Abstract
Thylakoid lipid composition in higher plants is characterized by a high level of fatty acid unsaturation. We have screened four mutants of Arabidopsis that have reduced levels of fatty acid unsaturation. Three of the mutant lines tested, fad5, fad6, and the fad3-2 fad7-2 fad8 triple mutant, were more susceptible to photoinhibition than wild-type Arabidopsis, whereas one mutant, fab1, was indistinguishable from wild type. The fad3-2 fad7-2 fad8 triple mutant, which contains no trienoic fatty acids in its thylakoid membranes, was most susceptible to photoinhibition. Detailed investigation of photoinhibition in the triple mutant revealed that the rate of photoinactivation of PSII was the same in wild-type and mutant plants. However, the recovery of photoinactivated PSII was slower in fad3-2 fad7-2 fad8, relative to wild type, at all temperatures below 27°C. These results indicate that trienoic fatty acids of thylakoid membrane lipids are required for low-temperature recovery from photoinhibition in Arabidopsis.
The chloroplast thylakoid membranes that are host to the light harvesting and electron transport reactions of photosynthesis have a characteristic and unusual lipid composition. The highly unsaturated fatty acids 18:3 and 16:3 account for approximately two-thirds of all the fatty acids in thylakoids and more than 90% of the fatty acids in monogalactosyldiacylglycerol, the most abundant chloroplast lipid. The atypical fatty acid, Δ3-trans-hexadecenoate (16:1,Δ3-trans), is present as a component of the major thylakoid phospholipid, phosphatidylglycerol (PG). Because these and other characteristics of chloroplast lipids are common to most or all higher plants, researchers have developed a series of hypotheses that propose that particular lipid structures have important roles in ensuring proper photosynthetic function (Siegenthaler and Murata, 1998).
Several lines of evidence indicate that thylakoid fatty-acid composition influences photoinhibition. Under most conditions, the major mechanism of photoinhibition is inactivation of the D1 protein of photosystem II (PSII; Aro et al., 1993). Damage to D1 is directly proportional to light intensity (Tyystjärvi and Aro, 1996), and inactivated protein molecules must be replaced by newly synthesized D1 to restore PSII activity (Kanervo et al., 1997; Kettunen et al., 1997; van Wijk et al., 1997; Zhang et al., 1999, 2000). The actual extent of photoinhibition in vivo depends on the balance between inactivation of D1 and the recovery process, which involves insertion of new D1 molecules into the thylakoid and their incorporation into the PSII complex (van Wijk et al., 1997). Recovery from photoinhibition is strongly temperature dependent (Greer et al., 1991; Wunschmann and Brand, 1992; Gombos et al., 1994), and photoinhibitory damage has been implicated as a major cause of chilling sensitivity in plants (Moon et al., 1995). Thylakoid fatty acid composition has been shown to influence photoinhibition in two specific ways. In cyanobacteria, the overall level of thylakoid unsaturation has been directly related to the capacity for D1 replacement and to the rate of recovery from photoinhibition at low temperatures (18°C–22°C; Gombos et al., 1992, 1994; Kanervo et al., 1995). In these studies, double mutants of Synechocystis sp. PCC6803, Fad6/DesA::Kmr and DesA−/DesD−, which lack all polyunsaturated fatty acids, were susceptible to photoinhibitory damage. However, the Fad6 mutant, which is substantially deficient in trienoic fatty acids but contains dienoic fatty acids, was indistinguishable from wild type (Gombos et al., 1992; Tasaka et al., 1996). Complementary to these results is the observation that the expression of the Δ12 desaturase encoded by DesA in a cyanobacterium that normally does not synthesize polyunsaturated fatty acids, Synechococcus sp. PCC7942, increased the low-temperature tolerance of PSII in this organism (Sippola et al., 1998).
