Abstract
We developed a novel method to isolate nonpigmented epithelial (NPE) cells from porcine eyes in order to examine Na,K-ATPase responses to nitric oxide (NO) donors specifically in the epithelium.
Cells were treated with NO donors and other test compounds for 20 min prior to Na,K-ATPase activity measurement.
NO donors, sodium nitroprusside (SNP, 1 μM–1 mM), sodium azide (100 nM–1 μM) and S-nitroso-N-acetylpenicillamine (1 μM–1 mM) caused significant concentration-dependent inhibition of Na, K-ATPase activity. Detection of nitrite in the medium of L-arginine and SNP-treated NPE confirmed NO generation.
Concentration-dependent inhibition of Na,K-ATPase was also obtained by L-arginine (1–3 mM), a physiological precursor of NO and 8p-CPT-cGMP (1–100 μM), a cell permeable analog of cGMP. The L-arginine effect was abolished when the NO synthesizing enzyme, NO-synthase, was inhibited by L-NAME (100 μM).
The inhibitory effect of SNP or sodium azide on Na,K-ATPase activity was suppressed by soluble guanylate cyclase (sGC) inhibitors, ODQ (10 μM) or methylene blue (10 μM).
The inhibitory effect of 8p-CPT-cGMP on Na,K-ATPase was abolished by protein kinase G (PKG) inhibitors, H-8 (1 μM) and H-9 (20 μM), but not by the protein kinase A (PKA) inhibitor H-89 (100 nM). H-8 and H-9 partially suppressed the inhibitory effect of SNP on Na,K-ATPase.
Taken together the results indicate that Na,K-ATPase inhibition response to NO donors involves activation of sGC, generation of cGMP and activation of PKG. These findings suggest that Na, K-ATPase inhibition in NPE may contribute to the ability of NO donors to reduce aqueous humor secretion.
Keywords: Aqueous humor; Na,K-ATPase; nitric oxide; porcine eye; protein kinase G
Introduction
Aqueous humor is secreted continuously into the eye by the double-layered ciliary epithelium (CE) of the ciliary body. The fluid is important as a source of nutrients for the avascular tissues in the anterior part of the eye. Aqueous humor secretion also gives rise to the intraocular pressure (IOP), which has a major influence on eye shape, eye development and structural integrity conducive to optical function. IOP is regulated by a balance between the rate of aqueous humor production and its exit through the outflow pathways at the anterior chamber angle. Abnormally high IOP causes death of retinal ganglion cells, structural changes in the optic nerve head, and vision loss, a condition known as glaucoma. Therapeutic measures for glaucoma work by lowering IOP, either by reducing aqueous humor secretion or by increasing its outflow. Clearly there is an argument for development of IOP-lowering drugs that have a neuroprotective effect on failing retinal ganglion cells (Hartwick, 2001; Naskar & Dreyer, 2001). In this respect, it is interesting that nitric oxide (NO) donors have been shown to reduce IOP in normal (Nathanson, 1992; Behar-Cohen et al., 1996; Kotikoski et al., 2003) and glaucomatous animals (Wang & Podos, 1995) as well as in humans (Chuman et al., 2000). NO donors have also been reported to have significant neuroprotective properties (Imai et al., 1997; Mohanakumar et al., 2002; Nakazawa et al., 2002; Kitaoka & Kumai, 2004).
Using an arterially perfused isolated bovine eye to study the mechanism of IOP responses to NO, we reported that sodium azide, a vasodilator drug which acts through the generation of NO, lowers IOP by reducing aqueous humor formation (Millar et al., 2001). Recently, using the in vitro pig eye model, we confirmed that the inhibitory effect of NO donors on aqueous humor secretion could be suppressed by ODQ, a specific inhibitor of soluble guanylate cyclase (sGC), suggesting that the effect involved the generation of cGMP. Importantly, the ability of NO donors to reduce aqueous humor formation was found to be independent of their effect on ocular vasculature (Shahidullah et al., 2005). It is noteworthy that NO has been shown to inhibit fluid transport in other tissues including kidney (Ortiz & Garvin, 2002) and salivary gland (Lomniczi et al., 1998).
Aqueous humor formation requires coordinated action of several different ion transport mechanisms and ion channels in the CE (Civan, 1998b; To et al., 2002). The CE is a bilayer that consists of two types of cells in an apex to apex orientation. The inner layer of pigmented ciliary epithelium (PE) faces the stromal blood and the outer nonpigmented ciliary epithelium (NPE) layer faces the aqueous humor in the posterior chamber of the eye. Solute transport across the bilayer gives rise to osmotic water movement, resulting in AH formation. The major transport proteins identified in aqueous humor formation include Na,K-ATPase, Na-K-2Cl co-transporter, Na+/H+ and Cl−/HCO3− exchangers and chloride channels (Jacob & Civan, 1996; Civan, 1998a; Counillon et al., 2000). While NPE and PE cells both express Na,K-ATPase at their basolateral membrane, Na,K-ATPase activity is two- to three-fold greater in NPE cells (Krupin et al., 1984; Riley & Kishida, 1986). The NPE layer appears to be specialized for Na,K-ATPase-mediated transport since the NPE expresses α1, α2 and α3 Na,K-ATPase isoforms in contrast to PE which expresses only the housekeeping α1 isoform (Ghosh et al., 1990) found in all tissues. In NPE, as in most epithelia, the electrochemical gradient established by Na,K-ATPase-mediated sodium-potassium transport provides the driving force for other transport mechanisms. On this basis, we reason that inhibition of Na,K-ATPase in the NPE should reduce the rate of AH formation. In the intact eye, aqueous humor formation is indeed reduced by the Na,K-ATPase inhibitor ouabain applied to the NPE side of the ciliary body (Shahidullah et al., 2003). Here, we consider the effect of NO donors on Na,K-ATPase activity in the NPE.
NO and NO donors have been shown to inhibit Na,K-ATPase in other secretory epithelium including choroids plexus (Ellis et al., 2000), trachea (de Oliveira Elias et al., 1999) and kidney tubule (Guzman et al., 1995; Seven et al., 2005). In a recent study NO donors were found to have a convincing inhibitory effect on Na,K-ATPase activity in ciliary process isolated from the bovine eye (Ellis et al., 2001). However, interpretation of this study was difficult because the ciliary process is a highly vascularized tissue (Funk & Rohen, 1990) in which the capillary bed accounts for a significant fraction of tissue mass. Vascular endothelium is responsive to NO and generally has a vigorous NO synthase activity. Thus, the reported response of ciliary processes to NO donors could represent responses of the vascular endothelium. In the present study, we developed a way to isolate NPE cells in sufficient quantity to determine whether Na,K-ATPase specifically in the NPE is sensitive to NO and to examine the response mechanism. In addition, we were able to test whether NPE cells themselves are able to generate NO.
Methods
Isolation of NPE cells
Fresh pig eyes were a kind gift from the Swift Company, Louisville KY. The eyes were transported from the abattoir to the laboratory on ice and used immediately. Extra-ocular tissues removed and the eyes were dissected open at the posterior pole by cross incisions made with a scalpel. The cornea was everted by pulling the cut flaps of the sclera backward and at the same time by gently pushing the cornea inward. This causes the vitreous to protrude with its base still attached to the pars plana of the ciliary body. One side of the vitreous was then pushed aside from the pars plana using curved forceps and the vitreous body was gently separated from the eye. In this way the entire ring of NPE remained attached to the vitreous, leaving the pigmented cell (PE) layer attached to the ciliary body. Histological sections of the NPE ring showed NPE with very few PE cells and no stroma or vascular tissue (Figure 1). With a small amount of the vitreous still attached, the cell ring was placed in an ice-cold centrifuge tube.
