Abstract
Chronic renal disease is associated with oxidative stress, reduced nitric oxide (NO) availability and soluble guanylate cyclase (sGC) dysfunction. Recently, we discovered BAY 58-2667, a compound activating heme-deficient or oxidized sGC in a NO-independent manner.
We assessed potential of BAY 58-2667 in preventing cardiac and renal target organ damage in rats with 5/6 nephrectomy.
Male Wistar rats were allocated to three groups: 5/6 nephrectomy, 5/6 nephrectomy treated with BAY 58-2667 and sham operation. Study period was 18 weeks: blood pressure and creatinine clearance were assessed repeatedly. At study end blood samples were taken and hearts and kidneys harvested for histological studies.
BAY 58-2667 markedly lowered blood pressure in animals with 5/6 nephrectomy (untreated versus treated animals: 189±14 versus 146±11 mmHg, P<0.001). Left ventricular weight, cardiac myocyte diameter as well as cardiac arterial wall thickness significantly decreased in comparison to untreated animals with 5/6 nephrectomy. Natriuretic peptide plasma levels were also improved by BAY 58-2667. Kidney function and morphology as assessed by creatinine clearance, glomerulosclerosis, interstitial and perivascular fibrosis of intrarenal arteries were likewise significantly improved by BAY 58-2667.
This is the first study showing that BAY 58-2667 effectively lowers blood pressure, reduces left ventricular hypertrophy and slows renal disease progression in rats with 5/6 nephrectomy by targeting mainly oxidized sGC. Therefore, BAY 58-2667 represents a novel pharmacological principle with potential clinical value in treatment of chronic renal disease.
Keywords: Soluble guanylate cyclase, heme, BAY 58-2667, cyclic GMP, 5/6 nephrectomy, hypertension, chronic renal failure, left ventricular hypertrophy, rat
Introduction
The prevalence of end stage renal disease in Europe is 700 cases per million inhabitants; the prevalence of chronic kidney disease in earlier stages is estimated to exceed this number by as much as 50 times thus creating a major health care and economic challenge (El Nahas & Bello, 2005). Moreover, cardiovascular disease (CVD) such as coronary disease, heart failure, peripheral vascular disease and cerebrovascular disease has a high prevalence in patients with chronic renal failure (CRF) as a study in the US with patients at the initiation of dialysis showed a prevalence of 52% (Foley et al., 2003; Haffner et al., 2005). Owing to pressure and volume overload in renal disease, left ventricular hypertrophy (LVH) is described as the most frequent cardiac alteration, which has an important impact on the mortality of these patients (Silberberg et al., 1989; Foley et al., 1995). Those facts create a need for new therapeutic strategies to slow renal disease progression and prevent the detrimental cardiac alterations in CRF patients.
Soluble guanylate cyclase (sGC) is the receptor for the ubiquitous nitric oxide (NO). NO exerts its effects by binding to the prosthetic heme group at the beta-subunit of the sGC and thereby activating the enzyme that catalyzes the conversion of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP) (Gruetter et al., 1979; 1981; Wedel et al., 1994; Hobbs, 1997). cGMP therefore is a second messenger which affects various physiological processes such as vasodilatation and inhibition of platelet aggregation (Radomski et al., 1990). Those actions make activation of the sGC a promising tool in treating cardiovascular diseases. Up to now organic nitrates, which mimic the action of NO, are used in the clinic for that purpose, but they require enzymatic bioactivation and suffer from the phenomenon of nitrate tolerance (Feelisch, 1998).
Recently, we identified a novel NO-independent activator of sGC BAY 58-2667 (4-[((4-carboxybutyl){2-[(4-phenethyl-benzyl)oxy]phenethyl}amino)-methyl[benzoic]acid) through an ultra-high-throughput screening (Stasch et al., 2002). This compound is characterized by the intriguing feature of activating sGC more potently after oxidation of its heme moiety via the sGC inhibitor and hemeoxidant, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ). BAY 58-2667 shows remarkable in vitro and in vivo effects on vasorelaxation and inhibition of platelet aggregation; the vasodilating effect was not altered by pre-existing nitrate tolerance. The oral administration of the compound to spontaneously hypertensive rats led to a long-lasting decrease in blood pressure. Interestingly, BAY 58-2667 exerts its vasorelaxing effect both on arterial and venous vessels (Stasch et al., 2002).
Taken those aspects together this compound might be a promising tool in treating hypertension and preventing hypertensive target organ damage such as LVH and ischemic heart disease associated with oxidative stress. As there is currently no data available on the potential of this compound to prevent cardiac target organ damage in hypertension, we conducted this study to investigate the effects of a long-term oral administration of BAY 58-2667 in a rat model of CRF with special attention on hemodynamic and cardiovascular effects.
Methods
Chemicals
The sGC activator BAY 58-2667 (4-[((4-carboxybutyl){2-[(4-phenethyl-benzyl)oxy]phenethyl}amino)-methyl[benzoic]acid) was prepared as recently described (Stasch et al., 2002). Unless otherwise stated all other reagents were of analytical grade and were purchased from SIGMA (Seelze, Germany), MERCK (Darmstadt, Germany) and ROTH (Karlsruhe, Germany).
