Abstract
The effects of L-glutamate on activation-induced cell death (AICD) of human activated (1 μg ml−1 phytohemagglutinin plus 2 U ml−1 interleukin-2; 8 days) T lymphocytes were studied by measuring anti-CD3 monoclonal antibody (10 μg ml−1; 18 h)-induced cell apoptosis (Annexin V and propidium iodide staining).
L-Glutamate (1 × 10−8–1 × 10−4 M) significantly (P⩽0.01) inhibited AICD in a concentration-dependent manner (EC50=6.3 × 10−8 M; maximum inhibition 54.8±6.3% at 1 × 10−6 M).
The L-glutamate inhibitory effect was pharmacologically characterized as mediated by group I mGlu receptors, since mGlu receptor agonists reproduced this effect. The EC50 values were: 3.2 × 10−7 M for (1S,3R)-ACPD; 4.5 × 10−8 M for quisqualate; 1.0 × 10−6 M for (S)-3,5-DHPG; 2.0 × 10−5 M for CHPG.
Group I mGlu receptor antagonists inhibited the effects of quisqualate 1.0 × 10−6 M. The IC50 values calculated were: 8.7 × 10−5, 4.3 × 10−6 and 6.3 × 10−7 M for AIDA, LY 367385 and MPEP, respectively.
L-Glutamate (1 × 10−6 M; 18 h) significantly (P⩽0.05) inhibited FasL expression (40.8±11.3%) (cytofluorimetric analysis), whereas it did not affect Fas signalling.
Expression of both mGlu1 and mGlu5 receptor mRNA by T lymphocytes and T-cell lines, as demonstrated by reverse transcriptase–PCR analysis, suggests that L-glutamate-mediated inhibition of AICD was exerted on T cells.
These data depict a novel role for L-glutamate in the regulation of the immune response through group I mGlu receptor-mediated mechanisms.
Keywords: L-Glutamate, metabotropic glutamate receptors, apoptosis, human T lymphocytes, Fas/FasL system, cell death
Introduction
L-Glutamate signalling is not restricted to the CNS since glutamate receptors have been identified in several non-neuronal cells including immune cells (Skerry & Genever, 2001; Hinoi et al., 2004; Boldyrev et al., 2005): human peripheral blood T cells posses high affinity L-glutamate binding sites on their surface (Kostanyan et al., 1997), and both rodent and human cells of the T lineage (thymocytes and lymphocytes) express glutamate receptors (Storto et al., 2000; Rezzani et al., 2003; Ganor et al., 2003; Pacheco et al., 2004; Boldyrev et al., 2005; Miglio et al., 2005b).
Two major types of glutamate receptors have been so far characterized: ionotropic (iGlu) and metabotropic (mGlu) receptors (Kew & Kemp, 2005). iGlu receptors are ligand-gated ion channels (Dingledine et al., 1999; McFeeters & Oswald, 2004), that, on the basis of their sequence homology and agonist preference, are classified into NMDA, AMPA and kainate receptor types. mGlu receptors are members of the family C of G-protein-coupled receptors (GPCR), including Ca2+ and Mg2+ receptors, GABAB receptors, a number of receptors found in the vomeronasal organ and receptors for sweet molecules (Pin et al., 2004). The eight mGlu receptor subtypes so far characterized are linked to several effector systems, and are accordingly divided into three groups (Conn & Pin, 1997). Group I (mGlu1 and mGlu5 receptor subtypes) stimulates formation of inositol 1,4,5-triphosphate (IP3) and diacylglicerol (DAG), group II (mGlu2 and mGlu3 receptor subtypes) and group III (mGlu4, mGlu6, mGlu7 and mGlu8 receptor subtypes) induce reduction of intracellular cAMP levels.
Through these receptors, L-glutamate modulates several lymphocyte functions: (i) it potentiates T-cell responses (intracellular Ca2+ ([Ca2+]i) rises) to anti-CD3 monoclonal antibodies (mAb) or phytohemagglutinin (PHA) by acting on NMDA and non-NMDA iGlu receptors (Lombardi et al., 2001); (ii) it induces reactive oxygen species formation and modulates T-cell activation by acting on NMDA receptors (Boldyrev et al., 2004; Miglio et al., 2005b); (iii) it increases lymphocyte adhesion to extracellular matrix proteins and cell motility by acting on AMPA iGlu receptors (Ganor et al., 2003); (iv) it modulates the properties of Kv1.3 channels by acting on mGlu receptors and non-NMDA iGlu receptors (Poulopoulou et al., 2005); (vi) it induces intracellular Ca2+ signals and early gene (c-jun and c-fos) expression by acting on group I mGlu receptors (Miglio et al., 2005a).
Upon T-cell receptor (TCR) triggering, resting T cells are activated to proliferate and to produce cytokines. Restimulation of activated T cells through the TCR induces an apoptosis program termed activation-induced cell death (AICD). AICD is a crucial mechanism for maintenance of peripheral tolerance and limiting ongoing immune responses (Lenardo et al., 1999; Green et al., 2003; Marrack & Kappler, 2004). Current data suggest that AICD consists of an inductive phase, triggered by TCR stimulation, followed by an effector phase, which activates cell death (Budd, 2001; Hildeman et al., 2002). Inhibition of AICD by actinomycin D or cycloheximide (Shi et al., 1990) suggests that the inductive phase induces transcription of a new set of genes required to activate the cell-death program. The interface between the two phases appears to be linked to molecules belonging to the tumour-necrosis factor (TNF) and TNF receptor superfamilies, whose expression and function are closely regulated by TCR stimulation (Kroemer et al., 1995). Several members of the TNF receptor superfamily act as ‘death receptors' and include the TNFR-I, the DR4 and DR5 receptors for tumour-necrosis factor-related apoptosis-inducing ligand (TRAIL), and Fas (CD95/APO-1). Binding of these receptors by their ligands activates a caspase cascade and induces apoptosis (Sharma et al., 2000). AICD is inhibited by microenvironmental signals acting on T cell costimulatory molecules, such as CD28, H4/ICOS, several adhesion molecules, and cytokine receptors (Aoudjit & Vuori, 2000; Palmer et al., 2001; Carreno & Collins, 2002; Kerstan & Hunig, 2004).
The aim of this study was to investigate the effect of L-glutamate on T-cell AICD. Results show that micromolar concentrations of L-glutamate significantly inhibit AICD; this effect is mediated by group I mGlu receptor activation and is partially achieved by down regulation of FasL expression.
Methods
Cell cultures
Peripheral blood mononuclear cells (PBMC) were isolated by gradient centrifugation onto Ficoll (Lymphoprep, Nycomed, Oslo, Norway) of heparinized venous blood obtained from healthy volunteers (Boyum, 1968). T cells were purified by panning to remove CD11b+, CD45RO+ and HLA-DR+ cells with the appropriate mAbs, followed by use of the CD4+/CD8+ T Cell Isolation Kit II (Miltenyi Biotec, Bergisch-Gladbach, Germany). This approach provided >99% cells displaying the phenotype CD3+CD4+CD8+CD45RA+CD14−CD16−, as assessed by flow cytometry. Cells were activated with PHA (1 μg ml−1) and cultured in RPMI 1640 supplemented with fetal calf serum (FCS; 10% v v−1), human recombinant IL-2 (2 U ml−1), L-glutamine (2 mM), penicillin (100 U ml−1) and streptomycin (100 μg ml−1).