In higher plants, the extent of low-temperature photoinhibition has been correlated with the level of saturated fatty acids (16:0 and 18:0) plus 16:1, Δ3-trans in PG (Moon et al., 1995). Wild-type tobacco (Nicotiana tabacum) plants contain 67% of these fatty acids in leaf PG, but in a transgenic line expressing a squash (Cucurbita pepo) acyl-ACP:glycerol-3-P acyltransferase (Rbcs-SQ), the proportion was increased to 88% (Moon et al., 1995). Photoinhibition in Rbcs-SQ plants was significantly higher than in wild-type controls. Additional experiments demonstrated that the recovery process (rather than D1 damage) was affected in Rbcs-SQ plants at 17°C and 25°C. As discussed by Moon et al. (1995), the significance of these results lies in the fact that the level of 16:0 + 18:0 + 16:1, Δ3-trans (sometimes referred to as high-melting-point fatty acids) is also correlated with chilling sensitivity in plants (Murata, 1983; Roughan, 1985). The implication is that delayed recovery from photoinhibition may be directly linked to the fatty acid composition of the chloroplast PG (Somerville, 1995).
The series of lipid mutants available in Arabidopsis have provided important information about the relationship between lipid structure and membrane function (Browse et al., 1985; Miquel et al., 1993; Wu et al., 1994; McConn and Browse, 1998). Here, we have used these mutants to investigate the effects of altered thylakoid fatty acid composition on damage and recovery processes during photoinhibition.
RESULTS
Initial Studies of Photoinhibition
The four mutant lines we selected for study all exhibit reductions in thylakoid membrane unsaturation compared with wild-type Arabidopsis. The fab1 (Wu et al., 1994) and fad5 (= fadB; Kunst et al., 1989) mutants show similar increases in 16:0 at the expense of polyunsaturated fatty acids. In the fad6 mutant (= fadC; Browse et al., 1989) trienoic fatty acids are reduced by 43% and replaced by 16:1 and 18:1. In the triple mutant fad3-2 fad7-2 fad8 (McConn and Browse, 1996), trienoic acids are completely eliminated and replaced by 16:2 and 18:2. We monitored potential quantum yield of PSII, Fv/Fm, in intact leaves of these plants using noninvasive chlorophyll fluorescence techniques. In Arabidopsis and other plants, Fv/Fm values have been shown to be well correlated with other measures of photoinhibition (Russell et al., 1995).
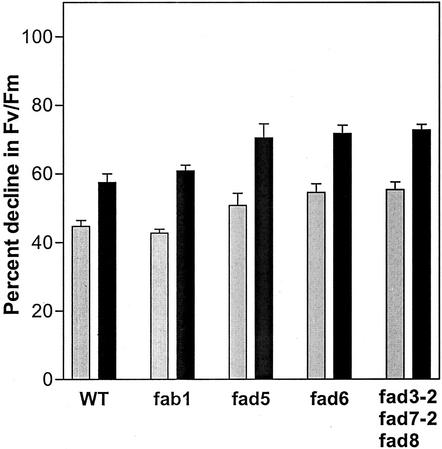
For plants grown at 22°C under 120 μmol quanta m−2 s−1 continuous illumination, there was no detectable difference in Fv/Fm between the mutants and wild-type controls (McConn and Browse, 1996; Wu et al., 1997). After leaves had been exposed to 1,200 μmol quanta m−2 s−1 of white light at 22°C for 2 h, Fv/Fm in wild-type leaves was reduced by 45%. When exposure to high light occurred at 17°C, Fv/Fm declined by 58% (Fig. 1). Photoinhibition of leaves from fab1 plants resulted in reductions in Fv/Fm that were the same as wild type. However, the other three mutants all experienced significantly greater photoinhibition than wild type and fab1 at both 22°C and 17°C. For example, at 17°C fad5 is photoinhibited 22% more than wild type and the triple mutant, fad3-2 fad7-2 fad8 shows a 26% greater photoinhibition relative to wild type (Fig. 1).
Figure 1.