Animal model
In order to perform 5/6 nephrectomy the animals were anesthetized with isoflurane and placed on a heated table to maintain normal body temperature. The right kidney was exposed via flank incision and removed. After a 2-week recovery period, the left kidney was exposed accordingly and 2/3 were surgically removed.
Study design
Animal studies were carried out in accordance with local ethical regulations for the use of laboratory animals. Male Wistar rats at the age of 9–10 weeks weighing 320–340 g were randomly allocated to three groups: 5/6 nephrectomy (n=15), 5/6 nephrectomy plus treatment with BAY 58-2667 (3000 parts per million (p.p.m). in the solid feed/about 50 mg day−1; n=12) and sham-operation (OP) (n=10). All animals received a commercial diet (Altromin®; Altromin Co., Lage, Germany) and water ad libitum during the study period. After the 5/6 nephrectomy the animals were given 1 week of recovery from surgery (week 0) before the oral administration of the substance was started; furthermore GFR and blood pressure were assessed during this period (see below) in order to exclude differences between uraemic animals before drug treatment. The duration of the study was 18 weeks. During the study period the animals were weighed weekly, blood pressure was assessed via the tail-cuff method during week 0, 2, 5, 9, 15. The animals were placed in metabolic cages to obtain 24 h urine samples at week 0, 4, 17; at the same time blood was taken from retro-orbital veins for the single purpose of measuring plasma creatinine levels and calculate creatinine clearance using standard formula. In week 18 animals were killed, blood samples were taken to assess plasma levels of aspartate amino transferase (AST), alanine amino transferase (ALT), alkaline phosphatase (AP), glutamate dehydrogenase (GLDH), lactate dehydrogenase (LDH), creatine kinase (CK), creatinin and as described before (Haffner et al., 2005). The hearts and kidneys were harvested for histological studies, organ weights were measured.
Laboratory chemistry
Plasma renin activity (PRA), atrial natriuretic peptide (ANP) and b-type natriuretic peptide (BNP) in plasma were measured at the end of the study period as previously described (Stasch et al., 2002; Dumitrascu et al., 2006).
Histological studies
Tissue samples were all embedded in paraffin, cut into 3 and 1 μm sections, subjected to hematoxylin–eosin (HE), Sirius red, perjodic acid-Schiff- (PAS) and Elastica-van Gieson staining. Quantitative stereology (i.e. intima/media and lumen area of the arteries) was analyzed using a computer-aided image analysis system as previously described (Hocher et al., 1999). Cardiac and renal morphology (interstitial fibrosis, perivascular fibrosis, glomerulosclerosis and media/lumen ratio of blood vessels) were measured as recently described (Hocher et al., 2000; Haffner et al., 2005). In brief, glomerulosclerosis was defined by the presence of PAS-positive material within the glomeruli. To quantify the amount of glomerulosclerosis a semiquantitative score was used; two investigators, who had no knowledge of the groups to which the rats belonged, judged the results.
The severity of interstitial fibrosis was evaluated after Sirius Red staining using computer-aided histomorphometry devices. In brief, at least 30 microscopic pictures per kidney/heart section were transferred to a PowerMAC via Hitachi-CCD-camera. After manually setting a threshold using a randomly chosen subset of the pictures, we measured the relationship of SR-stained area (connective tissue) to total area of the picture using ImageJ, an image processing software (shareware from the NIH).
Accordingly, microscopic pictures of kidney/heart sections after Elastica-van Gieson staining showing arterial blood vessels were generated. We measured the area contents of the media and the lumen of intrarenal/intracardial arteries using the ImageJ program; afterwards media/lumen ratio was calculated serving as marker for arterial wall thickening.
Using 1 μm-sections of the heart in HE-staining pictures were generated as described above and myocyte diameter was measured with ImageJ.
Perivascular fibrosis was judged after Sirius-Red staining using a semiquantitative score by two independent investigators blinded to the groups to which the animals belonged.
Plasma level of BAY 58-2667
Samples were subjected to high-performance liquid chromatography performed on a 2300 HTLC system (Cohesive Technologies, Franklin, U.S.A.) as described (Dumitrascu et al., 2006). Briefly, the mobile phase consisted of 10 mM ammonium acetate (pH 3.0) and acetonitrile. A linear gradient from 20 to 85% acetonitrile (vol vol−1) within 1 min was applied. Tandem mass spectrometry was performed on an API 3000 triple-quadrupole mass spectrometer (PE Sciex, Wellesley, U.S.A.) connected to the 2300 HTLC system through a turbospray interface. The lower limit for quantification of BAY 58-2667 was 0.5 μg l−1.
Statistical analysis
To detect any significant differences between the three groups the Kruskal–Wallis test was applied; the Mann–Whitney test was used to detect significant differences between two groups of interest. Results (given as mean±standard deviation (s.d.)) were considered significant when the probability error (P) was less than 0.05.
Results
Mortality
Treatment with BAY 58-2667 was tolerated well without any side effects. During the study, six out of 15 animals (40%) in the 5/6 NX group and three out of 12 animals (25%) of the 5/6 NX+BAY 58-2667 group died, whereas all sham-OP animals survived.