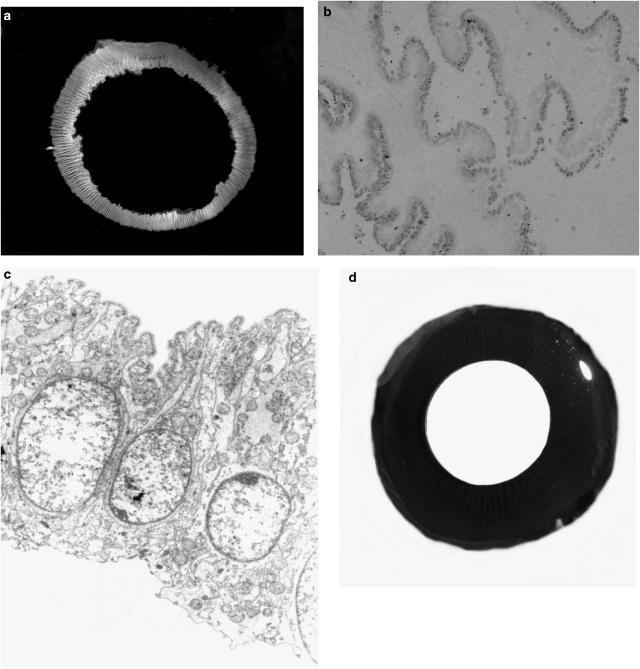
Figure 1.
(a) Photograph of the intact ring of NPE cells isolated from the porcine eye. (b) Histological section of the isolated NPE ring showing minimal contamination by PE cells. Examination under light microscope shows that the tissue samples comprised more than 99% NPE cells. (c) Electron microscopic section (× 4350) shows a single layer of cells with no apparent PE cells. Note that the basolateral side of the NPE is uppermost. There seems to be no obvious contamination with PE cell remnants or pigment granules. (d) Cross-section of the porcine eye exposing the ciliary body from behind. The ciliary body appears as radial black ridges that extend onto the posterior surface of the iris. The pupil appears as the disc in the center of the image. Abundant pigmentation is clearly visible in the intact ciliary body.
NPE-vitreous samples from three eyes were pooled and the volume was adjusted to 2 ml by adding cell free vitreous collected from the same eyes. The cell free vitreous was liquefied with an electronic homogenizer (Ultra Turrax T8, Germany) before adding to the sample. After this, the tube contents were transferred to round-bottomed, thin-walled, ultracentrifuge tubes used for the 20 min incubation period. Stock solutions of drugs in saline were added to the samples to obtain intended final concentrations. The samples were incubated for 20 min at 37°C in a humidified atmosphere of 95% O2 and 5% CO2. At the end of the incubation period, the samples were snap frozen in liquid nitrogen and stored at −70°C until Na,K-ATPase activity measurement.
Measurement of ATPase activity
NPE samples were homogenized in ice-cold homogenization buffer comprising 150 mM sucrose, 4.0 mM EGTA, 5.0 mM HEPES, 800 μM dithiothreitol (DTT) and protease inhibitors (100 μM phenylmethylsulfonyl fluoride (PMSF), 10 μg ml−1 antipain, 10 μg ml−1 leupeptin, 10 μg ml−1 pepstatin A and 2.0 μg ml−1 aprotinin). Use of low ionic strength buffer helps preserve the membrane from damage during freezing and thawing. Homogenization was carried out for 90 s (6 × 15 s) using the electric tissue homogenizer (Ultra Turrax T8, Germany). The homogenate was centrifuged at 85,000 × g for 60 min at 4°C and the supernatant discarded in order to obtain a crude particulate preparation as the pellete. After removing the supernatant, which contained the vitreous, soluble cellular components and the homogenization buffer, the pellet was resuspended in 1.0 ml of Na,K-ATPase assay buffer. The assay buffer contained 40 mM histidine, 100 mM NaCl, 5.0 mM KCl, 3 mM MgCl2 and 1.0 mM EGTA (pH 7.4). The resuspended pellet was rehomogenized (2 × 15 s) and used to assay Na,K-ATPase activity. Protein in the pellete was measured by the bicinchoninic acid (BCA) assay (Pierce Biotechnology, Rockford, IL, U.S.A.), using bovine serum albumin (BSA) as a standard. The average yield of protein from the tissue pellete of three rings was 1.0 mg (0.99±0.03 mg, n=120). Protein content of homogenized porcine vitreous was found to be 0.1%. When the vitreous was homogenized and then centrifuged in the same manner as the experimental samples, protein could not be detected in the pellete.
Na,K-ATPase activity was measured using a technique described previously (Delamere et al., 1997). Homogenate (∼40 μg protein) obtained from treated or control NPE was added to a tube containing of ice-cold Na,K-ATPase buffer. To ensure access of ions and ATP to membrane vesicles (Xie et al., 1989), alamethicin (10 μl of 12.5 mg ml−1) was added to each tube to give a final approximate concentration of 0.4 mg of alamethicin per mg protein. Ouabain, a specific inhibitor of Na,K-ATPase (Wallick & Schwartz, 1988), was added to half the tubes at a final concentration of 1.0 mM. The tubes were pre-incubated at 37°C with gentle agitation for 5 min, ATP was then added to each tube to give a final concentration of 2.0 mM. The total volume of the assay mixture was 400 μl. The ATP hydrolysis reaction was allowed to proceed for 45 min at 37°C, and then the reaction was stopped by adding 150 μl of 15% ice-cold trichloroacetic acid (TCA) and placing the tubes on ice.
ATP hydrolysis was quantified by measuring the amount of inorganic phosphate released in each reaction tube. The tubes were centrifuged at 3000 r.p.m. for 7.5 min at 4°C then 400 μl of the supernatant was removed and added to 400 μl of 4.0% FeSO4 solution in ammonium molybdate (1.25 g of ammonium molybdate in 100 ml of 2.5 N sulphuric acid, Sigma diagnostic, St Louis, MO, U.S.A.). NaH2PO4 standard solutions (equivalent to 0, 62.5, 125, 250 and 625 nmoles of PO4) were treated similarly. The mixture was allowed to sit for 10 min at room temperature then absorbance was measured at 750 nm using a microplate reader (PowerWave X, Biotech Instruments Inc., Vermont, NE, U.S.A.). Na,K-ATPase activity was calculated as the difference between ATP hydrolysis in the presence and absence of ouabain. ATPase activity was expressed as nanomoles ATP hydrolyzed per milligram protein per minute.
The data are expressed as percent of control because baseline Na-K-ATPase activity varied from one day to another. The variation of control values is unlikely to influence the results because each experiment (control+test groups) was repeated using at least three different sets of tissue samples.
Measurement of nitrite
Nitrite, a stable end product of NO metabolism, was measured using a Griess assay kit (Promega Corp., Madison, WI, U.S.A.). Reagents included a sodium nitrite standard (0.1 M in water), sulphanilamide (1 in 5% phosphoric acid) and N-1-napthylethylenediamine dihydrochloride (NED) (0.1% in water) solutions. Sulphanilamide and NED solutions were allowed to equilibrate at room temperature before use. For each sample, four replicate 50 μl aliquots were placed in separate wells of a 96-well plate alongside 50 μl aliquots of nitrite standards in the concentration range 1–100 μM. Sulphanilamide solution (50 μl) was added to each well and the plate was kept in the dark at room temperature for 10 min. Then, 50 μl of NED solution was added to each well and the plate was kept in the dark at room temperature for a further 10 min before measurement of absorbance at a wavelength of 448 nm (PowerWave × plate reader, Biotech Instruments Inc., Vermont, NE, U.S.A.) to determine nitrite concentration.