Human monocytes were isolated from heparinized venous blood of healthy volunteers as previously described (Brunelleschi et al., 2001) and their purity was assessed with the pan-leukocyte anti-CD45 (HLE-1) and the anti-CD14 (Leu-M3) monoclonal antibodies (Ziegler-Heitbrock, 2000). Monocyte-derived macrophages (MDM) were prepared by culturing monocytes for 7–8 days in RPMI 1640 medium containing FCS (10% v v−1), L-glutamine (2 mM), HEPES (10 mM), penicillin (100 U ml−1) and streptomycin (100 μg ml−1). MDM were defined as macrophage-like cells by evaluating the decreased expression of CD14 and absence of CD1a according to Gantner et al. (1997).
Jurkat (clone E6-1, human leukaemic T cells), FRO (human leukaemic T cells), SUP-T1 (VB) (human leukaemic T cells), H9 (human cutaneous T cell lymphoma), HuT-78 (human cutaneous T-cell lymphoma), and THP-1 (human leukaemic monocytic cells) cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, U.S.A.). Jurkat, FRO, SUP-T1, H9, and THP-1 cells were maintained in RPMI 1640 medium containing FCS (10% v v−1), L-glutamine (2 mM), NaHCO3 (1.5 g l−1), glucose (4.5 g l−1), HEPES (10 mM), sodium piruvate (1.0 mM), penicillin (100 U ml−1) and streptomycin (100 μg ml−1). HuT-78 cells were maintained in RPMI 1640 medium containing FCS (20% v v−1), L-glutamine (4 mM), NaHCO3 (1.5 g l−1), penicillin (100 U ml−1) and streptomycin (100 μg ml−1).
L(tk−), mouse fibroblast cells, stable transfected with recombinant human mGlu1b receptor or human mGlu5a receptor (Lin et al., 1997) were a generous gift from Professor F. Moroni (University of Florence, Italy). They were cultured in Dulbecco's Modified Eagle Medium supplemented with dialyzed FCS (10% v v−1), L-glutamine (2 mM), penicillin (100 U ml−1), streptomycin (100 μg ml−1) and G418 (500 μg ml−1). To induce the expression of recombinant receptors, the cells were treated with dexamethasone (1 × 10−6 M) for 18 h.
Apoptosis assays
In the AICD assay, activated T cells (day 8 of culture) were washed twice in phosphate-buffered saline and then cultured (5 × 104 cell well−1) in 96-well flat-bottomed plates coated with anti-CD3 mAb (10 μg ml−1) in L-glutamate free medium (Neurobasal medium), supplemented with B27 supplement, penicillin (100 U ml−1) and streptomycin (100 μg ml−1) in the absence or presence of different drug concentrations. After a 18 h incubation, live cells were counted with the Trypan blue exclusion test or by flow cytometric detection of apoptotic cells after staining with propidium iodide and Annexin V. Assays were performed in triplicate and analysed blind by an observer. Annexin V staining was performed using the Annexin-V-Fluos kit (Boehringer Mannhein, Gmbh, Germany). Briefly, cells from each well were stained with Annexin V (Annexin-V-Fluos kit, Boehringer Mannhein, Gmbh, Germany) 20 μg ml−1 and propidium iodide (1 μg ml−1) in 140 mM NaCl, 5 mM CaCl2 and 10 mM HEPES pH 7.4 and analysed by flow cytometry, that was set to acquire cells for 10 s. Live cells were those not displaying shrunken/hypergranular morphology and unstained by propidium iodide or Annexin V (Ramenghi et al., 2000). Results are expressed as percentage of relative cell loss calculated as follows: 100−(total live cell count in the assay well/total live cell count in the respective control well) × 100. Cells in the control wells were treated with experimental medium alone. Spontaneous cell loss in the control wells was always <10% of the seeded cells.
Evaluation of Fas-induced apoptosis was performed using the same protocol on activated T cells cultured for 18 h with the anti-Fas mAb (CH11 IgM; 1 μg ml−1).
FasL expression
FasL expression was evaluated by cytofluorimetric analysis using a fluorescein isothiocyanate (FITC)-conjugated anti-FasL mAb (Ancell, Bayport, MN, U.S.A.). Non-specific background fluorescence was evaluated with the appropriate isotype-matched control mAb (Becton Dickinson, San Jose, CA, U.S.A.). Levels of membrane-bound FasL were increased by blocking FasL cleavage before treatement with 3 × 10−5 M of GM6001, a broad spectrum matrix metalloproteinase inhibitor (Meng et al., 2004). FasL expression was expressed as median fluorescence intensity ratio (MFI-R) of total T lymphocytes according to the following formula: MFI-R=MFI of sample histogram (arbitrary units)/MFI of control histogram (arbitrary units).
RNA isolation and reverse transcriptase (RT)–PCR
Total RNA was isolated using the GenElute™ mammalian total RNA miniprep kit (Sigma-Aldrich, Milan, Italy) according to the manufacturer's instructions. Total RNA (5 μm) were reverse-transcribed using the ThermoScript™ RT–PCR kit (Invitrogen, Milan, Italy). Aliquots were amplified by eminested (mGlu5 receptor) or nested (mGlu1 receptor) PCR with 2.5 U of Taq polymerase (Invitrogen, Milan, Italy). The primers and protocols we used were reported in Table 1. Amplification products were separated by electrophoresis on 2% agarose gels and visualized with ethidium bromide.
Table 1.
PCR primers and protocols used in this study
| Template | Primers | Exon | Size (bp) | Denaturation | Annealing | Extension | Cycles |
|---|---|---|---|---|---|---|---|
| HmGluR1 |
External |
|
|
|
|
|
|
| NM_000838a |
Forward 5′-TTCGAGATGAGAAGGATGGG-3′ |
1 |
707 |
94°C for 30 s |
60°C for 30 s |
72°C for 60 s |
30 |
| |
Reverse 5′-CTCGTGTTAGTGTCCAGCC-3′ |
3 |
|
|
|
|
|
| |
Internal |
|
|
|
|
|
|
| |
Forward 5′-ACAAGCATCGACCTGAGTGA-3′ |
1 |
234 |
96°C for 15 s |
63°C for 30 s |
72°C for 30 s |
35 |
| |
Reverse 5′-GCGTTGCTGTAGATTTTGTCA-3′ |
2 |
|
|
|
|
|
| |
|
|
|
|
|
|
|
| HmGluR5 |
External |
|
|
|
|
|
|
| NM_000842a |
Forward 5′-CGATCCTATTCGATGAGAATGGA-3′ |
4 |
1075 |
94°C for 30 s |
TDb 65-60°C for 30 s |
72°C for 90 s |
30 |
| |
Reverse 5′-GTGGCACTGAGGCTGACCGAGAAA-3′ |
7 |
|
|
|
|
|
| |
Internal |
|
|
|
|
|
|
| |
Reverse 5′-GACCCCAGTTGGCATGCCTTGC-3′ |
6 |
310 |
94°C for 20 s |
TDb 65-60°C for 20 s |
72°C for 20 s |
30 |
| |
|
|
|
|
|
|
|
| GAPDH |
Forward 5′-GGTCGGAGTCAACAACGGATTTGG-3′ |
2 |
1000 |
96°C for 30 s |
60°C for 30 s |
72°C for 45 s |
25 |
| NM_002046a | Reverse 5′-ACCACCCTGTTGCTGTAGCCA-3′ | 9 |
Accession number NCBI sequence database (GenBank).