Laboratory results
At the end of the 18 week study period, the animals were killed, organs were harvested for histological studies and blood samples were obtained. The laboratory results from the blood samples are shown in Table 1. There were no significant or clinically relevant differences between the groups regarding AST, ALT, AP, GLDH, LDH and CK. Both groups with 5/6 nephrectomy had higher plasma levels of creatinine and urea compared with the sham-OP group, but in the group treated with BAY 58-2667 this increase was significantly diminished compared with the untreated animals. Plasma levels of protein were lower in both uraemic groups compared with the sham group. Regarding PRA we observed that in both nephrectomized groups the PRA was significantly suppressed compared to sham animals. BNP levels were significantly elevated in both uraemic groups compared with sham controls, but in the group treated with BAY 58-2667 there was a strong trend (P=0.05) towards lower levels compared with untreated uraemic animals. A similar trend was observed with respect to ANP, however, without reaching statistical significance.
Table 1.
Plasma laboratory results and body weights at the end of the study
| Parameter (unit) | 5/6 NX (n=9) | 5/6 NX+BAY 58-2667 (n=9) | Sham OP (n=10) |
|---|---|---|---|
| Body weight (g) |
415.0±49.3* |
391.2±50.7* |
502.0±75.4 |
| AST (U/l) |
51.0±28.7 |
51.6±29.5 |
55.5±11.1 |
| ALT (U/l) |
33.5±14.1 |
46.3±22.0 |
40.8±9.8 |
| AP (U/l) |
77.6±21.4 |
95.4±28.7 |
88.7±11.0 |
| GLDH (U/l) |
14.4±14.7 |
10.1±10.4 |
11.4±11.6 |
| LDH (U/l) |
107.3±83.8 |
104.1±70.1 |
132.8±45.5 |
| CK (U/l) |
96.1±42.0 |
105.2±53.4 |
109.5±25.3 |
| Crea (μmol/l) |
209.3±110.1** |
97.8±29.0**,† |
51.7±2.4 |
| Urea (mmol/l) |
52.8±46.9** |
18.5±6.0**,† |
6.0±0.9 |
| Protein (g/l) |
55.0±5.0** |
54.4±2.7** |
65.7±1.7 |
| PRA (ng/ml/h) |
0.7±0.5** |
1.3±1.0* |
2.6±1.2 |
| ANP (pg/ml) |
487.3±162.1 |
346.6±160.8 |
319.0±162.8 |
| BNP (pg/ml) | 47.3±19.3** | 33.3±11.0*,(†) | 16.7±7.9 |
Abbreviations: AP=alkaline phosphatase; ALT=alanine amino transferase; ANP=atrial natriuretic peptide; AST=aspartate amino transferase; BNP=B-type natriuretic peptide; CK=creatine kinase; Crea=creatinin; GLDH=glutamate dehydrogenase; LDH=lactate dehydrogenase; PRA=plasma renin activity.
Values are given as mean±s.d.
P<0.05/0.001 versus Sham OP;
P<0.05/0.001 versus Sham OP;
P<0.05 versus 5/6 NX;
P=0.05 versus 5/6 NX.
Creatinine clearance and albuminuria
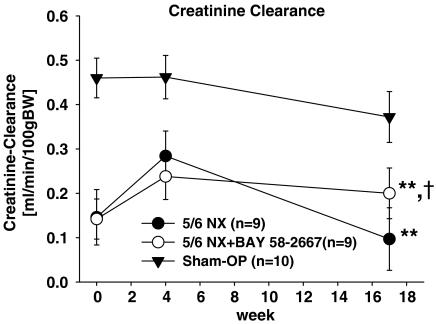
The creatinine clearance was calculated from blood and urine creatinine at week 0, 4, 17. The results are illustrated in Figure 1. Both groups with 5/6 nephrectomy had markedly lower GFR than the sham group during the time course of the experiment. Both uraemic groups started with the same GFR, but at the end of the experiment the group treated with BAY 58-2667 had a significantly higher GFR than the untreated group.
Figure 1.
Creatinine clearance. All values are given as mean±s.d. **P<0.001 versus sham OP. †P<0.05 versus 5/6 NX.
At the last urine collection (week 17) urinary albumin excretion was measured. The sham-OP animals exhibited a significantly lower urinary albumin excretion (30.7±30.4 mg 24 h−1) compared to both uraemic groups; no difference was detected between uraemic animals treated with BAY 58-2667 (297.9±102.3 mg 24 h−1) and without treatment (274.1±131.2 mg 24 h−1).
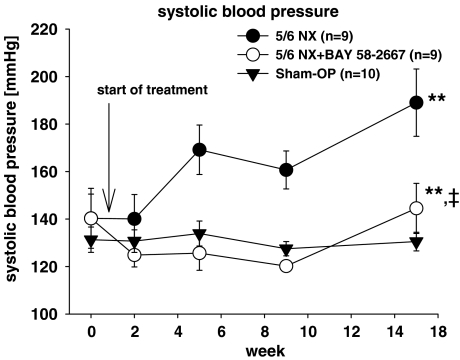
Blood pressure
The blood pressure was assessed via the tail-cuff method during week 0, 2, 5, 9, 15. As shown in Figure 2 the systolic blood pressure increased markedly in the untreated nephrectomized group compared with the sham controls. Treatment with BAY 58-2667 remarkably diminished this effect during the observation period.