Photoinhibition of PSII in excised leaves of wild-type and mutant Arabidopsis. Data are presented as the percent decrease in Fv/Fm after 2 h of photoinhibitory treatment under 1,200 μmol m−2 s−1 white light at 22°C (gray bars) and 17°C (black bars). The initial Fv/Fm ratios in wild-type and mutant samples varied from 0.80 to 0.81. Data are mean ± se; n = 10.
Photoinhibition and Recovery in fab1 Plants
The fab1 mutant contains increased 16:0 in PG and, as a result, the proportion of high-melting-point fatty acids (16:0 + 18:0 + 16:1,Δ3-trans) is 69% in this lipid compared with only 55% in PG from wild type. Levels of high-melting-point fatty acids above 60% in PG have been correlated with chilling sensitivity in plants and, as described above, with decreased rates of recovery from photoinhibition (Moon et al., 1995; Nishida and Murata, 1996). For this reason, we carried out further experiments to compare photoinhibitory damage and recovery between fab1 and wild-type plants.
In Arabidopsis and other plants, the D1 recovery process is blocked during short-term experiments in which plants grown at 18°C to 25°C are exposed to high light at temperatures below 5°C (Aro et al., 1994; Russell et al., 1995). By contrast, damage to D1 and the resulting photoinactivation of PSII is substantially independent of temperature (Tyystjärvi and Aro, 1996). When leaves from fab1 and wild-type plants were exposed to 1,200 μmol quanta m−2 s−1 at 2°C, Fv/Fm declined at the same rate in both sets of samples. After 3 h of exposure, Fv/Fm had fallen to 35% of the starting value (Fig. 2). These results indicate that the rate of photodamage to the D1 protein in fab1 was indistinguishable from the rate in wild type under these conditions.
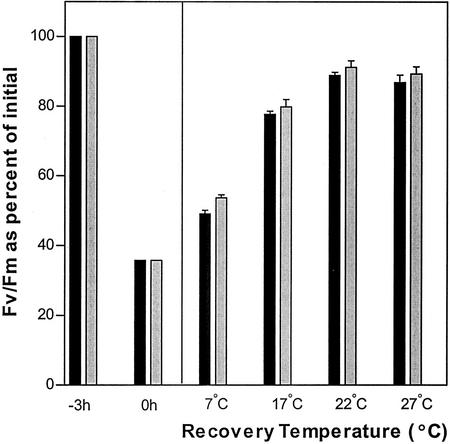
Figure 2.
Effect of 3 h of photoinhibition (PI) at 2°C (to) followed by 4 h of recovery at different temperatures on Fv/Fm activity in the leaves of wild-type Arabidopsis (black bars) and fab1 (gray bars) plants. Photoinhibition was carried out under 1,200 μmol quanta m−2 s−1 of white light; recovery was under 70 μmol quanta m−2 s−1. Values are averages of 15 leaves from three different experiments. The average initial value of Fv/Fm in wild-type and mutant plants varied between 0.82 and 0.83.
We then carried out a series of experiments in which photoinhibited leaves were allowed to recover under low light at different temperatures. At the four temperatures tested, 7°C, 17°C, 22°C, and 27°C, the kinetics of recovery were indistinguishable between fab1 and wild-type samples. The results are summarized in Figure 2 by showing the extent of recovery after 4 h at each temperature. The slightly better performance shown by fab1 is not statistically significant. Taken together, our results indicate that the increased proportion of high-melting-point PG found in fab1 plants (Wu and Browse, 1995) does not increase the susceptibility of this mutant to photoinhibition.