TD: Touch down program (annealing temperature decreases of 1°C for the first 10 cycles.
Statistical analysis
Results are expressed as means±s.e.m. of n experiments. Significance was assessed with Student's t-test for paired varieties with P⩽0.05 as the cutoff. Data were fitted as sigmoidal concentration–response curves and analysed with a four-parameter logistic equation. The molar concentration of an agonist that produces 50% of the maximal possible effect of that agonist (EC50) and the molar concentration of an antagonist that reduces the response to an agonist by 50% (IC50) values were determined with a nonlinear regression model using the software Origin version 6.0 (Microcal Software, Northampton, MA, U.S.A.).
Drugs and chemicals
(1S,3R)-1-Aminocyclopentan-1,3-dicarboxylic acid ((1S,3R)-ACPD), AMPA, (RS)-1-aminoindan-1,5-dicarboxylic acid (AIDA), L-α-amino-4-phosphonobutyrate (L-AP4), (2S,3S,4S)-α-(carboxycyclopropyl)glycine (L-CCG-I), (RS)-2-chloro-5-hydroxyphenylglycine (CHPG), (S)-3,5-dihydroxyphenylglycine ((S)-3,5-DHPG), kainate, (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY 367385), NMDA, 2-methyl-6-(2-phenyl-1-ethynyl)-pyridine (MPEP) and quisqualate were purchased from Tocris Cookson Ltd (Northpoint, U.K.). GM6001 was from Calbiochem (Milan, Italy). Dulbecco's Modified Eagle Medium, RPMI 1640, Neurobasal medium, FCS, dialyzed FCS and B27 supplement were obtained from Gibco (Life Technologies, Milan, Italy). Recombinant human IL-2 was from Biogen (Geneva, Switzerland). Anti-CD3 (OKT3), anti-CD11b (OKM1), anti-HLA-DR (L243), and anti-CD45RO (UCHL1) mAbs were purified by affinity chromatography on protein G-Sepharose 4 fast flow columns (Amersham Pharmacia Biotech, Uppsala, Sweden) from the hybridoma supernatants. Anti-CD14 (Leu-M3), anti-CD1a (HI149) and anti-CD45 (HLE-1) mAbs were from Becton Dickinson (San Jose, CA, U.S.A.). Anti-Fas IgM CH-11 mAb was obtained from MBL (Nagoya, Japan). All other drugs or chemicals were purchased from Sigma-Aldrich (Milan, Italy).
Results
Effects of L-glutamate on T cell AICD
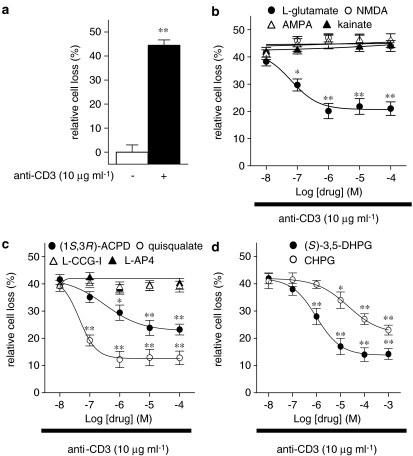
To study the effects of L-glutamate on AICD of human T lymphocytes, freshly isolated PBMC were treated with PHA (1 μg ml−1) in the presence of recombinant human IL-2 (2 U ml−1) for 8 days to activate and expand T cells and sensitize them to AICD (see Methods). Then, T cells were stimulated with anti-CD3 mAb (10 μg ml−1; 18 h) in the absence or presence of increasing concentrations (1 × 10−8–1 × 10−4 M) of L-glutamate and the surviving cells were counted by cytofluorimetric analysis after staining with Annexin V and propidium iodide. CD3 stimulation induced a 44.5±2.2% of AICD, expressed as percentage of relative cell loss (see Methods) in the L-glutamate un-treated cells (Figure 1a). When the cells were exposed to L-glutamate the percentage of relative cell loss was significantly (P⩽0.01; n=10) reduced in a concentration-dependent manner (EC50=6.3 × 10−8 M). The maximum effect (20.1±2.8% of AICD) was obtained at 1 × 10−6 M of L-glutamate (Figure 1b). The protective effect of L-glutamate was due to AICD inhibition and not to increased cell proliferation since no cell count increase was detected in any culture condition during the 18 h of AICD assay (data not shown).
Figure 1.
Concentration–response curves for the L-glutamate or iGlu/mGlu receptor agonists inhibitory effects on AICD of human T lymphocytes. Activated T cells were stimulated with anti-CD3 mAb (10 μg ml−1; 18 h) (a); in the presence of increasing concentrations (1 × 10−8–1 × 10−3 M) of L-glutamate, NMDA, AMPA or kainate (b), (1S,3R)-ACPD, quisqualate, L-CGG-I or L-AP4 (c), (S)-3,5-DHPG or CHPG (d), and the surviving cells were counted by cytofluorimetric analysis after staining with Annexin V plus propidium iodide. CD3 stimulation induced a 44.5±2.2% of AICD in comparison to anti-CD3-untreated cells. The EC50 values were: 6.3 × 10−8 M for L-glutamate, 3.2 × 10−7 M for (1S,3R)-ACPD, 4.5 × 10−8 M for quisqualate, 1.0 × 10−6 M for (S)-3,5-DHPG and 2.0 × 10−5 M for CHPG. The white and dashed bars show the relative cell loss in anti-CD3-untreated and -treated cells in the absence of other drugs (a). The results are expressed as the mean±s.e.m. of at least ten experiments. *P⩽0.05; **P⩽0.01 versus anti-CD3 mAb-treated cells.
Effects of iGlu and mGlu receptor agonists on T cell AICD
To determine whether the L-glutamate-mediated inhibition of AICD of human T cells is mediated by specific activation of iGlu receptors, we evaluated the effect of increasing concentrations (1 × 10−8–1 × 10−4 M) of NMDA, AMPA or kainate, selective iGlu receptor agonists. All selective iGlu receptor agonists were ineffective at all concentrations tested (1 × 10−8–1 × 10−4 M) (Figure 1b). On the contrary, (1S,3R)-ACPD, the prototype agonist of mGlu receptors, significantly (P⩽0.01; n=10) reduced the percentage of relative cell loss in a concentration-dependent manner (EC50=3.2 × 10−7 M) (Figure 1c). The maximum effect (23.2±2.0% of AICD) was measured at 1 × 10−4 M of (1S,3R)-ACPD.