Figure 2.
Systolic blood pressure. All values are given as mean±s.d.. **P<0.001 versus sham OP. ‡P<0.001 versus 5/6 NX.
Body and organ weight
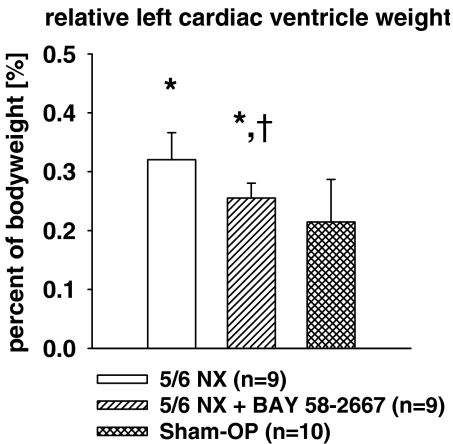
Body weight at the time of killing significantly differed between the groups: as shown in Table 1 both uraemic groups weighted less than the sham-OP controls. Left cardiac ventricle weight – expressed as a percentage of body weight – increased significantly in both uraemic groups compared to sham controls, but the effect was significantly diminished by treatment with BAY 58-2667 (Figure 3).
Figure 3.
Relative left cardiac ventricle weight. All values are given as mean±s.d.. *P<0.05 versus sham OP. †P<0.05 versus 5/6 NX.
Plasma levels of BAY 58-2667
Food intake and body weight were rising constantly during the study period thus indicating a stable drug administration throughout the study. Plasma levels of BAY 58-2667 were 233±29.2 μg l−1 in the treatment group at the end of the study.
Cardiac histology
At the end of the study period the hearts were harvested for histological study; the results are illustrated in Table 2.
Table 2.
Histology results of the heart
| Parameter (unit) | 5/6 NX (n=9) | 5/6 NX+BAY 58-2667 (n=9) | Sham OP (n=10) |
|---|---|---|---|
| Myocyte diameter (μm) |
19.0±4.6** |
14.8±2.3*,† |
12.0±0.9 |
| Media-lumen-ratio |
2.7±1.1* |
1.5±0.8† |
1.5±0.6 |
| Interstitial fibrosis (% of section) |
2.3±1.3 |
1.9±0.8 |
2.4±0.7 |
| Perivascular fibrosis (score) | 2.1±0.5 | 1.8±0.4 | 1.9±0.5 |
Values are given as mean±s.d.
P<0.05/0.001 versus Sham-OP;
P<0.05/0.001 versus Sham-OP;
P<0.05 versus 5/6 NX.
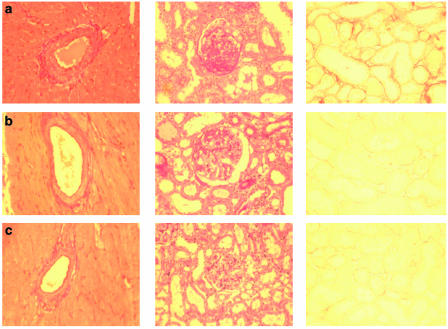
The media-lumen-ratio of cardiac arteries was calculated using computer-aided morphometry devices (for illustration, see Figure 4). The media-lumen-ratio of untreated uraemic animals increased significantly compared to sham controls. Treatment with BAY 58-2667 completely abolished this effect. The diameter of cardiac myocytes was accordingly measured using 1 μm slices in HE staining. We discovered that in both uraemic groups the mean diameter of cardiomyocytes increased significantly versus sham controls, but treatment with BAY 58-2667 effectively diminished this increase. Perivascular and interstitial fibrosis of the hearts were judged using 3 μm slices in Sirius-Red-staining; no statistically significant differences between all three study groups could be observed.
Figure 4.
Typical sections of heart and kidney. (a) 5/6 NX; (b) 5/6 NX+BAY 58-2667; (c) sham OP. First column: typical cardiac arteries in Elastica-van Gieson staining, Magnitude × 200. Second column: typical kidney sections in PAS staining, Magnitude × 200. Presence of PAS-positive material (pink color) within glomeruli indicates glomerulosclerosis. Third column: typical kidney sections in Sirius-Red-staining, Magnitude × 200. Red color indicates fibrotic areas.
Renal histology
At the end of the study period the kidneys were harvested for histological study; the results are shown in Table 3. Regarding the extent of glomerulosclerosis using a semiquantitative scoring system on tissue slices in PAS staining (for illustration, see Figure 4), we found a significant increase in both uraemic groups versus sham controls, but this effect was markedly diminished in animals treated with BAY 58-2667 compared with untreated animals. The same pattern was present when we investigated renal perivascular fibrosis in Sirius-Red staining. Using computer-aided histomorphometry devices we also measured the extent of interstitial fibrosis in the kidney (for illustration, see Figure 4). We observed significant increase in renal interstitial fibrosis in untreated uraemic groups versus sham controls, but this effect was completely abolished by treatment with BAY 58-2667. No significant differences between all study groups existed regarding the media-lumen-ratio of intrarenal arteries.
Table 3.