Kinetics of Photoinhibition in the Triple Mutant, fad3-2 fad7-2 fad8
The fad5, fad6, and fad3-2 fad7-2 fad8 mutants all showed increased photoinhibition relative to wild type in our preliminary assessment (Fig. 1). Subsequent experiments confirmed this finding and also revealed that the fad3-2 fad7-2 fad8 triple mutant consistently exhibited the most severe photoinhibition phenotype of the three lines. For this reason, we will report here the results of comparisons between the triple mutant and wild type. Previous studies have established that under light intensities that are normally used for growth of Arabidopsis (120–150 μmol quanta m−2 s−1), the triple mutant is indistinguishable from wild type in photosynthesis and growth at 22°C and shows only small differences (approximately 10%) during short-term experiments at temperatures as low as 5°C (McConn and Browse, 1996; Routaboul et al., 2000).
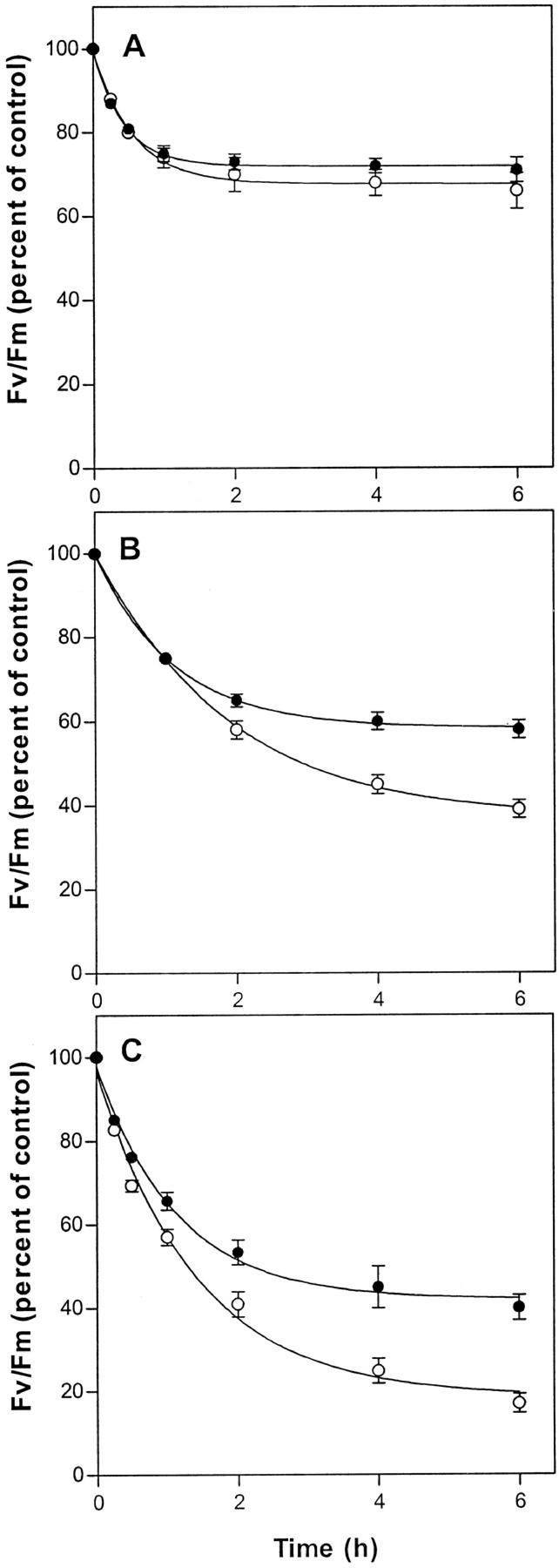
We first of all compared the time-course of decline in Fv/Fm for wild-type and mutant leaves exposed to 1,600 μmol quanta m−2 s−1 of white light (Fig. 3). At 27°C, the wild type and mutant both exhibited a decline in Fv/Fm during the 1st h of exposure followed by stabilization at approximately 70% of the starting value. At 22°C, a more extensive initial decline occurred, but whereas Fv/Fm in the wild type stabilized at 50% of the starting value, the mutant continued a slow decline to reach 40% after 6 h of high-light treatment. At 17°C, separation of the Fv/Fm curves for wild type and mutant occurred earlier in the experiment, and after 6 h of exposure, Fv/Fm in fad3-2 fad7-2 fad8 leaves averaged only 18% of the to value compared with 40% in the wild type.