Drugs and chemicals
Sodium azide, sodium nitroprusside (SNP), S-nitroso-N-acetyl-penicillamine (SNAP), N-nitro-L-arginine methyl ester hydrochloride (L-NAME), L-arginine hydrochloride, D-arginine monohydrochloride, glutathione, 8-para-chlorophenyl-thioguanosine-3′,5′-cyclic guanosine monophosphate (8-pCPT-cGMP), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), methylene blue, allopurinol, ouabain, EGTA, dimethyl sulfoxide (DMSO), ethanol, ATP, alamethicin, aprotinin, pepstatin A, phenylmethylsulfonyl fluoride (PMSF), antipain hydrochloride and leupeptin were purchased from the Sigma Chemical Co. (St Louis, MO, U.S.A.). H-8 dihydrochloride, H-9 dihydrochloride, H-89 dihydrochloride and peroxynitrite were purchased from Calbiochem (EDM biosciences Inc., Darmstadt, Germany). Griess assay reagents were purchased from Promega (Madison, WI, U.S.A.) and the BCA protein assay kit from Pierce Biotechnology (Rockford, IL, U.S.A.). All other chemicals were obtained either from the Sigma Chemical Co. or from Fisher Scientific Products (Pittsburgh, PA, U.S.A.). Stock solutions of test agents were prepared by dissolving either in distilled water, ethanol or in DMSO according to their solubility.
Statistical analysis of data
Results were expressed as the mean±s.e.m. of data from a specified number of independent experiments. Statistical comparisons were made by analysis of variance followed by the Bonferroni post hoc multiple comparison tests. A probability (P) value of <0.05 was considered significant.
Results
NPE cell preparation
NPE was isolated from the porcine eye as described in the Methods. A sheet of cells in the form of a ring was obtained from each eye. Lack of pigment signified the low amount of PE cells that remained attached to the NPE (Figure 1a). Histological sections confirmed the tissue samples had more than 99% NPE cells and no vascular cells (Figure 1b). Electron microscopic sections (× 4350) show a single layer of cells with no apparent PE cells. There seems to be no obvious contamination with PE cell remnants or pigment granules. (Figure 1c). A cross section of porcine eye exposing the ciliary body from behind shows abundant pigmentation (Figure 1d).
Na,K-ATPase activity in control samples was 105.0±2.0 (n=84) nmoles mg−1 protein min−1 with the maximum and minimum values of 138.9 and 62.2, respectively. Ouabain insensitive Na,K-ATPase activity was 48.2±1.7 (n=84) nmoles mg−1 membrane protein min−1, with the maximum and minimum values of 77.7 and 25.8, respectively.
Effect of L-arginine and L-NAME on Na,K-ATPase activity
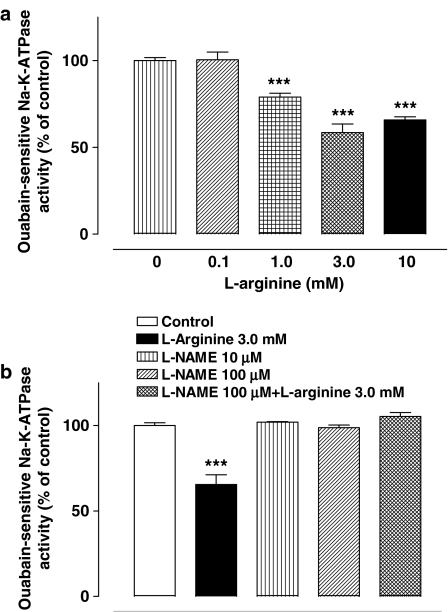
NPE cells were exposed for 20 min to L-arginine (1–3 mM) prior to Na,K-ATPase activity measurement. L-arginine, a substrate for NO synthase (NOS), caused significant and concentration-dependent reduction of Na,K-ATPase activity (Figure 2a). D-Arginine (1 mM), which is not a NOS substrate, failed to alter Na,K-ATPase activity (data not shown). In some experiments, NPE was exposed to L-NAME (100 μM), a NOS inhibitor. Added alone L-NAME had no effect on the Na,K-ATPase activity. However, when L-NAME (100 μM) was added together with L-arginine (1 mM), the effect of L-arginine on Na,K-ATPase activity was abolished (Figure 2b). The ouabain-insensitive component of the ATPase activity, which represented less than one-third of the total ATPase activity, remained unchanged in cells exposed to L-arginine or L-NAME (data not shown).
Figure 2.
(a) Concentration-dependent inhibition of Na,K-ATPase by L-arginine. NPE cells were incubated with or without (control) L-arginine for 20 min, then homogenized and Na,K-ATPase activity in control and treated samples was measured under identical conditions. The results are the mean±s.e.m. of nine independent measurements at each concentration of L-arginine. (b) Shows results from cells that were incubated for 20 min with both L-NAME (100 μM) and L-arginine (1 mM) or with either L-NAME or L-arginine alone. The results are the mean±s.e.m. of data from six independent measurements. Statistical comparisons were made by analysis of variance followed by the Bonferroni post hoc multiple comparison test. ***Significantly different from the control value; P<0.001.
Effect of sodium azide, SNP and SNAP on Na-K-ATPase activity
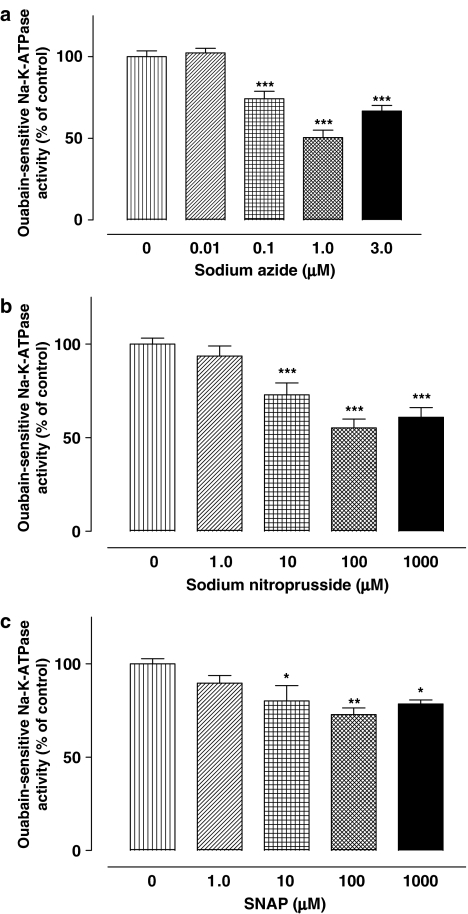
NPE cells were exposed to sodium azide (1 μM–100 nM), a vasodilator that acts through the generation of NO. Sodium azide has previously been found to reduce aqueous humor formation in the porcine eye by a mechanism involving cGMP and NO (Shahidullah et al., 2005). Here, sodium azide reduced Na,K-ATPase activity in a concentration-dependent manner (Figure 3a). The NO donors SNP (10 μM–1 mM) and SNAP (10 μM–1 mM) also reduced Na,K-ATPase activity in a concentration-dependent fashion (Figure 3b and c). Ouabain-insensitive ATPase activity was unchanged by sodium azide, SNP or SNAP (data not shown).
Figure 3.
Inhibition of Na,K-ATPase activity by sodium azide (a), SNP (b) or SNAP (c). Cells were incubated with or without (control) test compounds for 20 min, then homogenized and Na,K-ATPase activity in control and treated samples was measured under identical conditions. The results are the mean±s.e.m. of data from six independent experiments (a and c), or 12–18 experiments (b). Statistical comparisons were made by analysis of variance followed by the Bonferroni post hoc multiple comparison tests. *,**and ***indicate significant differences from the control value at P<0.05, P<0.01 and P<0.01, respectively.