Histology results of the kidney
| Parameter (unit) | 5/6 NX (n=9) | 5/6 NX+BAY 58-2667 (n=9) | Sham OP (n=10) |
|---|---|---|---|
| Glomerulosclerosis (score) |
2.4±0.6** |
1.4±0.3*,† |
1.0±0.04 |
| Media-lumen-ratio |
3.5±0.7 |
3.1±0.8 |
3.3±0.8 |
| Interstitial fibrosis (% of section) |
2.4±1.3** |
0.4±0.3† |
0.2±0.2 |
| Perivascular fibrosis (score) | 2.1±0.3** | 1.6±0.3*,† | 1.2±0.2 |
Values are given as mean±s.d.
P<0.05/0.001 versus Sham OP;
P<0.05/0.001 versus Sham OP;
P<0.05/0.001 versus 5/6 NX.
Discussion
This study is the first to evaluate and describe the renal and cardiovascular consequences of long-term sGC activation by the novel NO-independent sGC activator BAY 58-2667 in a rat model of CRF (Stasch et al., 2002; Schmidt et al., 2004). BAY 58-2667 is characterized by activating sGC in an NO-independent manner and even more potently activating sGC after oxidation of its heme group. We recently showed that the activity of BAY 58-2667 is also potentiated in cells, aortas from different species and in vivo under oxidative stress conditions (Rothkegel et al., 2005). CRF is associated with diminished NO availability which is caused by a combination of reduced NO production (Vaziri et al., 1998; Roczniak et al., 1999; Schmidt & Baylis, 2000), depletion/inactivation of NO by reactive oxygen species (Vallance et al., 1992; Vaziri et al., 2002; Vaziri, 2004b) and by a dysfunction of sGC (Sindhu et al., 2004; Vaziri, 2004a). The associated NO deficiency and dysfunctional sGC, in turn, promotes hypertension and accelerates progression of renal disease (Himmelfarb et al., 2002; Vaziri, 2004b). We, therefore, hypothesized that targeting dysfunctional sGC in an NO-independent manner by BAY 58-2667 may attenuate hypertension and retard progression of renal disease by raising the intracellular cGMP.
Regarding plasma parameters provided in Table 1, which were intended to screen for main organ system dysfunctions, we can exclude major side effects of treatment with Bay 58-2667 such as cardio-, nephro- or hepatotoxicity and rhabdomyolysis. With regard to mortality we did not observe increased mortality in the group treated with BAY 58-2667 when compared to untreated animals. In rats with renal mass ablation PRA was markedly suppressed, whereas BNP, urea and creatinine in plasma were significantly elevated in the nephrectomized, uraemic groups compared to the sham-OR group. These findings are in good agreement with published data of this model of CRF (Strauch & Gretz, 1988; Gretz, 1995).
The NO-independent sGC activator BAY 58-2667 caused lower rise of BNP, urea and creatinine levels within the treated 5/6 nephrectomized group suggesting cardiorenal protective effects of this compound. This is consistent with our data regarding creatinine clearance: both uraemic groups started at the same level, but at the end of the study period the group treated with BAY 58-2667 showed a significantly higher clearance than the untreated group, thus indicating that BAY 58-2667 also slowed the progression of renal disease in our animal model of CRF.
However, this beneficial effect on renal function was not extended on albuminuria; in our study treatment with BAY 58-2667 failed to exert an antiproteinuric effect. This is consistent with literature describing compounds (e.g. certain calcium antagonist subclasses), which are lacking specific antiproteinuric effects despite potent antihypertensive action (Nathan et al., 2005). Further studies are needed to confirm and elucidate the effects of sGC agonistic compounds on proteinuria.
BNP and ANP are released in conditions related to increased cardiac wall stretch (Angermann & Ertl, 2004; McCullough, 2004). BNP compared to ANP is recognized as the superior marker for left ventricular dysfunction and has a powerful diagnostic and prognostic value in patients with CRF and is also responsive to medical treatment (Hocher et al., 2004; Silver et al., 2004; Takami et al., 2004). Suggesting that a rise in BNP levels in both uraemic groups reflects a cardiac impairment due to uraemia, lower plasma BNP levels in animals treated with BAY 58-2667 underline the cardiorenal protective effects of this compound.
Regarding cardiac target organ damage we were able to demonstrate a beneficial effect of BAY 58-2667 on morphology, by left ventricular weight and myocyte diameter reduction, as well as on vasculature, by completely abolishing cardiac arterial wall thickening compared with the untreated uraemic group. We suggest that these findings can mainly be attributed to the observed reduction of systemic of blood pressure in the BAY 58-2667 treated group, although there is evidence that arterial wall thickening in CRF also occurs independently from blood pressure (Kakinuma et al., 1992; Amann et al., 1995; Tornig et al., 1996). In addition, it should be noted that animals treated with BAY 58-2667 have a better kidney function as compared to untreated uraemic rats. This might also have a beneficial effect on the cardiac vasculature.
The improved kidney function and morphology in ureamic rats treated with BAY 58-2667 too can most likely be attributed to reduction of systemic blood pressure. Blood pressure is known to be of major impact on renal disease progression in the setting of CRF (Bidani & Griffin, 2004). The antihypertensive potential of BAY 58-2667 in our study is equal to the effect of established antihypertensive agents like enalapril (Okada et al., 2004) or candesartan (Noda et al., 1999) in a similar study design. However, we state that interpretations regarding blood pressure in our study are limited to the observation period: at the last measurement (week 15) blood pressure in the treatment group increased significantly versus untreated uraemic animals; as we have no data beyond that point, further studies are needed to elucidate if blood pressure and target organ damage can effectively be controlled by treatment with BAY 58-2667 in long-term studies.