As (1S,3R)-ACPD is not a subtype-specific agonist, to pharmacologically characterize the mGlu receptor subtype(s) involved in the L-glutamate-induced AICD inhibition, we evaluated the effects of increasing concentrations (1 × 10−8–1 × 10−4 M) of quisqualate, L-CCG-I, and L-AP4, selective agonists of group I, II or III mGlu receptors, respectively. Quisqualate significantly (P⩽0.01; n=10) protected cells from AICD, whereas L-CCG-I and L-AP4 were ineffective at all concentrations tested (1 × 10−8–1 × 10−4 M) (Figure 1c). Quisqualate was more potent and effective than (1S,3R)-ACPD (EC50: 4.5 × 10−8 versus 3.2 × 10−7 M; maximum effects: 12.9±3.0 versus 23.8±2.8% of AICD at 1.0 × 10−5 M). We obtained similar results with both (S)-3,5-DHPG, a selective group I mGlu receptor agonist acting on both mGlu1 and mGlu5 receptors, and CHPG, a highly selective mGlu5 receptor agonist (Figure 1d). (S)-3,5-DHPG was more potent and effective than CHPG (EC50: 1.0 × 10−6 versus 2.0 × 10−5 M; maximum effects: 14.1±2.5% versus 23.0±1.8% AICD at 1.0 × 10−3 M).
These data indicate that L-glutamate-induced AICD inhibition is mediated by group I mGlu receptor activation.
Effects of group I mGlu receptor antagonists on T cell AICD
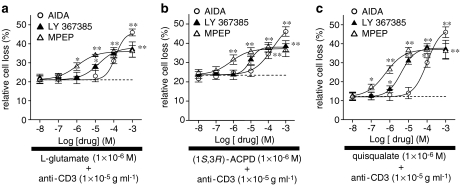
To asses the relative contribution of mGlu1 and mGlu5 receptor subtypes, we evaluated the effects of increasing concentrations (1 × 10−8–1 × 10−3 M) of AIDA, a selective group I mGlu receptor antagonist, LY 367385, a selective mGlu1 receptor antagonist, and MPEP, a selective mGlu5 receptor antagonist (Kew & Kemp, 2005), on AICD inhibition mediated by L-glutamate (1 × 10−6 M) (Figure 2a), (1S,3R)-ACPD (1 × 10−4 M) (Figure 2b) or quisqualate (1 × 10−6 M) (Figure 2c). All antagonists significantly (P⩽0.01; n=8) antagonized the inhibitory effects of mGlu receptor agonists in a concentration-dependent manner: the IC50 values calculated on quisqualate effect were: 8.7 × 10−5, 4.3 × 10−6 and 6.3 × 10−7 M for AIDA, LY 367385 and MPEP, respectively. AIDA (1 × 10−3 M) abolished the effects of L-glutamate, (1S,3R)-ACPD, and quisqualate; LY 367385 and MPEP, however, only partially antagonized the protective effects of the agonists (maximum effects calculated on quisqualate effect was 38.4±0.5 and 37.8±1.2% of AICD at 1 × 10−3 M).
Figure 2.
Inhibition of the L-glutamate, (1S,3R)-ACPD or quisqualate protective effects on AICD of human T lymphocytes. Activated T cells were stimulated with anti-CD3 mAb (10 μg ml−1; 18 h) in the presence of (1 × 10−6 M) L-glutamate (a), (1 × 10−4 M) (1S,3R)-ACPD (b), (1 × 10−6 M) quisqualate (c) and increasing concentrations (1 × 10−8–1 × 10−3 M) of AIDA, LY 367385 or MPEP. Surviving cells were counted by cytofluorimetric analysis after staining with Annexin V plus propidium iodide. The IC50 values, calculated when quisqualate was used, were: 8.7 × 10−5 M for AIDA; 4.3 × 10−6 M for LY 367385 and 6.3 × 10−7 M for MPEP. Dot lines represent the relative cell loss in anti-CD3 mAb-treated cells in the presence of L-glutamate (1 × 10−6 M) (a), (1S,3R)-ACPD (1 × 10−4 M) (b) quisqualate (1 × 10−6 M) (c). The results are expressed as the mean±s.e.m. of cell death of at least eight experiments. *P⩽0.05, **P⩽0.01 versus anti-CD3 mAb-treated cells in the presence of mGlu receptor agonists.
These results demonstrate that both group I mGlu receptor subtypes contribute to the L-glutamate-mediated inhibition of AICD of human T lymphocytes.
Effects of L-glutamate on the Fas/FasL system
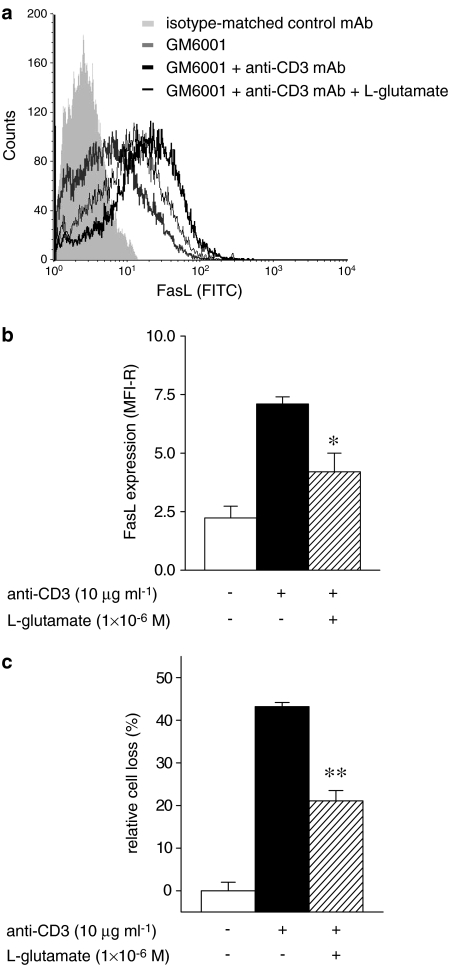
AICD is partly due to recruitment of the Fas/FasL system (Green et al., 2003). To assess whether L-glutamate acts on this system, we evaluated its effect on FasL expression and Fas-induced T-cell death.
FasL expression was evaluated by direct immunofluorescence and cytofluorimetric analysis on activated T cells (day 8) cultured for 18 h in the presence of GM6001 (3 × 10−5 M), a broad spectrum matrix metalloproteinase inhibitor used to maximize FasL expression (Meng et al., 2004) and in the absence or presence of anti-CD3 mAb (10 μg ml−1) and L-glutamate (1 × 10−6 M). Results showed that stimulation of activated T cells with anti-CD3 mAb increased FasL expression, but the upregulation was significantly higher in the absence than in the presence of L-glutamate (MFI-R were 7.1±0.3 versus 4.2±0.8, respectively, P⩽0.05; n=4) (Figure 3a and b). In the same experiments, L-glutamate inhibited AICD induced by anti-CD3 mAb at levels comparable to the inhibition of FasL expression (cell loss in the absence and presence of L-glutamate was 43.2±1.2 versus 21.1±2.4%, P⩽0.01; n=4) (Figure 3c).