Nitrite generation by NPE
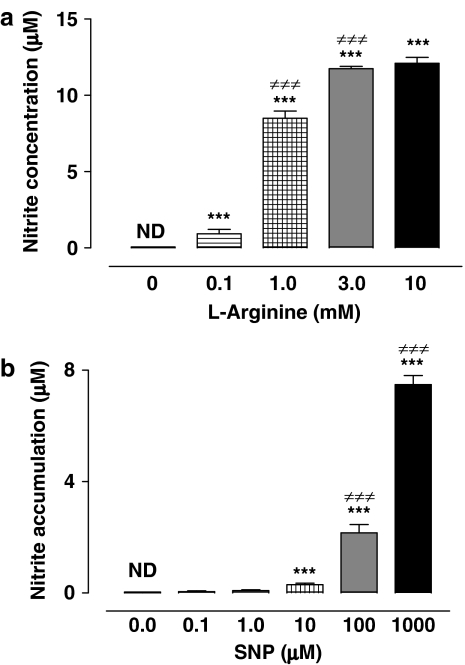
NO is short lived, being metabolized to generate nitrite as a stable end product. Using the Griess assay, nitrite was measured in the incubation medium of NPE cells exposed to either L-arginine or SNP for 20 min (Figure 4). Both L-arginine and SNP caused a significant concentration-dependent increase in the concentration of nitrite. Nitrite was undetectable in medium taken from control cells incubated for 20 min without either L-arginine or SNP. Nitrite was also undetectable when SNP was added to medium alone (cell free) for 20 min.
Figure 4.
Accumulation of nitrite in the incubation medium of NPE cells exposed to L-arginine (a) or SNP (b). NPE cells were incubated with or without (control) test compounds for 20 min and then the concentration of nitrite was measured in the incubation medium. Nitrite concentration (μM) was shown as the mean±s.e.m. of 12 independent measurements at each concentration of L-arginine and 12–16 measurements at each concentration of SNP. Statistical comparisons were made by analysis of variance followed by the Bonferroni post hoc multiple comparison tests. ***and ≠≠≠indicate significant differences from the control values and from values for the preceeding concentration of each drug at P<0.001, respectively.
The role of cGMP and sGC
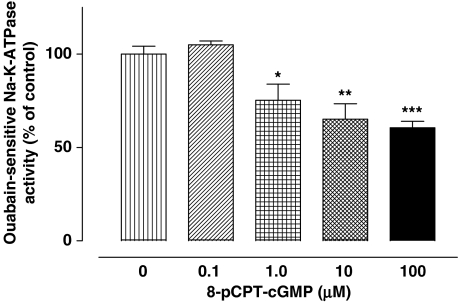
In many tissues, the effects of NO on cell function follow a rise in cGMP due to activation of sGC. To determine whether cGMP is capable of bringing about a change of Na,K-ATPase activity, NPE cells were exposed to 8-p-CPT-cGMP, a cell permeable cGMP analog. 8-p-CPT-cGMP caused significant inhibition of Na,K-ATPase activity that was concentration-dependent (Figure 5).
Figure 5.
Inhibition of Na,K-ATPase activity in by 8-pCPT-cGMP, a cell permeable analog of cGMP. Cells were incubated with or without (control) 8-pCPT-cGMP for 20 min, then homogenized and Na,K-ATPase activity in control and treated samples was measured under identical conditions. The results are the mean±s.e.m. of data from six independent measurements at each concentration of 8-pCPT-cGMP. Statistical comparisons were made by analysis of variance followed by the Bonferroni post hoc multiple comparison tests. *,**and ***indicate significant differences from the control value at P<0.05, P<0.01 and P<0.01, respectively.
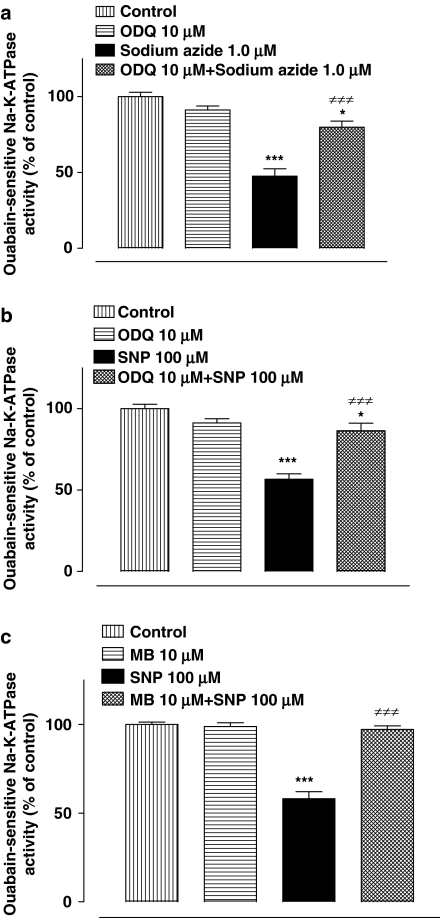
To examine the possible involvement of sGC in the Na,K-ATPase response to NO, NPE cells were exposed to ODQ (10 μM) an inhibitor of sGC. In the presence of ODQ, the magnitude of the inhibitory effect of sodium azide (1.0 μM) on Na,K-ATPase activity was significantly reduced (Figure 6a). ODQ also suppressed the Na,K-ATPase inhibition caused by SNP (100 μM) (Figure 6b). Methylene blue (10 μM), a different inhibitor of sGC, was similarly found to suppress the inhibition of Na,K-ATPase activity caused by SNP (Figure 6c).
Figure 6.
The effect of sGC inhibitors on the Na,K-ATPase activity response to sodium azide and SNP. (a and b) Show the Na,K-ATPase response to sodium azide and SNP in the presence or absence of ODQ. (c) Shows the Na,K-ATPase response to SNP in the presence or absence of methylene blue. Cells were incubated with or without (control) ODQ or methylene blue for 15 min and then sodium azide or SNP was added for a further 20 min. After this, the cells were homogenized and Na,K-ATPase activity in control and treated samples was measured under identical conditions. The results are the mean±s.e.m. of data from six independent measurements for each experimental group. Statistical comparisons were made by analysis of variance followed by the Bonferroni post hoc multiple comparison tests. *and ***indicate a significant difference from the normalized control value at P<0.05 and P<0.001, respectively. ≠≠≠Indicates a significant difference from sodium azide or SNP-treated samples at P<0.001.
Inhibition of protein kinase G
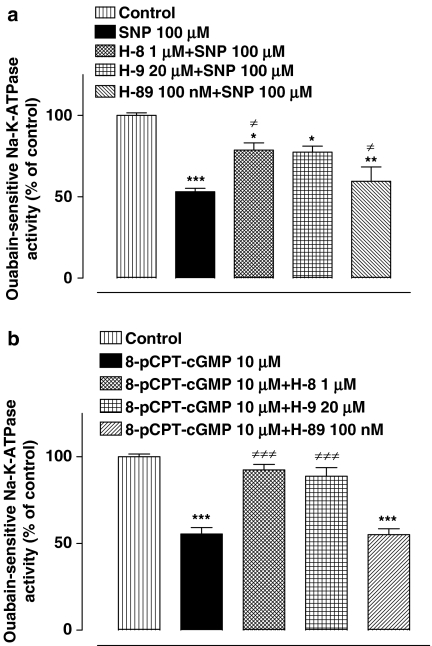
The above results point to a role for cGMP. As protein kinase G (PKG) is activated by cGMP, experiments were conducted to examine the need for PKG activation in the chain of events that lead to Na,K-ATPase inhibition in SNP-treated cells. The NPE was exposed to either H-8 hydrochloride (1.0 μM) or H-9 hydrochloride (20 μM), both of which are reported to be relatively selective inhibitors of PKG (Inagaki et al., 1985, 1986). In the presence of H-8 or H-9, the inhibitory effect of SNP on Na,K-ATPase activity was significantly, but not fully, suppressed (Figure 7a). H-89, a different protein kinase inhibitor which is selective for protein kinase A (PKA) (de Rooij et al., 1998), failed to alter the magnitude of Na,K-ATPase inhibition elicited by SNP. The effect of PKG inhibition was also examined in NPE exposed to 8-p-CPT-cGMP, which can activate PKG directly. The Na,K-ATPase inhibition response to 8-p-CPT-cGMP was abolished in the presence of H-8 or H-9 (Figure 7b). The PKA inhibitor H-89 failed to alter magnitude of Na,K-ATPase inhibition by 8-pCPT-cGMP.