Considering that sGC activating compounds are known to have specific antifibrotic properties in the kidney (Wang et al., 2005), we assume that in our study there might have been additional beneficial effects independent from blood pressure reduction. However, as we observed major differences regarding systemic blood pressure between our groups, our study cannot give evidence for putative blood pressure independent effects of BAY 58-2667. Further studies with low doses of BAY 58-2667 which leave systemic blood pressure unaffected are warranted to clarify this point.
Very recently it has been shown that inhibition of phosphodiesterase 5 by sildenafil (ViagraR) treatment prevented hypertension and deterioration of renal function, reduced histologic damage, inflammation and apoptosis, delayed the onset of proteinuria, and preserved renal capillary integrity in 5/6 nephrectomy by increased availability of cGMP (Rodriguez-Iturbe et al., 2005). Moreover, in a second protocol sildenafil was compared with losartan and the combination of both drugs in established renal disease, starting these drugs 4 weeks after 5/6 nephrectomy. Delayed sildenafil treatment failed to improve proteinuria and glomerulosclerosis but ameliorated hypertension and azotemia (Rodriguez-Iturbe et al., 2005).
In conclusion, our study demonstrated for the first time that treatment with the new NO-independent sGC activator BAY 58-2667 in a setting of CRF effectively lowers blood pressure, reduces LVH and preserves renal function and morphology. Therefore, NO-independent activation of sGC as exemplified by BAY 58-2667 is a novel pharmacological principle and has the potential clinical value in the treatment of chronic renal disease.
Acknowledgments
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) (Ho 1665/5-2) to Dr B Hocher and a grant from the Dr-Werner-Jackstaedt-Stiftung to Dr P Kalk. We thank Dr Mark Jean Gnoth for measuring BAY 58-2667 plasma levels. The technical assistance of Norbert Schulz, Ralf Hartkopf and Yvonne Keim is appreciated.
Abbreviations
- ALT
alanine amino transferase
- ANP
atrial natriuretic peptide
- AP
alkaline phosphatase
- AST
aspartate amino transferase
- BNP
brain natriuretic peptide
- cGMP
cyclic guanosine monophosphate
- CK
creatine kinase
- CRF
chronic renal failure
- CVD
cardiovascular disease
- GLDH
glutamate dehydrogenase
- GTP
guanosine triphosphate
- HE
hematoxylin eosin
- LDH
lactate dehydrogenase
- LVH
left ventricular hypertrophy
- NO
nitric oxide
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PAS
periodic acid Schiff
- p.p.m.
parts per million
- sGC
soluble guanylate cyclase
Conflict of interest:
As stated in the affiliations, some authors are research employees of Bayer Healthcare, Wuppertal.
References
- AMANN K., NEUSUSS R., RITZ E., IRZYNIEC T., WIEST G., MALL G. Changes of vascular architecture independent of blood pressure in experimental uremia. Am. J. Hypertens. 1995;8:409–417. doi: 10.1016/0895-7061(94)00248-a. [DOI] [PubMed] [Google Scholar]
- ANGERMANN C.E., ERTL G. Natriuretic peptides – new diagnostic markers in heart disease. Herz. 2004;29:609–617. doi: 10.1007/s00059-004-2619-8. [DOI] [PubMed] [Google Scholar]
- BIDANI A.K., GRIFFIN K.A. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension. 2004;44:595–601. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- DUMITRASCU R., WEISSMANN N., GHOFRANI H.A., DONY E., BEUERLEIN K., SCHMIDT H., STASCH J.P., GNOTH M.J., SEEGER W., GRIMMINGER F., SCHERMULY R.T. Activation of soluble guanylate cyclase reverses experimental pulmonary hypertension and vascular remodeling. Circulation. 2006;113:286–295. doi: 10.1161/CIRCULATIONAHA.105.581405. [DOI] [PubMed] [Google Scholar]
- EL NAHAS M., BELLO A.K. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–340. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- FEELISCH M. The use of nitric oxide donors in pharmacological studies. Naunyn Schmiedebergs Arch. Pharmacol. 1998;358:113–122. doi: 10.1007/pl00005231. [DOI] [PubMed] [Google Scholar]
- FOLEY R.N., HERZOG C.A., COLLINS A.J. Smoking and cardiovascular outcomes in dialysis patients: the United States Renal Data System Wave 2 study. Kidney Int. 2003;63:1462–1467. doi: 10.1046/j.1523-1755.2003.00860.x. [DOI] [PubMed] [Google Scholar]