Figure 3.
Photoinhibition under 1,600 μmol quanta m−2 s−1 of white light in excised leaves of wild type (●) and fad3-2 fad7-2 fad8 mutant plants (○) at 27°C (A), 22°C (B), and 17°C (C). Data are averages of 15 leaves from three independent experiments. Vertical bars represent se of measurement. Initial values for wild-type and mutant leaves were 0.80 and 0.82, respectively.
Photoinhibition in the Absence of D1 Repair
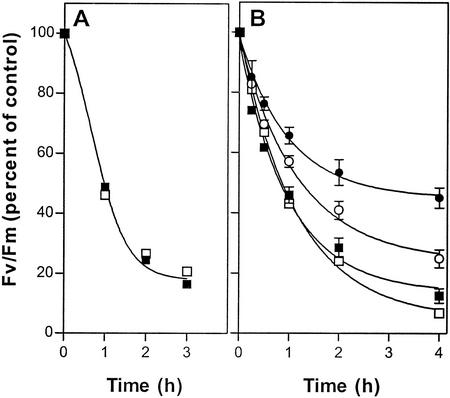
When leaves were photoinhibited at 3°C, the decline in Fv/Fm followed the same time course in both the mutant and wild type (Fig. 4A), and this is consistent with the conclusion that removal and replacement of damaged D1 does not occur at low temperature. To confirm this interpretation, we repeated the photoinhibition experiment at 17°C but included wild-type and mutant leaves that had been treated with lincomycin to inhibit chloroplast protein synthesis (Tyystjärvi and Aro, 1996). Compared with untreated leaves, lincomycin allowed more extensive photoinhibition of PSII and very largely eliminated the differential between wild-type and mutant responses at this temperature (Fig. 4B). The accelerated rate of Fv/Fm decline in lincomycin-treated leaves at 17°C was almost identical to that observed in untreated leaves at 3°C, and these data reflect the kinetics of PSII damage in the absence of D1 synthesis and reassembly of functional PSII complexes.
Figure 4.
Photoinactivation of PSII under 1,600 μmol quanta m−2 s−1 in leaves from wild-type (solid symbols) and fad3-2 fad7-2 fad8 mutant (open symbols) plants at 3°C (A) and at 17°C (B) either untreated (● and ○) or after treatment with 1 mm lincomycin (▪ and □). Data are averages of 15 leaves from three independent experiments expressed as a percentage of initial Fv/Fm. Vertical bars represent se of measurement. Initial values for wild-type and mutant leaves were 0.80 and 0.82, respectively.
Photoinhibition in Isolated Thylakoids
Photoinactivation of PSII occurs much more rapidly in isolated thylakoid preparations than in intact leaves (e.g. Moon et al., 1995) even under conditions where the thylakoids maintain activity after long periods in the dark or in low light. To uncover any differences between the fad3-2 fad7-2 fad8 mutant and wild type in this more rapid in vitro assay, we incubated thylakoids prepared from plants of each line either under 2,000 μmol quanta m−2 s−1 of white light or in darkness and then assayed for PSII activity. In the light, PSII activity declined rapidly in thylakoids from both wild type and mutant (Fig. 5). Thylakoids maintained in darkness before assay retained high rates of PSII electron transport throughout the experiment (Fig. 5) and for several hours afterward (not shown).