Figure 7.
The effect of protein kinase inhibitors on the Na,K-ATPase activity response to SNP (a) and 8-pCPT-cGMP (b). Cells were incubated with or without (control) PKG inhibitors H-8 or H-9 or the specific PKA inhibitor H-89 for 15 min. and then SNP or 8-pCPT-cGMP was added for a further 20 min. After this, the cells were homogenized and Na,K-ATPase activity in control and treated samples was measured under identical conditions. The results are the mean±s.e.m. of data from six independent measurements for each experimental group. Statistical comparisons were made by analysis of variance followed by the Bonferroni post hoc multiple comparison tests. *,**and ***indicate significant differences from the control value at P<0.05, P<0.01 and P<0.001, respectively. ≠Indicates a significant difference from SNP-treated samples.
Effect of peroxynitrite on Na-Ka-ATPase
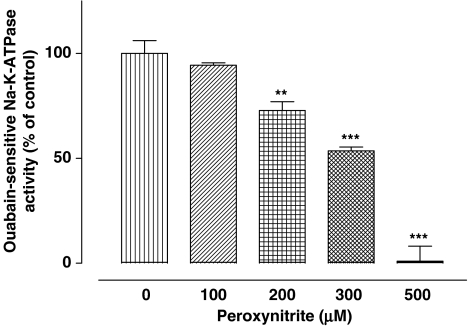
While the above results point to a role for cGMP and PKG in the mechanism of the Na,K-ATPase response to NO donors, other investigators have proposed that some enzymes are susceptible to peroxynitrite generated in NO donor-treated tissues (Guzman et al., 1995; Sato et al., 1997). Thus, NPE cells were incubated for 20 min with peroxynitrite in the concentration range 100–500 μM. At a concentration of 200 μM and higher, peroxynitrite caused a significant reduction of Na,K-ATPase activity (Figure 8). Na,K-ATPase activity was almost abolished in cells exposed to 500 μM peroxynitrite.
Figure 8.
Inhibition of Na,K-ATPase activity by peroxynitrite. Cells were incubated with or without (control) peroxynitrite for 20 min, then homogenized and Na,K-ATPase activity in control and treated samples was measured under identical conditions. The results are the mean±s.e.m. of data from six independent measurements at each concentration of peroxynitrite. Statistical comparisons were made by analysis of variance followed by the Bonferroni post hoc multiple comparison tests. **and ***indicate a significant difference from the control value at P<0.01 and P<0.001, respectively.
Discussion
It had been reported previously that NO donors elevate cGMP and cause inhibition of Na,K-ATPase activity in the bovine ciliary process (Ellis et al., 2001). In that study, it was not possible to specify the degree to which the observed NO responses to donors stemmed from vascular tissue since the interior of each ciliary process has an elaborate network of blood vessels (Funk & Rohen, 1990). Moreover, part of the Na,K-ATPase activity measured in ciliary processes derives from vascular endothelium.
Here, we developed a way to isolate NPE cells from porcine eyes in order to examine Na,K-ATPase responses to NO donors specifically in the epithelium. The technique enabled us to obtain the NPE layer with minimal contamination by PE. Importantly, there was no contamination with ciliary process stroma and vascular endothelium that could respond itself to NO donors and generate NO through the activity of endogenous NO synthase. NO donors, sodium azide, SNP and S-nitroso-N-acetyl-penicillamine (SNAP) produced significant and concentration-dependent inhibition of the Na,K-ATPase activity in the NPE. The ability of NO donors to inhibit NPE Na,K-ATPase activity suggests that such Na, K-ATPase inhibition may contribute to the previously observed reduction of aqueous humor secretion elicited by NO donors (Shahidullah et al., 2005). Although aqueous humor secretion is supported by the coordinated operation of multiple ion transport mechanisms, studies with arterially perfused eyes suggest that selective inhibition of NPE Na, K-ATPase may indeed be capable of causing a reduction in aqueous formation; the rate of aqueous secretion was reduced significantly by ouabain delivered to the NPE-facing aqueous humor compartment of the eye (Shahidullah et al., 2003). Inhibition of CE Na,K-ATPase activity by NO donors also is consistent with the finding that the IOP-lowering effect of NO donors is independent of any vascular effect of NO donors (Millar et al., 2001). It is noteworthy that ODQ, an inhibitor of sGC, suppresses both the IOP-lowering effect of NO donors (Shahidullah et al., 2005) and the Na,K-ATPase response to NO donors observed in this study.
NPE cells are not unique in their display of an Na,K-ATPase inhibition response to NO donors. NO donors have also been shown to inhibit purified Na,K-ATPase obtained from pig brain (Sato et al., 1995; 1997). Moreover, NO or NO donors have been reported to inhibit Na,K-ATPase-linked ion transport and fluid secretion in intestinal epithelium (Suzuki et al., 2005), kidney (Ortiz & Garvin, 2002; Varela et al., 2004), erythrocytes (Muriel et al., 2003), brain (Ellis et al., 2000; Wyse et al., 2001) and in the gill of brown trout (Tipsmark & Madsen, 2003). To elucidate the mechanism of action of NO on the NPE, cells were exposed to L-arginine, which acts as the substrate for NO production by NOS. L-Arginine caused a concentration-dependent reduction of ouabain-sensitive Na, K-ATPase activity. L-NAME, a NOS inhibitor abolished the inhibitory effect of L-arginine on Na,K-ATPase activity. From studies in other tissues it has been established that NOS contributes to the generation of NO from L-arginine (Knowles & Moncada, 1994; Sessa, 1994). The findings thus point to NOS activity in porcine NPE. Concentration-dependent accumulation of nitrite, a stable end product of NO metabolism, was detected in the incubation medium of L-arginine and SNP-treated NPE cells, confirming the ability of the NPE to generate NO. The L-arginine-induced increase in nitrite formation points to expression of a constitutive NOS isoform in porcine NPE. This is consistent with an earlier report that nNOS and iNOS are expressed in porcine NPE (Meyer et al., 1999). In another study, using porcine ciliary process it has been shown that expression of nNOS was six times higher than eNOS and fifteen times higher than iNOS (Liu et al., 2002).
The inhibitory effect of NO donors SNP and sodium azide on Na,K-ATPase was suppressed by ODQ and by methylene blue, two inhibitors of sGC. These results suggest that NO effects on Na,K-ATPase lie downstream of sGC activation and elevation of cGMP. Consistent with this mechanism, Na, K-ATPase activity was inhibited in cells treated with the cell-permeable cGMP analog 8-pCPT-cGMP. Elevation of cGMP has been reported to inhibit Na,K-ATPase activity in choroid plexus (Ellis et al., 2000) and in bovine ciliary processes (Ellis et al., 2001). If NPE Na,K-ATPase activity is inhibited by cGMP, it follows that elevation of cGMP in the ciliary body might be expected to reduce aqueous humor secretion and IOP. This seems to be the case in the rabbit (Korenfeld & Becker, 1989; Takashima et al., 1996) bovine (Millar et al., 1997; Shahidullah & Wilson, 1999) and monkey eye (Kee et al., 1994).