- FOLEY R.N., PARFREY P.S., HARNETT J.D., KENT G.M., MURRAY D.C., BARRE P.E. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J. Am. Soc. Nephrol. 1995;5:2024–2031. doi: 10.1681/ASN.V5122024. [DOI] [PubMed] [Google Scholar]
- GRETZ N. The development of hypertension in the remnant kidney model after either pole resection or partial infarction of the kidney. J. Am. Soc. Nephrol. [Letter] 1995;5:1839–1840. doi: 10.1681/ASN.V5101839. [DOI] [PubMed] [Google Scholar]
- GRUETTER C.A., BARRY B.K., MCNAMARA D.B., GRUETTER D.Y., KADOWITZ P.J., IGNARRO L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J. Cyclic. Nucleotide. Res. 1979;5:211–224. [PubMed] [Google Scholar]
- GRUETTER C.A., KADOWITZ P.J., IGNARRO L.J. Methylene blue inhibits coronary arterial relaxation and guanylate cyclase activation by nitroglycerin, sodium nitrite, and amyl nitrite. Can. J. Physiol Pharmacol. 1981;59:150–156. doi: 10.1139/y81-025. [DOI] [PubMed] [Google Scholar]
- HAFFNER D., HOCHER B., MULLER D., SIMON K., KONIG K., RICHTER C.M., EGGERT B., SCHWARZ J., GODES M., NISSEL R., QUERFELD U. Systemic cardiovascular disease in uremic rats induced by 1,25(OH)2D3. J. Hypertens. 2005;23:1067–1075. doi: 10.1097/01.hjh.0000166849.72721.1c. [DOI] [PubMed] [Google Scholar]
- HIMMELFARB J., STENVINKEL P., IKIZLER T.A., HAKIM R.M. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- HOBBS A.J. Soluble guanylate cyclase: the forgotten sibling. Trends Pharmacol. Sci. 1997;18:484–491. doi: 10.1016/s0165-6147(97)01137-1. [DOI] [PubMed] [Google Scholar]
- HOCHER B., GEORGE I., DIEKMANN F., ZART R., REBSTOCK J., SCHWARZ A., THONE-REINEKE C., NEUMAYER H.H., BAUER C. ETA receptor blockade induces fibrosis of the clipped kidney in two-kidney-one-clip renovascular hypertensive rats. J. Hypertens. 2000;18:1807–1814. doi: 10.1097/00004872-200018120-00015. [DOI] [PubMed] [Google Scholar]
- HOCHER B., GEORGE I., REBSTOCK J., BAUCH A., SCHWARZ A., NEUMAYER H.H., BAUER C. Endothelin system-dependent cardiac remodeling in renovascular hypertension. Hypertension. 1999;33:816–822. doi: 10.1161/01.hyp.33.3.816. [DOI] [PubMed] [Google Scholar]
- HOCHER B., ZIEBIG R., KRAUSE R., ASMUS G., NEUMAYER H.H., LIEFELDT L., STASCH J.P. Relaxin is an independent risk factor predicting death in male patients with end-stage kidney disease. Circulation. 2004;109:2266–2268. doi: 10.1161/01.CIR.0000128598.72920.B5. [DOI] [PubMed] [Google Scholar]
- KAKINUMA Y., KAWAMURA T., BILLS T., YOSHIOKA T., ICHIKAWA I., FOGO A. Blood pressure-independent effect of angiotensin inhibition on vascular lesions of chronic renal failure. Kidney Int. 1992;42:46–55. doi: 10.1038/ki.1992.259. [DOI] [PubMed] [Google Scholar]
- MCCULLOUGH P.A. Clinical applications of B-type natriuretic peptide levels in the care of cardiovascular patients. Minerva Cardioangiol. 2004;52:479–489. [PubMed] [Google Scholar]
- NATHAN S., PEPINE C.J., BAKRIS G.L. Calcium antagonists: effects on cardio-renal risk in hypertensive patients. Hypertension. 2005;46:637–642. doi: 10.1161/01.HYP.0000184541.24700.c7. [DOI] [PubMed] [Google Scholar]
- NODA M., MATSUO T., FUKUDA R., OHTA M., NAGANO H., SHIBOUTA Y., NAKA T., NISHIKAWA K., IMURA Y. Effect of candesartan cilexetil (TCV-116) in rats with chronic renal failure. Kidney Int. 1999;56:898–909. doi: 10.1046/j.1523-1755.1999.00614.x. [DOI] [PubMed] [Google Scholar]
- OKADA K., OKAWA E., SHIBAHARA H., MARUYAMA T., MARUYAMA N., MATSUMOTO K., TAKAHASHI S. Combination therapy with angiotensin-converting enzyme inhibitor and oral adsorbent of uremic toxins can delay the appearance of glomerular sclerosis and interstitial fibrosis in established renal failure. Kidney Blood Press Res. 2004;27:218–225. doi: 10.1159/000079869. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M., MONCADA S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. U.S.A. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROCZNIAK A., FRYER J.N., LEVINE D.Z., BURNS K.D. Downregulation of neuronal nitric oxide synthase in the rat remnant kidney. J. Am. Soc. Nephrol. 1999;10:704–713. doi: 10.1681/ASN.V104704. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ-ITURBE B., FERREBUZ A., VANEGAS V., QUIROZ Y., ESPINOZA F., PONS H., VAZIRI N.D. Early treatment with cGMP phosphodiesterase inhibitor ameliorates progression of renal damage. Kidney Int. 2005;68:2131–2142. doi: 10.1111/j.1523-1755.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- ROTHKEGEL C., SCHMIDT PM., KUMAR A., STOLL F., LAPP H., WUNDER F., SCHRODER H., RINKE M., SCHMIDT H.H., STASCH J.P. Beyond NO and heme: biochemical and pharmacological opportunities. BMC Pharmacol. 2005;5 Suppl 1:S18. [Google Scholar]
