREEN A.R., COLADO M.I. 3,4-Methylenedioxymethamphetamine increases interleukin-1beta levels and activates microglia in rat brain: studies on the relationship with acute hyperthermia and 5-HT depletion. J. Neurochem. 2004;89:1445–1453. doi: 10.1111/j.1471-4159.2004.02443.x. [DOI] [PubMed] [Google Scholar]
- O'SHEA E., GRANADOS R., ESTEBAN B., COLADO M.I., GREEN A.R. The relationship between the degree of neurodegeneration of rat brain 5-HT nerve terminals and the dose and frequency of administration of MDMA (‘ecstasy') Neuropharmacology. 1998;37:919–926. doi: 10.1016/s0028-3908(98)00029-x. [DOI] [PubMed] [Google Scholar]
- SABOL K.E., LEW R., RICHARDS J.B., VOSMER G.L., SEIDEN L.S. Methylenedioxymethamphetamine-induced serotonin deficits are followed by partial recovery over a 52-week period. Part I: synaptosomal uptake and tissue concentrations. J. Pharmacol. Exp. Ther. 1996;276:846–854. [PubMed] [Google Scholar]
- SANCHEZ V., O'SHEA E., SAADAT K.S., ELLIOTT J.M., COLADO M.I., GREEN A.R. Effect of repeated (‘binge') dosing of MDMA to rats housed at normal and high temperature on neurotoxic damage to cerebral 5-HT and dopamine neurones. J. Psychopharmacol. 2004;18:412–416. doi: 10.1177/026988110401800312. [DOI] [PubMed] [Google Scholar]
- SCANZELLO C.R., HATZIDIMITRIOU G., MARTELLO A.L., KATZ J.L., RICAURTE G.A. Serotonergic recovery after (±)-3,4-(methylenedioxy) methamphetamine injury: observations in rats. J. Pharmacol. Exp. Ther. 1993;264:1484–1491. [PubMed] [Google Scholar]
- SCHMIDT C.J., TAYLOR V.L. Depression of rat brain tryptophan hydroxylase following the acute administration of methylenedioxymethamphetamine. Biochem. Pharmacol. 1987;36:4095–4102. doi: 10.1016/0006-2952(87)90566-1. [DOI] [PubMed] [Google Scholar]
- SHANKARAN M., YAMAMOTO B.K., GUDELSKY G.A. Mazindol attenuates the 3,4-methylenedioxymethamphetamine-induced formation of hydroxyl radicals and long-term depletion of serotonin in the striatum. J. Neurochem. 1999;72:2516–2522. doi: 10.1046/j.1471-4159.1999.0722516.x. [DOI] [PubMed] [Google Scholar]
- STONE D.M., JOHNSON M., HANSON G.R., GIBB J.W. Role of endogenous dopamine in the central serotonergic deficits induced by 3,4-methylenedioxymethamphetamine. J. Pharmacol. Exp. Ther. 1988;247:79–87. [PubMed] [Google Scholar]
- STONE D.M., JOHNSON M., HANSON G.R., GIBB J.W. Acute inactivation of tryptophan hydroxylase by amphetamine analogs involves the oxidation of sulfhydryl sites. Eur. J. Pharmacol. 1989;172:93–97. doi: 10.1016/0922-4106(89)90048-5. [DOI] [PubMed] [Google Scholar]
- STONE D.M., MERCHANT K.M., HANSON G.R., GIBB J.W. Immediate and long-term effects of 3,4-methylenedioxymethamphetamine on serotonin pathways in brain of rat. Neuropharmacology. 1987;12:1677–1683. doi: 10.1016/0028-3908(87)90117-1. [DOI] [PubMed] [Google Scholar]
- STONE D.M., STAHL D.C., HANSON G.R., GIBB J.W. The effects of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. Eur. J. Pharmacol. 1986;12:41–48. doi: 10.1016/0014-2999(86)90555-8. [DOI] [PubMed] [Google Scholar]
- SUMNALL H.R., O'SHEA E., MARSDEN C.A., COLE J.C. The effects of MDMA pretreatment on the behavioural effects of other drugs of abuse in the rat elevated plus-maze test. Pharmacol. Biochem. Behav. 2004;77:805–814. doi: 10.1016/j.pbb.2004.02.007. [DOI] [PubMed] [Google Scholar]
- WANG X., BAUMANN M.H., XU H., MORALES M., ROTHMAN R.B. 3,4-methylenedioxymethamphetamine (MDMA) administration to rats does not decrease levels of the serotonin transporter protein or alter its distribution between endosomes and the plasma membrane. J. Pharmacol. Exp. Ther. 2005;314:1002–1012. doi: 10.1124/jpet.105.088476. [DOI] [PubMed] [Google Scholar]
- WANG X., BAUMAN M.H., XU H., ROTHMAN R.B. 3,4-methylenedioxymethamphetamine (MDMA) administration to rats decreases brain tissue serotonin but not serotonin transporter protein and glial fibrillary acidic protein. Synapse. 2004;53:240–248. doi: 10.1002/syn.20058. [DOI] [PubMed] [Google Scholar]
- YUAN J., CORD B.J., MCCANN U.D., CALLAHAN T., RICAURTE G.A. Effect of depleting vesicular and cytoplasmic dopamine on methylenedioxymethamphetamine neurotoxicity. J. Neurochem. 2002;80:960–969. doi: 10.1046/j.0022-3042.2002.00758.x. [DOI] [PubMed] [Google Scholar]
- XIE T., TONG L., MCLANE M.W., HATZIDIMITRIOU G., YUAN J., MCCANN U., RICAURTE G.Loss of serotonin transporter protein after MDMA and other ring-substituted amphetamines Neuropsychopharmacology 2006 10.1038/sj.npp.1301031doi [DOI] [PubMed]