As cGMP is a known activator of PKG, studies were carried out to test whether PKG is involved in the Na,K-ATPase response to NO donors. PKG inhibitors H-8 and H-9 were both found to cause significant, but incomplete, inhibition of the Na,K-ATPase response to the NO donor SNP. The findings point to a situation where Na,K-ATPase inhibition following PKG activation occurs subsequent to cGMP elevation in cells treated with NO donors. This mechanism fits with the observation that H-8 and H-9 also suppressed the inhibitory effect of 8-pCPT-cGMP on Na,K-ATPase activity. It remains to be determined whether PKG alters Na,K-ATPase function directly by causing phosphorylation of Na,K-ATPase protein or whether Na, K-ATPase activity is altered indirectly as the result of PKG-induced phosphorylation of other regulatory proteins. In the choroid plexus (Snyder et al., 1992) and in the substantia nigra (Tsou et al., 1993), cGMP elicits phosphorylation of dopamine and cAMP-regulated phosphoprotein (DARPP-32) and protein phosphatase-1 inhibitor (inhibitor-1), which in their phosphorylated state are potent inhibitors of protein phophatase type 1 (a serine/threonine phosphatase). Protein phosphatase-1 has been shown to be involved in the regulation of Na,K-ATPase in kidney (Li et al., 1995). It is noteworthy that there is evidence pointing to Na,K-ATPase activity modulation in the CE by a different serine–threonine protein kinase, the cAMP-dependent PKA (Delamere & King, 1992). In the present study, however, the PKA inhibitor H-89 did not change Na,K-ATPase responses to NO donors or 8-pCPT-cGMP, pointing to distinction between the cAMP signaling pathway and the cGMP pathway activated by NO. However, we cannot rule out involvement of PKC. In opposum kidney cells it has been reported that PKC activation by NO donors inhibits Na,K-ATPase activity (Liang & Knox, 1999).
At the same concentrations that abolished the 8-pCPT-cGMP effect on Na,K-ATPase activity, neither H-8, H-9 nor ODQ fully inhibited the Na,K-ATPase response to SNP. This leaves open the possibility that NO donors may cause Na,K-ATPase activity inhibition in part via another mechanism that acts in addition to the sGC-cGMP-PKG pathway. In other tissues, the generation of peroxynitrite has been found to cause biochemical changes subsequent to NO donor exposure. In the present study, application of exogenous peroxynitrite to NPE cells was found to cause concentration-dependent inhibition of Na,K-ATPase activity. This raises the possibility that peroxynitrite generation during oxidative stress might influence aqueous humor secretion since peroxynitrite formed during oxidative stress would theoretically inhibit Na-K-ATPase activity. Na,K-ATPase inhibition by ouabain reduces aqueous humor secretion in isolated porcine eye (Shahidullah et al., 2005). In the rat exocrine pancreas, it has been shown that acute oxidative stress reduced fluid and salt secretion although the involvement of peroxynitrite was not examined (Sweiry et al., 1999). Peroxynitrite generation, following NO treatment, had been shown to inhibit Na,K-ATPase activity in several other tissues (Guzman et al., 1995; Sato et al., 1997; Zhang et al., 2002; Kocak-Toker et al., 2005). Using FeTPPS, which isomerizes peroxynitrite to form nitrite, we tried to block the peroxynitrite effect in cells treated with NO donors. The studies were inconclusive due to the significant inhibition of Na,K-ATPase activity caused by FeTPPS itself (data not shown). It remains to be determined whether peroxynitrite contributes to the H-8-, H-9-, or ODQ-resistant component of the Na,K-ATPase response to NO donors.
Taken together, the findings in this study suggest that NO inhibits Na,K-ATPase activity in NPE cells via a mechanism that involves activation of sGC, generation of cGMP and the activation of PKG. Inhibition of NPE Na,K-ATPase has the potential to reduce aqueous humor formation as evidenced by the ability of intraocular ouabain to slow aqueous humor secretion in the perfused eye (Shahidullah et al., 2003). On this basis, we suggest that Na,K-ATPase inhibition may contribute to the previously reported ability of NO donors to reduce aqueous humor formation in the porcine eye (Shahidullah et al., 2005).
Acknowledgments
This work was supported by NIH Grant EY06915, Research to Prevent Blindness Inc. and the Kentucky Lions Eye Foundation.
Abbreviations
- BCA
bicinchoninic acid
- BSA
bovine serum albumin
- cAMP
cyclic AMP
- CE
ciliary epithelium
- cGMP
cyclic GMP
- DMSO
dimethyl sulfoxide
- L-NAME
N-nitro-L-arginine methyl ester
- NED
N-1-napthylethylenediamine dihydrochloride
- NO
nitric oxide
- NOS
nitric oxide synthase
- NPE
nonpigmented ciliary epithelium
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- 8-pCPT-cGMP
8-para-chlorophenyl-thioguanosine-3′,5′-cyclic guanosine monophosphate
- PE
pigmented ciliary epithelium
- PKA
protein kinase A
- PKC
protein kinase C
- PKG
protein kinase G
- sGC
soluble guanylate cyclase
- SNP
sodium nitroprusside
References
- BEHAR-COHEN F.F., GOUREAU O., D'HERMIES F., COURTOIS Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Invest. Ophthalmol. Visual Sci. 1996;37:1711–1715. [PubMed] [Google Scholar]
- CHUMAN H., CHUMAN T., NAO-I N., SAWADA A. The effect of L-arginine on intraocular pressure in the human eye. Curr. Eye Res. 2000;20:511–516. [PubMed] [Google Scholar]
- CIVAN M.M. The Eye's Aqueous Humor: from Secretion to Glaucoma. San Diego, London, Boston, New York, Sydney, Tokyo, Toronto: Academic Press; 1998a. [Google Scholar]
- CIVAN M.M.Transport components of net secretion of the aqueous humour and their integrated regulation The Eye's Aqueous Humor: from Secretion to Glaucoma 1998bSan Diego, London, Boston, New York, Sydney, Tokyo, Toronto: Academic Press; 1–24.Civan, M.M. (ed), pp [Google Scholar]
- COUNILLON L., TOURET N., BIDET M., PETERSON-YANTORNO K., COCA-PRADOS M., STUART-TILLEY A., WILHELM S., ALPER S.L., CIVAN M.M. Na+/H+ and CI-/HCO3-antiporters of bovine pigmented ciliary epithelial cells. Pflugers Arch. – Eur. J. Physiol. 2000;440:667–678. doi: 10.1007/s004240000302. [DOI] [PubMed] [Google Scholar]
- DE OLIVEIRA ELIAS M., TAVARES DE LIMA W., VANNUCHI Y.B., MARCOURAKIS T., DA SILVA Z.L., TREZENA A.G., SCAVONE C. Nitric oxide modulates Na+, K+-ATPase activity through cyclic GMP pathway in proximal rat trachea. Eur. J. Pharmacol. 1999;367:307–314. doi: 10.1016/s0014-2999(98)00928-5. [DOI] [PubMed] [Google Scholar]