Figure 3.
FasL expression in human T lymphocytes. Activated T cells were stimulated with anti-CD3 mAb (10 μg ml−1; 18 h), in the presence of GM6001 (3 × 10−5 M), and in the absence or presence of L-glutamate (1 × 10−6 M). FasL expression evaluated by direct immunofluorescence and cytofluorimetric analysis using a fluorescein isothiocyanate (FITC)-conjugated anti-FasL mAb (a). (b) FasL expression of L-glutamate (1 × 10−6 M) untreated or treated T cells expressed as MFI-R (see Methods) of total T lymphocytes. Activated T cells were stimulated with anti-CD3 mAb (10 μg ml−1; 18 h) in the presence of GM6001 (3 × 10−5 M) and in the absence or presence of L-glutamate (1 × 10−6 M). Surviving cells were counted by cytofluorimetric analysis after staining with Annexin V plus propidium iodide (c). The results are expressed as the mean±s.e.m. of cell death of at least four experiments. *P⩽0.05, **P⩽0.01 versus anti-CD3 mAb-treated cells in the absence of L-glutamate.
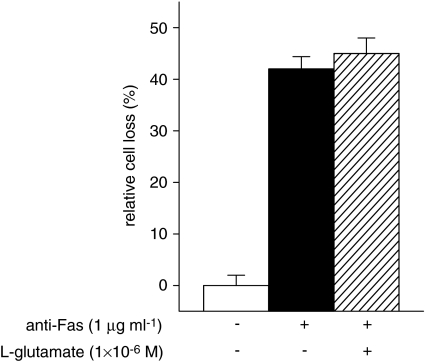
Fas-induced T-cell death was then evaluated by treating activated T cells (day 8) with an anti-Fas mAb (1 μg ml−1) in the absence or presence of L-glutamate (1 × 10−6 M) and counting surviving cells after 18 h as in the AICD assay. L-Glutamate did not inhibit Fas-induced cell death (Figure 4), which indicates that it does not affect Fas signalling.
Figure 4.
Fas-induced T-cell death. Activated T cells were stimulated with anti-Fas mAb (1 μg ml−1; 18 h), in the absence or presence of L-glutamate (1 × 10−6 M). Surviving cells were counted by cytofluorimetric analysis after staining with Annexin V plus propidium iodide. The results are expressed as the mean±s.e.m. of cell death of at least four experiments.
These results suggest that L-glutamate protects T cells from AICD by inhibiting FasL expression.
Expression of group I mGlu receptors by human T lymphocytes
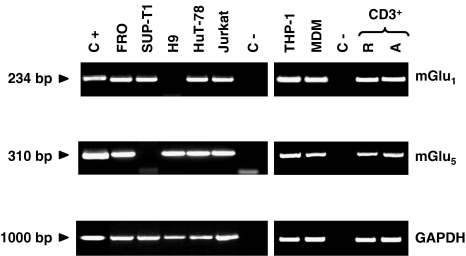
To verify whether L-glutamate-mediated inhibition of AICD is directly exerted on T cells, we evaluated the expression of mGlu1 and mGlu5 mRNA in resting and activated PBMC, purified T cells, MDM (see Methods), several T cell lines (FRO, SUP-T1, H9, HuT-78 and Jurkat), and the THP-1 monocytic cell line by RT–PCR (Figure 5). mGlu1- and mGlu5-specific amplification fragments were obtained from all samples, except for mGlu1 in the H9 line and mGlu5 in the SUP-T1 line. Identity of the amplified fragments was confirmed by direct sequencing (data not shown).
Figure 5.
Expression of mGlu1 and mGlu5 receptors in human lymphoid and myeloid cells. RT–PCRs were performed on total RNA isolated from mouse fibroblasts L(tk−) cells stably transfected with human mGlu1 or mGlu5 receptor cDNA (C+), FRO, SUP-T1, H9, HuT-78, Jurkat, THP-1 cell lines, human MDM, human resting T cell (R-CD3+) or human PHA (1 μg ml−1) plus IL-2 (2 U ml−1)-activated T cells (A-CD3+) using specific primers; in the negative control (C-) reverse trascriptase was omitted. GAPDH was used as house keeping gene to normalize cDNA amount. The results are representative of at least five experiments.
Discussion
This work shows that L-glutamate at low concentration range (10−7–10−4 M) protects T cells from AICD by acting on group I mGlu receptors (mGlu1 and mGlu5 receptor subtypes). In fact: (i) the protective effect was induced by (1S,3R)-ACPD, the prototype agonist of mGlu receptors, but not by NMDA, AMPA and kainate, specific iGlu receptor agonists; (ii) it was induced by quisqualate, (S)-3,5-DHPG, and CHPG, group I-selective mGlu receptor agonists, but not by L-CCG-I and L-AP4, group II- or III-selective mGlu receptor agonists; (iii) the protective effects of (1S,3R)-ACPD and quisqualate were antagonized by AIDA, LY 367385, and MPEP, group I-selective mGlu receptor antagonists (Kew & Kemp, 2005). These results are in line with those of other authors showing that T cells express mGlu1 and mGlu5 receptors (Pacheco et al., 2004). Moreover, stimulation of group I mGlu receptors protects neuronal and non-neuronal cells from apoptosis induced by different insults (Copani et al., 1998; Allen et al., 2000; Lin & Maiese, 2001; Pizzi et al., 2000; Rong et al., 2003).
Cell protection from AICD could be mediated by direct activation of glutamate receptors expressed on T cells or on bystander cells, such as, macrophages. However, the relative contribution of macrophages should be low in our system, since AICD assays contained <0.5% residual macrophages.
AICD is a complex death response involving upregulation of both Fas and FasL, and sensitization of Fas connection to caspase cascades activating the apoptotic response. However, direct inhibition of Fas signalling was not involved in the L-glutamate-mediated antiapoptotic effects, since L-glutamate did not inhibit T-cell death induced by direct triggering of Fas. By contrast, the L-glutamate effects may be mediated by inhibition of FasL expression, which is supported by the observation that L-glutamate induced comparable inhibition of AICD and FasL expression. However, our data do not rule out the possibility that L-glutamate may also act on other receptors, such as those of TNF, TRAIL or possibly IFN-γ, whose role in AICD has been postulated (Sytwu et al., 1996; Tucek-Szabo et al., 1996; Martinez-Lorenzo et al., 1998; Refaeli et al., 2002).