- SCHMIDT P.M., SCHRAMM M., SCHRODER H., WUNDER F., STASCH J.P. Identification of residues crucially involved in the binding of the heme moiety of soluble guanylate cyclase. J. Biol. Chem. 2004;279:3025–3032. doi: 10.1074/jbc.M310141200. [DOI] [PubMed] [Google Scholar]
- SCHMIDT R.J., BAYLIS C. Total nitric oxide production is low in patients with chronic renal disease. Kidney Int. 2000;58:1261–1266. doi: 10.1046/j.1523-1755.2000.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILBERBERG J.S., BARRE P.E., PRICHARD S.S., SNIDERMAN A.D. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36:286–290. doi: 10.1038/ki.1989.192. [DOI] [PubMed] [Google Scholar]
- SILVER M.A., MAISEL A., YANCY C.W., MCCULLOUGH P.A., BURNETT J.C., JR, FRANCIS G.S., MEHRA M.R., PEACOCK W.F., FONAROW G., GIBLER W.B., MORROW D.A., HOLLANDER J. BNP Consensus Panel 2004: a clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases. Congest. Heart Fail. 2004;10:1–30. doi: 10.1111/j.1527-5299.2004.03271.x. [DOI] [PubMed] [Google Scholar]
- SINDHU R.K., EHDAIE A., VAZIRI N.D., ROBERTS C.K. Effects of chronic renal failure on caveolin-1, guanylate cyclase and AKT protein expression. Biochim. Biophys. Acta. 2004;1690:231–237. doi: 10.1016/j.bbadis.2004.06.013. [DOI] [PubMed] [Google Scholar]
- STASCH J.P., SCHMIDT P., ALONSO-ALIJA C., APELER H., DEMBOWSKY K., HAERTER M., HEIL M., MINUTH T., PERZBORN E., PLEISS U., SCHRAMM M., SCHROEDER W., SCHRODER H., STAHL E., STEINKE W., WUNDER F. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br. J. Pharmacol. 2002;136:773–783. doi: 10.1038/sj.bjp.0704778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUCH M., GRETZ N. Animal models to induce renal failure: a historical survey. Contrib. Nephrol. 1988;60:1–8. doi: 10.1159/000414783. [DOI] [PubMed] [Google Scholar]
- TAKAMI Y., HORIO T., IWASHIMA Y., TAKIUCHI S., KAMIDE K., YOSHIHARA F., NAKAMURA S., NAKAHAMA H., INENAGA T., KANGAWA K., KAWANO Y. Diagnostic and prognostic value of plasma brain natriuretic peptide in non-dialysis-dependent CRF. Am. J. Kidney Dis. 2004;44:420–428. [PubMed] [Google Scholar]
- TORNIG J., AMANN K., RITZ E., NICHOLS C., ZEIER M., MALL G. Arteriolar wall thickening, capillary rarefaction and interstitial fibrosis in the heart of rats with renal failure:the effects of ramipril, nifedipine and moxonidine. J. Am. Soc. Nephrol. 1996;7:667–675. doi: 10.1681/ASN.V75667. [DOI] [PubMed] [Google Scholar]
- VALLANCE P., LEONE A., CALVER A., COLLIER J., MONCADA S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- VAZIRI N.D. Oxidative stress in uremia: nature, mechanisms, and potential consequences. Semin. Nephrol. 2004a;24:469–473. doi: 10.1016/j.semnephrol.2004.06.026. [DOI] [PubMed] [Google Scholar]
- VAZIRI N.D. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr. Opin. Nephrol. Hypertens. 2004b;13:93–99. doi: 10.1097/00041552-200401000-00013. [DOI] [PubMed] [Google Scholar]
- VAZIRI N.D., NI Z., OVEISI F., LIANG K., PANDIAN R. Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension. 2002;39:135–141. doi: 10.1161/hy0102.100540. [DOI] [PubMed] [Google Scholar]
- VAZIRI N.D., NI Z., WANG X.Q., OVEISI F., ZHOU X.J. Downregulation of nitric oxide synthase in chronic renal insufficiency: role of excess PTH. Am. J. Physiol. 1998;274:F642–F649. doi: 10.1152/ajprenal.1998.274.4.F642. [DOI] [PubMed] [Google Scholar]
- WANG Y., KRAMER S., LOOF T., MARTINI S., KRON S., KAWACHI H., SHIMIZU F., NEUMAYER H.H., PETERS H. Stimulation of soluble guanylate cyclase slows progression in anti-thy1-induced chronic glomerulosclerosis. Kidney Int. 2005;68:47–61. doi: 10.1111/j.1523-1755.2005.00380.x. [DOI] [PubMed] [Google Scholar]
- WEDEL B., HUMBERT P., HARTENECK C., FOERSTER J., MALKEWITZ J., BOHME E., SCHULTZ G., KOESLING D. Mutation of His-105 in the beta 1 subunit yields a nitric oxide-insensitive form of soluble guanylyl cyclase. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2592–2596. doi: 10.1073/pnas.91.7.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]