- DE ROOIJ J., ZWARTKRUIS F.J., VERHEIJEN M.H., COOL R.H., NIJMAN S.M., WITTINGHOFER A., BOS J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP (see comment) Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- DELAMERE N.A., KING K.L. The influence of cyclic AMP upon Na,K-ATPase activity in rabbit ciliary epithelium. Invest. Ophthalmol. Visual Sci. 1992;33:430–435. [PubMed] [Google Scholar]
- DELAMERE N.A., PARKERSON J., HOU Y. Indomethacin alters the Na,K-ATPase response to protein kinase C activation in cultured rabbit nonpigmented ciliary epithelium. Invest. Ophthalmol. Visual Sci. 1997;38:866–875. [PubMed] [Google Scholar]
- ELLIS D.Z., NATHANSON J.A., RABE J., SWEADNER K.J. Carbachol and nitric oxide inhibition of Na,K-ATPase activity in bovine ciliary processes. Invest. Ophthalmol. Visual Sci. 2001;42:2625–2631. [PubMed] [Google Scholar]
- ELLIS D.Z., NATHANSON J.A., SWEADNER K.J. Carbachol inhibits Na(+)-K(+)-ATPase activity in choroid plexus via stimulation of the NO/cGMP pathway. Am. J. Physiol. – Cell Physiol. 2000;279:C1685–C1693. doi: 10.1152/ajpcell.2000.279.6.C1685. [DOI] [PubMed] [Google Scholar]
- FUNK R., ROHEN J.W. Scanning electron microscopic study on the vasculature of the human anterior eye segment, especially with respect to the ciliary processes. Exp. Eye Res. 1990;51:651–661. doi: 10.1016/0014-4835(90)90049-z. [DOI] [PubMed] [Google Scholar]
- GHOSH S., FREITAG A.C., MARTIN-VASALLO P., COCA-PRADOS M. Cellular distribution and differential gene expression of the three alpha subunit isoforms of the Na,K-ATPase in the ocular ciliary epithelium. J. Biol. Chem. 1990;265:2935–29340. [PubMed] [Google Scholar]
- GUZMAN N.J., FANG M.Z., TANG S.S., INGELFINGER J.R., GARG L.C. Autocrine inhibition of Na+/K(+)-ATPase by nitric oxide in mouse proximal tubule epithelial cells. J. Clin. Invest. 1995;95:2083–2088. doi: 10.1172/JCI117895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTWICK A.T. Beyond intraocular pressure: neuroprotective strategies for future glaucoma therapy. Optomet. Vision Sci. 2001;78:85–94. doi: 10.1097/00006324-200102000-00008. [DOI] [PubMed] [Google Scholar]
- IMAI N., TSUYAMA Y., MURAYAMA K., ADACHI-USAMI E. Protective effect of nitric oxide on ischemic retina. Nippon Ganka Gakkai Zasshi – Acta Societatis Ophthalmol. Japonicae. 1997;101:639–643. [PubMed] [Google Scholar]
- INAGAKI M., KAWAMOTO S., ITOH H., SAITOH M., HAGIWARA M., TAKAHASHI J., HIDAKA H. Naphthalenesulfonamides as calmodulin antagonists and protein kinase inhibitors. Mol. Pharmacol. 1986;29:577–581. [PubMed] [Google Scholar]
- INAGAKI M., WATANABE M., HIDAKA H. N-(2-Aminoethyl)-5-isoquinolinesulfonamide, a newly synthesized protein kinase inhibitor, functions as a ligand in affinity chromatography. Purification of Ca2+-activated, phospholipid-dependent and other protein kinases. J. Biol. Chem. 1985;260:2922–2925. [PubMed] [Google Scholar]
- JACOB T.J., CIVAN M.M. Role of ion channels in aqueous humor formation. Am. J. Physiol. 1996;271:C703–C720. doi: 10.1152/ajpcell.1996.271.3.C703. [DOI] [PubMed] [Google Scholar]
- KEE C., KAUFMAN P.L., GABELT B.T. Effect of 8-Br cGMP on aqueous humor dynamics in monkeys. Invest. Ophthalmol. Visual Sci. 1994;35:2769–2773. [PubMed] [Google Scholar]
- KITAOKA Y., KUMAI T. Modulation of retinal dopaminergic cells by nitric oxide. A protective effect on NMDA-induced retinal injury. In Vivo. 2004;18:311–315. [PubMed] [Google Scholar]
- KNOWLES R.G., MONCADA S. Nitric oxide synthases in mammals. Biochem. J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCAK-TOKER N., GIRIS M., TULUBAS F., UYSAL M., AYKAC-TOKER G. Peroxynitrite induced decrease in Na+, K+-ATPase activity is restored by taurine. World J. Gastroenterol. 2005;11:3554–3557. doi: 10.3748/wjg.v11.i23.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORENFELD M.S., BECKER B. Atrial natriuretic peptides. Effects on intraocular pressure, cGMP, and aqueous flow. Invest. Ophthalmol. Visual Sci. 1989;30:2385–2392. [PubMed] [Google Scholar]
- KOTIKOSKI H., VAPAATALO H., OKSALA O. Nitric oxide and cyclic GMP enhance aqueous humor outflow facility in rabbits. Curr. Eye Res. 2003;26:119–123. doi: 10.1076/ceyr.26.2.119.14511. [DOI] [PubMed] [Google Scholar]
- KRUPIN T., REINACH P.S., CANDIA O.A., PODOS S.M. Transepithelial electrical measurements on the isolated rabbit iris-ciliary body. Exp. Eye Res. 1984;38:115–123. doi: 10.1016/0014-4835(84)90096-4. [DOI] [PubMed] [Google Scholar]
- LI D., APERIA A., CELSI G., DA CRUZ E SILVA E.F., GREENGARD P., MEISTER B. Protein phosphatase-1 in the kidney: evidence for a role in the regulation of medullary Na(+)-K(+)-ATPase. Am. J. Physiol. 1995;269:F673–F680. doi: 10.1152/ajprenal.1995.269.5.F673. [DOI] [PubMed] [Google Scholar]
- LIANG M., KNOX F.G. Nitric oxide activates PKCalpha and inhibits Na+-K+-ATPase in opossum kidney cells. Am. J. Physiol. 1999;277:F859–F865. doi: 10.1152/ajprenal.1999.277.6.F859. [DOI] [PubMed] [Google Scholar]
- LIU R., FLAMMER J., HAEFLIGER I.O. Forskolin upregulation of NOS I protein expression in porcine ciliary processes: a new aspect of aqueous humor regulation. Klinische Monatsblatter fur Augenheilkunde. 2002;219:281–283. doi: 10.1055/s-2002-30643. [DOI] [PubMed] [Google Scholar]
- LOMNICZI A., SUBURO A.M., ELVERDIN J.C., MASTRONARDI C.A., DIAZ S., RETTORI V., MCCANN S.M. Role of nitric oxide in salivary secretion. Neuroimmunomodulation. 1998;5:226–233. doi: 10.1159/000026342. [DOI] [PubMed] [Google Scholar]
- MEYER P., CHAMPION C., SCHLOTZER-SCHREHARDT U., FLAMMER J., HAEFLIGER I.O. Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr. Eye Res. 1999;18:375–380. doi: 10.1076/ceyr.18.5.375.5355. [DOI] [PubMed] [Google Scholar]
- MILLAR J.C., SHAHIDULLAH M., WILSON W.S. Atriopeptin lowers aqueous humor formation and intraocular pressure and elevates ciliary cyclic GMP but lacks uveal vascular effects in the bovine perfused eye. J. Ocular Pharmacol. Therapeut. 1997;13:1–11. doi: 10.1089/jop.1997.13.1. [DOI] [PubMed] [Google Scholar]
- MILLAR J.C., SHAHIDULLAH M., WILSON W.S. Intraocular pressure and vascular effects of sodium azide in bovine perfused eye. J. Ocular Pharmacol. Therapeut. 2001;17:225–234. doi: 10.1089/108076801750295263. [DOI] [PubMed] [Google Scholar]
- MOHANAKUMAR K.P., THOMAS B., SHARMA S.M., MURALIKRISHNAN D., CHOWDHURY R., CHIUEH C.C. Nitric oxide: an antioxidant and neuroprotector. Ann. NY Acad. Sci. 2002;962:389–401. doi: 10.1111/j.1749-6632.2002.tb04083.x. [DOI] [PubMed] [Google Scholar]