Group I mGlu receptor stimulation leads to signalling through several pathways that may interfere with TCR signalling, such as the phospholipase C/protein kinase C (Pin & Duvoisin, 1995; Hermans & Challiss, 2001; Miglio et al., 2005b), the mitogen-activated protein kinase (MAPK) (Karim et al., 2001; Thandi et al., 2002), the phosphoinositide 3-kinases (PI3K) (Rong et al., 2003), and the cAMP/protein kinase A pathways (Reid et al., 1996; Balazs et al., 1998). It is worth noting that similar pathways are used by T-cell costimulatory receptors that can deliver antiapoptotic signals. For instance, CD28 triggers the PI3K/MAPK pathways and inhibits AICD by decreasing FasL expression in some experimental systems (Collette et al., 1998). However, it is relevant that, differently from CD28-mediated costimulation, group-I mGlu receptors display a specific effect on AICD without influencing cell proliferation (Lombardi et al., 2004) or response to other apoptotic signals, such as Fas triggering.
The antiapoptotic effect is detectable at low concentrations of L-glutamate with maximal effect at concentrations >10−6 M and with the EC50 at 6.3 × 10−8 M. It is difficult to hypothesize physiological significance for these concentrations, since L-glutamate plasma concentrations are physiologically between 3 and 10 × 10−5 M (i.e. in the maximal effective range). One possibility is that glutamate receptor activity is strictly regulated during the T-cell response by controlling their surface expression (Miglio et al., 2005b) or connection with the signalling pathways; this would allow a temporal specificity of L-glutamate-mediated signalling and modulate T-cell sensitivity to AICD in different activation conditions. A second possibility is that mGlu receptors are located in specialized regions of the T-cell membrane, such as the immunological synapse between T cells and antigen-presenting cells, and are sensitive to small changes of L-glutamate concentrations in this narrow space. In this regard, the immunological synapse may be similar to the nervous synapse, where Na+-dependent high-affinity L-glutamate transporters may reuptake L-glutamate and reduce the extracellular concentrations to the levels required for a high signal-to-noise ratio (Rothstein et al., 1994; Danbolt, 2001). It is intriguing that macrophages express glutamate transporters, similar to those present in the CNS, able to take up high amounts of L-glutamate under stimuli (Rimaniol et al., 2000). A third possibility is that mGlu receptor activation is responsible for a tonic survival signal that decreases cell sensitivity to AICD in the common body microenvironments where L-glutamate concentrations are high (>1 × 10−6 M); by contrast T cells would be more sensitive to AICD in specific microenvironments where L-glutamate concentrations are low (<1 × 10−6 M), such as in the CNS. This model could imply that L-glutamate plays a role in the immunological privilege of these tissues, but this is contradicted by the mean extracellular L-glutamate concentrations present in the CNS (∼1 × 10−6 M), which is effective in protecting T lymphocytes from AICD (on the border of the maximal effect plateau). However, the experimental L-glutamate concentrations may be higher than the physiological CNS extracellular levels, because of the release of intracellular L-glutamate by either apoptotic or damaged cells.
Together with previous reports, these data depict a complex role of L-glutamate in regulation of the immune response. Micromolar concentrations may have a receptor-mediated effect capable to modulate TCR signalling by acting on both iGlu and mGlu receptors. Stimulation of mGlu receptors may sustain the immune response by decreasing cell susceptibility to AICD, whereas stimulation of non-NMDA or NMDA iGlu receptors may trigger cell adhesion to extracellular matrix glycoproteins, chemotactic migration (Ganor et al., 2003) and T-cell activation (Miglio et al., 2005b), respectively. By contrast, millimolar concentrations, reached in damaged tissues during acute and chronic inflammation, may have a metabolic effect possibly by modification of the intracellular levels of thiol compounds, resulting in modulation of the immune response with decreased cell proliferation and increased secretion of IFN-γ and IL-10 (Lombardi et al., 2004).
Acknowledgments
We thank Professor F. Moroni, University of Florence, Italy, for providing L(tk−), mouse fibroblast cells, stable transfected with recombinant human mGlu1b receptor or human mGlu5a receptor. This study was supported by Eastern Piedmont University (Vercelli, Italy), PRIN Projects by ‘Ministero dell'Istruzione, dell'Università e della Ricerca' (Rome, Italy), Fondazione CARIPLO (Milan, Italy), and Project no. GGP04071 by Telethon (Rome, Italy).
Abbreviations
- (1S,3R)-ACPD
(1S,3R)-1-aminocyclopentan-1,3-dicarboxylic acid
- AICD
activation-induced cell death
- AIDA
(RS)-1-aminoindan-1,5-dicarboxylic acid
- CHPG
(RS)-2-chloro-5-hydroxyphenylglycine
- DAG
diacylglicerol
- (S)-3,5-DHPG
(S)-3,5-dihydroxyphenylglycine
- FCS
foetal calf serum
- FITC
fluorescein isothiocyanate
- GPCR
G-protein-coupled receptors
- iGlu
ionotropic glutamate
- IL
interleukin
- INF
interferon
- IP3
inositol 1,4,5-triphosphate
- L-AP4
L-α-amino-4-phosphonobutyrate
- L-CCG-I
(2S,3S,4S)-α-(carboxycyclopropyl)glycine
- LY 367385
(S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid
- mAb
monoclonal antibodies
- MFI-R
median fluorescence intensity ratio
- mGlu
metabotropic glutamate
- MAPK
mitogen-activated protein kinases
- MPEP
2-methyl-6-(2-phenyl-1-ethynyl)-pyridine
- PBMC
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
- PI3K
phosphoinositide 3-kinases
- TCR
T cell receptor
- TNF
tumour-necrosis factor
- TRAIL
tumour-necrosis factor-related apoptosis-inducing ligand
References
- ALLEN J.W., KNOBLACH S.M., FADEN A.I. Activation of group I metabotropic glutamate receptors reduces neuronal apoptosis but increases necrotic cell death in vitro. Cell Death Differ. 2000;7:470–476. doi: 10.1038/sj.cdd.4400678. [DOI] [PubMed] [Google Scholar]
- AOUDJIT F., VUORI K. Engagement of the α2β1 integrin inhibits Fas ligand expression and activation-induced cell death in T cells in a focal adhesion kinase-dependent manner. Blood. 2000;95:2044–2051. [PubMed] [Google Scholar]
- BALAZS R., MILLER S., CHUN Y., O'TOOLE J., COTMAN C.W. Metabotropic glutamate receptor agonists potentiate cyclic AMP formation induced by forskolin or β-adrenergic receptor activation in cerebral cortical astrocytes in culture. J. Neurochem. 1998;70:2446–2458. doi: 10.1046/j.1471-4159.1998.70062446.x. [DOI] [PubMed] [Google Scholar]
- BOLDYREV A.A., CARPENTER D.O., JOHNSON P. Emerging evidence for a similar role of glutamate receptors in the nervous and immune systems. J. Neurochem. 2005;95:913–918. doi: 10.1111/j.1471-4159.2005.03456.x. [DOI] [PubMed] [Google Scholar]
- BOLDYREV A.A., KAZEY V.I., LEINSOO T.A., MASHKINA A.P., TYULINA O.V., JOHNSON P., TUNEVA J.O., CHITTUR S., CARPENTER D.O. Rodent lymphocytes express functionally active glutamate receptors. Biochem. Biophys. Res. Commun. 2004;324:133–139. doi: 10.1016/j.bbrc.2004.09.019. [DOI] [PubMed] [Google Scholar]
- BOYUM A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Lab. Clin. Invest. 1968;21:77–89. [PubMed] [Google Scholar]
- BRUNELLESCHI S., PENENGO L., LAVAGNO L., SANTORO C., COLANGELO D., VIANO I., GAUDINO G. Macrophage stimulating protein (MSP) evokes superoxide anion production by human macrophages of different origin. Br. J. Pharmacol. 2001;134:1285–1295. doi: 10.1038/sj.bjp.0704356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUDD R.C. Activation-induced cell death. Curr. Opin. Immunol. 2001;13:356–362. doi: 10.1016/s0952-7915(00)00227-2. [DOI] [PubMed] [Google Scholar]
- CARRENO B.M., COLLINS M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- COLLETTE Y., BENZIANE A., RAZANAJAONA D., OLIVE D. Distinct regulation of T-cell death by CD28 depending on both its aggregation and T-cell receptor triggering: a role for Fas-FasL. Blood. 1998;92:1350–1363. [PubMed] [Google Scholar]
- CONN P.J., PIN J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- COPANI A., CASABONA G., BRUNO V., CARUSO A., CONDORELLI D.F., MESSINA A., DI GIORGI GEREVINI V., PIN J.P., KUHN R., KNOPFEL T., NICOLETTI F. The metabotropic glutamate receptor mGlu5 controls the onset of developmental apoptosis in cultured cerebellar neurons. Eur. J. Neurosci. 1998;10:2173–2184. doi: 10.1046/j.1460-9568.1998.00230.x. [DOI] [PubMed] [Google Scholar]
- DANBOLT N.C. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- DINGLEDINE R., BORGES K., BOWIE D., TRAYNELIS S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- GANOR Y., BESSER M., BEN-ZAKAY N., UNGER T., LEVITE M. Human T cells express a functional ionotropic glutamate receptor GluR3, and glutamate by itself triggers integrin-mediated adhesion to laminin and fibronectin and chemotactic migration. J. Immunol. 2003;170:4362–4372. doi: 10.4049/jimmunol.170.8.4362. [DOI] [PubMed] [Google Scholar]
- GANTNER F., KUPFERSCHMIDT R., SCHUDT C., WENDEL A., HATZELMANN A. In vitro differentiation of human monocytes to macrophages: change of PDE profile and its relationship to suppression of tumour necrosis factor-alpha release by PDE inhibitors. Br. J. Pharmacol. 1997;121:221–231. doi: 10.1038/sj.bjp.0701124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D.R., DROIN N., PINKOSKI M. Activation-induced cell death in T cells. Immunol. Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- HERMANS E., CHALLISS R.A. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem. J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILDEMAN D.A., ZHU Y., MITCHELL T.C., KAPPLER J., MARRACK P. Molecular mechanisms of activated T cell death in vivo. Curr. Opin. Immunol. 2002;14:354–359. doi: 10.1016/s0952-7915(02)00335-7. [DOI] [PubMed] [Google Scholar]
- HINOI E., TAKARADA T., UESHIMA T., TSUCHIHASHI Y., YONEDA Y. Glutamate signaling in peripheral tissues. Eur. J. Biochem. 2004;271:1–13. doi: 10.1046/j.1432-1033.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- KARIM F., WANG C.C., GEREAU R.W., IV Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J. Neurosci. 2001;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERSTAN A., HUNIG T. Cutting edge: distinct TCR- and CD28-derived signals regulate CD95L, Bcl-xL, and the survival of primary T cells. J. Immunol. 2004;172:1341–1345. doi: 10.4049/jimmunol.172.3.1341. [DOI] [PubMed] [Google Scholar]
- KEW J.N., KEMP J.A. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology. 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- KOSTANYAN I.A., MERKULOVA M.I., NAVOLOTSKAYA E.V., NURIEVA R.I. Study of interaction between L-glutamate and human blood lymphocytes. Immunol. Lett. 1997;58:177–180. doi: 10.1016/s0165-2478(97)00086-2. [DOI] [PubMed] [Google Scholar]
- KROEMER G., PETIT P., ZAMZAMI N., VAYSSIERE J.-C., MIGNOTTE B. The biochemistry of programmed cell death. FASEB J. 1995;9:1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- LENARDO M.J., CHAN K.M., HORMUNG F., MCFARLAND H., SIEGEL R., WANG J., ZHENG L. Mature T lymphocyte apoptosis: immune regulation in a dynamic and unpredictable antigen enviroment. Annu. Rev. Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- LIN F.F., VARNEY M., SACAAN A.I., JACHEC C., DAGGETT L.P., RAO S., FLOR P., KUHN R., KERNER J.A., STANDAERT D., YOUNG A.B., VELICELEBI G. Cloning and stable expression of the mGluR1b subtype of human metabotropic receptors and pharmacological comparison with the mGluR5a subtype. Neuropharmacology. 1997;36:917–931. doi: 10.1016/s0028-3908(97)00078-6. [DOI] [PubMed] [Google Scholar]
- LIN S.H., MAIESE K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J. Cereb. Blood Flow Metab. 2001;21:262–275. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- LOMBARDI G., DIANZANI C., MIGLIO G., CANONICO P.L., FANTOZZI R. Characterization of ionotropic glutamate receptors in human lymphocytes. Br. J. Pharmacol. 2001;133:936–944. doi: 10.1038/sj.bjp.0704134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOMBARDI G., MIGLIO G., DIANZANI C., MESTURINI R., VARSALDI F., CHIOCCHETTI A., DIANZANI U., FANTOZZI R. Glutamate modulation of human lymphocyte growth: in vitro studies. Biochem. Biophys. Res. Commun. 2004;318:496–502. doi: 10.1016/j.bbrc.2004.04.053. [DOI] [PubMed] [Google Scholar]