- MURIEL P., CASTANEDA G., ORTEGA M., NOEL F. Insights into the mechanism of erythrocyte Na+/K+-ATPase inhibition by nitric oxide and peroxynitrite anion. J. Appl. Toxicol. 2003;23:275–278. doi: 10.1002/jat.922. [DOI] [PubMed] [Google Scholar]
- NAKAZAWA T., TOMITA H., YAMAGUCHI K., SATO Y., SHIMURA M., KUWAHARA S., TAMAI M. Neuroprotective effect of nipradilol on axotomized rat retinal ganglion cells. Curr. Eye Res. 2002;24:114–122. doi: 10.1076/ceyr.24.2.114.8162. [DOI] [PubMed] [Google Scholar]
- NASKAR R., DREYER E.B.New horizons in neuroprotection Surv. Ophthalmol. 200145S250–S255.discussion S273–S276 [DOI] [PubMed] [Google Scholar]
- NATHANSON J.A. Nitrovasodilators as a new class of ocular hypotensive agents. J. Pharmacol. Exp. Therapeut. 1992;260:956–965. [PubMed] [Google Scholar]
- ORTIZ P.A., GARVIN J.L. Role of nitric oxide in the regulation of nephron transport. Am. J. Physiol. – Renal Fluid Electrolyte Physiol. 2002;282:F777–F784. doi: 10.1152/ajprenal.00334.2001. [DOI] [PubMed] [Google Scholar]
- RILEY M.V., KISHIDA K. ATPases of ciliary epithelium: cellular and subcellular distribution and probable role in secretion of aqueous humor. Exp. Eye Res. 1986;42:559–568. doi: 10.1016/0014-4835(86)90046-1. [DOI] [PubMed] [Google Scholar]
- SATO T., KAMATA Y., IRIFUNE M., NISHIKAWA T. Inhibition of purified (Na+,K+)-ATPase activity from porcine cerebral cortex by NO generating drugs. Brain Res. 1995;704:117–120. doi: 10.1016/0006-8993(95)01165-x. [DOI] [PubMed] [Google Scholar]
- SATO T., KAMATA Y., IRIFUNE M., NISHIKAWA T. Inhibitory effect of several nitric oxide-generating compounds on purified Na+,K(+)-ATPase activity from porcine cerebral cortex. J. Neurochem. 1997;68:1312–1318. doi: 10.1046/j.1471-4159.1997.68031312.x. [DOI] [PubMed] [Google Scholar]
- SESSA W.C. The nitric oxide synthase family of proteins. J. Vasc. Res. 1994;31:131–143. doi: 10.1159/000159039. [DOI] [PubMed] [Google Scholar]
- SEVEN I., TURKOZKAN N., CIMEN B. The effects of nitric oxide synthesis on the Na+,K(+)-ATPase activity in guinea pig kidney exposed to lipopolysaccharides. Mol. Cell. Biochem. 2005;271:107–112. doi: 10.1007/s11010-005-5616-1. [DOI] [PubMed] [Google Scholar]
- SHAHIDULLAH M., WILSON W.S. Atriopeptin, sodium azide and cyclic GMP reduce secretion of aqueous humour and inhibit intracellular calcium release in bovine cultured ciliary epithelium. Br. J. Pharmacol. 1999;127:1438–1446. doi: 10.1038/sj.bjp.0702681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAHIDULLAH M., WILSON W.S., YAP M., TO C.H. Effects of ion transport and channel-blocking drugs on aqueous humor formation in isolated bovine eye. Invest. Ophthalmol. Visual Sci. 2003;44:1185–1191. doi: 10.1167/iovs.02-0397. [DOI] [PubMed] [Google Scholar]
- SHAHIDULLAH M., YAP M.K., TO C.H. cGMP, sodiun nitroprusside and sodium azide reduce aqueous humour formation in the isolated arterially perfused pig eye. Br. J. Pharmacol. 2005;144:1–9. doi: 10.1038/sj.bjp.0706156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNYDER G.L., GIRAULT J.A., CHEN J.Y., CZERNIK A.J., KEBABIAN J.W., NATHANSON J.A., GREENGARD P. Phosphorylation of DARPP-32 and protein phosphatase inhibitor-1 in rat choroid plexus: regulation by factors other than dopamine. J. Neurosci. 1992;12:3071–3783. doi: 10.1523/JNEUROSCI.12-08-03071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI Y., LU Q., XU D.Z., SZABO C., HASKO G., DEITCH E.A. Na+,K+-ATPase activity is inhibited in cultured intestinal epithelial cells by endotoxin or nitric oxide. Int. J. Mol. Med. 2005;15:871–877. [PubMed] [Google Scholar]
- SWEIRY J.H., SHIBUYA I., ASADA N., NIWA K., DOOLABH K., HABARA Y., KANNO T., MANN G.E. Acute oxidative stress modulates secretion and repetitive Ca2+ spiking in rat exocrine pancreas. Biochim. Biophys. Acta. 1999;1:19–30. doi: 10.1016/s0925-4439(99)00021-6. [DOI] [PubMed] [Google Scholar]
- TAKASHIMA Y., TANIGUCHI T., YOSHIDA M., HAQUE M.S., YOSHIMURA N., HONDA Y. Ocular hypotensive mechanism of intravitreally injected brain natriuretic peptide in rabbit. Invest. Ophthalmol. Visual Sci. 1996;37:2671–2677. [PubMed] [Google Scholar]
- TIPSMARK C.K., MADSEN S.S. Regulation of Na+/K+-ATPase activity by nitric oxide in the kidney and gill of the brown trout (Salmo trutta) J. Exp. Biol. 2003;206:1503–1510. doi: 10.1242/jeb.00284. [DOI] [PubMed] [Google Scholar]
- TO C.H., KONG C.W., CHAN C.Y., SHAHIDULLAH M., DO C.W. The mechanism of aqueous humour formation. Clin. Exp. Optomet. 2002;85:335–349. [PubMed] [Google Scholar]
- TSOU K., SNYDER G.L., GREENGARD P. Nitric oxide/cGMP pathway stimulates phosphorylation of DARPP-32, a dopamine- and cAMP-regulated phosphoprotein, in the substantia nigra. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3462–3465. doi: 10.1073/pnas.90.8.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARELA M., HERRERA M., GARVIN J.L. Inhibition of Na-K-ATPase in thick ascending limbs by NO depends on O2- and is diminished by a high-salt diet. Am. J. Physiol. Renal Physiol. 2004;287:F224–F230. doi: 10.1152/ajprenal.00427.2003. [DOI] [PubMed] [Google Scholar]
- WALLICK E.T., SCHWARTZ A. Interaction of cardiac glycosides with Na+,K+-ATPase. Methods Enzymol. 1988;156:201–213. doi: 10.1016/0076-6879(88)56022-6. [DOI] [PubMed] [Google Scholar]
- WANG R.F., PODOS S.M. Effect of the topical application of nitroglycerin on intraocular pressure in normal and glaucomatous monkeys. Exp. Eye Res. 1995;60:337–339. doi: 10.1016/s0014-4835(05)80116-2. [DOI] [PubMed] [Google Scholar]
- WYSE A.T., BAVARESCO C.S., BANDINELLI C., STRECK E.L., FRANZON R., DUTRA-FILHO C.S., WAJNER M. Nitric oxide synthase inhibition by L-NAME prevents the decrease of Na+,K+-ATPase activity in midbrain of rats subjected to arginine administration. Neurochem. Res. 2001;26:515–520. doi: 10.1023/a:1010912929042. [DOI] [PubMed] [Google Scholar]
- XIE Z.J., WANG Y.H., GANJEIZADEH M., MCGEE R., JR, ASKARI A. Determination of total (Na+ + K+)-ATPase activity of isolated or cultured cells. Anal. Biochem. 1989;183:215–219. doi: 10.1016/0003-2697(89)90470-3. [DOI] [PubMed] [Google Scholar]
- ZHANG C., IMAM S.Z., ALI S.F., MAYEUX P.R. Peroxynitrite and the regulation of Na(+),K(+)-ATPase activity by angiotensin II in the rat proximal tubule. Nitric Oxide. 2002;7:30–35. doi: 10.1016/s1089-8603(02)00003-4. [DOI] [PubMed] [Google Scholar]