- MARRACK P., KAPPLER J. Control of T cell viability. Annu. Rev. Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- MARTINEZ-LORENZO M.J., ALAVA M.A., GAMEN S., KIM K.J., CHUNTHARAPAI A., PINEIRO A., NAVAL J., ANEL A. Involvement of APO2 ligand/TRAIL in activation-induced death of Jurkat and human peripheral blood T cells. Eur. J. Immunol. 1998;28:2714–2725. doi: 10.1002/(SICI)1521-4141(199809)28:09<2714::AID-IMMU2714>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- MCFEETERS R.L., OSWALD R.E. Emerging structural explanations of ionotropic glutamate receptor function. FASEB J. 2004;18:428–438. doi: 10.1096/fj.03-0873rev. [DOI] [PubMed] [Google Scholar]
- MENG Y., GRAVES L., DO T.V., SO J., FISHMAN D.A. Upregulation of FasL by LPA on ovarian cancer cell surface leads to apoptosis of activated lymphocytes. Gynecol. Oncol. 2004;95:488–495. doi: 10.1016/j.ygyno.2004.07.052. [DOI] [PubMed] [Google Scholar]
- MIGLIO G., VARSALDI F., DIANZANI C., FANTOZZI R., LOMBARDI G. Stimulation of group I mGlu receptors evokes Ca2+ signals and c-jun and c-fos gene expression in human T cells. Biochem. Pharmacol. 2005a;70:189–199. doi: 10.1016/j.bcp.2005.04.038. [DOI] [PubMed] [Google Scholar]
- MIGLIO G., VARSALDI F., LOMBARDI G. Human T lymphocytes express N-methyl-D-aspartate receptors functionally active in controlling T cell activation. Biochem. Biophys. Res. Commun. 2005b;338:1875–1883. doi: 10.1016/j.bbrc.2005.10.164. [DOI] [PubMed] [Google Scholar]
- PACHECO R., CIRUELA F., CASADO V., MALLOL J., GALLART T., LLUIS C., FRANCO R. Group I metabotropic glutamate receptors mediate a dual role of glutamate in T cell activation. J. Biol. Chem. 2004;279:33352–33358. doi: 10.1074/jbc.M401761200. [DOI] [PubMed] [Google Scholar]
- PALMER E.M., FARROKH-SIAR L., MAGUIRE VAN SEVENTER J., VAN SEVENTER G.A. IL-12 decreases activation-induced cell death in human naive Th cells costimulated by intercellular adhesion molecule-1. I. IL-12 alters caspase processing and inhibits enzyme function. J. Immunol. 2001;167:749–758. doi: 10.4049/jimmunol.167.2.749. [DOI] [PubMed] [Google Scholar]
- PIN J.P., DUVOISIN R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- PIN J.P., KNIAZEFF J., GOUDET C., BESSIS A.S., LIU J., GALVEZ T., ACHER F., RONDARD P., PREZEAU L. The activation mechanism of class-C G-protein coupled receptors. Biol. Cell. 2004;96:335–342. doi: 10.1016/j.biolcel.2004.03.005. [DOI] [PubMed] [Google Scholar]
- PIZZI M., BENARESE M., BORONI F., GOFFI F., VALERIO A., SPANO P.F. Neuroprotection by metabotropic glutamate receptor agonists on kainate-induced degeneration of motor neurons in spinal cord slices from adult rat. Neuropharmacology. 2000;39:903–910. doi: 10.1016/s0028-3908(99)00257-9. [DOI] [PubMed] [Google Scholar]
- POULOPOULOU C., MARKAKIS I., DAVAKI P., NIKOLAOU C., POULOPOULOS A., RAPTIS E., VASSILOPOULOS D. Modulation of voltage-gated potassium channels in human T lymphocytes by extracellular glutamate. Mol. Pharmacol. 2005;67:856–867. doi: 10.1124/mol.67.3.. [DOI] [PubMed] [Google Scholar]
- RAMENGHI U., BONISSONI S., MIGLIARETTI G., DEFRANCO S., BOTTAREL F., GAMBARUTO C., DIFRANCO D., PRIORI R., CONTI F., DIANZANI I., VALESINI G., MERLETTI F., DIANZANI U. Deficiency of the Fas apoptosis pathway without Fas gene mutations is a familial trait predisposing to development of autoimmune diseases and cancer. Blood. 2000;95:3176–3182. [PubMed] [Google Scholar]
- REFAELI Y., VAN PARIJS L., ALEXANDER S.I., ABBAS A.K. Interferon gamma is required for activation-induced death of T lymphocytes. J. Exp. Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REID S.N., DAW N.W., GREGORY D.S., FLAVIN H. cAMP levels increased by activation of metabotropic glutamate receptors correlate with visual plasticity. J. Neurosci. 1996;16:7619–7626. doi: 10.1523/JNEUROSCI.16-23-07619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REZZANI R., CORSETTI G., RODELLA L., ANGOSCINI P., LONATI C., BIANCHI R. Cyclosporine-A treatment inhibits the expression of metabotropic glutamate receptors in rat thymus. Acta Histochem. 2003;105:81–87. doi: 10.1078/0065-1281-00688. [DOI] [PubMed] [Google Scholar]
- RIMANIOL A.C., HAIK S., MARTIN M., LE GRAND R., BOUSSIN F.D., DEREUDDRE-BOSQUET N., GRAS G., DORMONT D. Na+-dependent high-affinity glutamate transport in macrophages. J. Immunol. 2000;164:5430–5438. doi: 10.4049/jimmunol.164.10.5430. [DOI] [PubMed] [Google Scholar]
- RONG R., AHN J.Y., HUANG H., NAGATA E., KALMAN D., KAPP J.A., TU J., WORLEY P.F., SNYDER S.H., YE K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat. Neurosci. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN J.D., MARTIN L., LEVEY A.I., DYKES-HOBERG M., JIN L., WU D., NASH N., KUNCL R.W. Localisation of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- SHARMA K., WANG R.X., ZHANG L.Y., YIN D.L., LUO W.Y., SOLOMON J.C., JIANG R.F., MARKOS K., DAVIDSON W., SCOTT D.W., SHI Y.F. Death the Fas way: regulation and pathophysiology of CD95 and its ligand. Pharmacol. Ther. 2000;88:333–347. doi: 10.1016/s0163-7258(00)00096-6. [DOI] [PubMed] [Google Scholar]
- SHI Y.F., SZALAY M.G., PASKAR L., SAHAI B.M., BOYER M, SINGH B., GREEN D.R. Activation-induced cell death in T cell hybridomas is due to apoptosis. Morphologic aspects and DNA fragmentation. J. Immunol. 1990;144:3326–3333. [PubMed] [Google Scholar]
- SKERRY T.M., GENEVER P.G. Glutamate signalling in non-neuronal tissues. Trends Pharmacol. Sci. 2001;22:174–181. doi: 10.1016/s0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- STORTO M., DE GRAZIA U., BATTAGLIA G., FELLI M.P., MARODER M., GULINO A., RAGONA G., NICOLETTI F., SCREPANTI I., FRATI L., CALOGERO A. Expression of metabotropic glutamate receptors in murine thymocytes and thymic stromal cells. J. Neuroimmunol. 2000;109:112–120. doi: 10.1016/s0165-5728(00)00269-1. [DOI] [PubMed] [Google Scholar]
- SYTWU H.K., LIBLAU R.S., MCDEVITT H.O. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- THANDI S., BLANK J.L., CHALLISS R.A. Group-I metabotropic glutamate receptors, mGlu1a and mGlu5a, couple to extracellular signal-regulated kinase (ERK) activation via distinct, but overlapping, signalling pathways. J. Neurochem. 2002;83:1139–1153. doi: 10.1046/j.1471-4159.2002.01217.x. [DOI] [PubMed] [Google Scholar]
- TUCEK-SZABO C.L., ANDJELIC S., LACY E., ELKON K.B., NIKOLIC-ZUGIC J. Surface T cell Fas receptor/CD95 regulation, in vivo activation, and apoptosis. Activation-induced death can occur without Fas receptor. J. Immunol. 1996;156:192–200. [PubMed] [Google Scholar]
- ZIEGLER-HEITBROCK H.W. Definition of human blood monocytes. J. Leukoc. Biol. 2000;67:603–606. doi: 10.1002/jlb.67.5.603. [DOI] [PubMed] [Google Scholar]