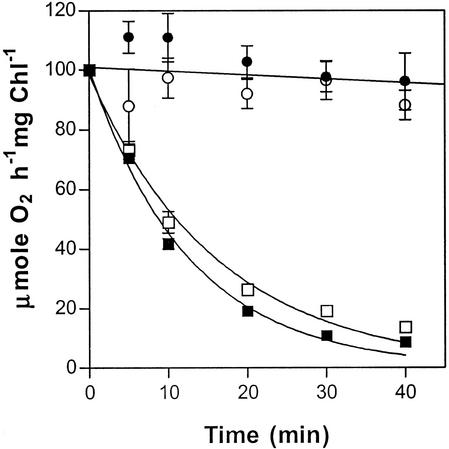
Figure 5.
Photoinactivation of PSII activity in thylakoids isolated from wild-type (▪) and fad3-2 fad7-2 fad8 mutant (□) plants by 2,000 μmol quanta m−2 s−1 white light at 22°C. PSII activity was measured as the rate of DCPIP-dependent oxygen evolution at saturating light intensity in thylakoids that were previously photoinhibited in the absence of the electron donor. The PSII activity of control samples of wild-type (●) and mutant (□) thylakoids, which were incubated in darkness, are also shown. The initial rate of DCPIP-dependent oxygen evolution was 253 and 260 μmol O2 h−1 mg−1 chlorophyll in wild-type and mutant, respectively. Data are averages of three independent measurements. Vertical bars represent se.
Measuring Recovery from Photoinhibition
The results reported above are consistent with a model in which a relatively constant and temperature-insensitive rate of photoinactivation of D1 competes with a highly temperature-dependent process of D1 replacement. To measure the rate of recovery, we incubated wild-type and mutant leaves under 1,600 μmol quanta m−2 s−1 at 3°C for 3 h so that Fv/Fm was reduced to only 20% of the value measured in untreated controls and then followed recovery of Fv/Fm at different temperatures under low light of 70 μmol m−2 s−1. Recovery from photoinhibition does not occur in darkness, but the process is maximal at all temperatures under as little as 20 μmol quanta m−2 s−1 (Aro et al., 1994).
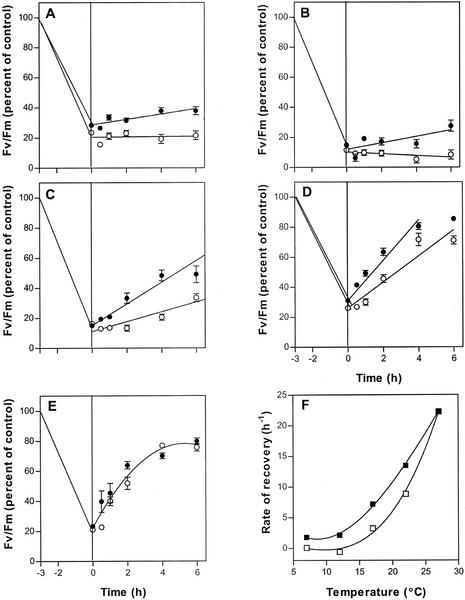
At both 7°C and 12°C, recovery of Fv/Fm is negligible in fad3-2 fad7-2 fad8 plants, but recovery is also extremely slow in wild-type plants (Fig. 6, A and B). A much larger difference in recovery rates is seen at 17°C (Fig. 6C). At this temperature, Fv/Fm measured in wild-type leaves increased steadily with time, so that by the end of the experiment at 8 h (data not shown) Fv/Fm was 70% of the pretreatment value. In mutant leaves, Fv/Fm rose much more slowly than in wild type. After 8 h Fv/Fm in the mutant was still less than 40% of the pretreatment value (data not shown). At 22°C, the rates of recovery were higher in both wild-type and mutant leaves, but a large difference remained between the two lines (Fig. 6D). However, at 27°C, the rate of recovery in mutant plants was substantially the same as that in wild type (Fig. 6E). These results allow the construction of temperature-response curves for the recovery process in the mutant and wild type, and these are shown in Figure 6F.
Figure 6.
Temperature dependence of recovery from photoinhibition. Leaves of wild-type (●) and fad3-2 fad7-2 fad8 mutant (○) plants were photoinactivated for 3 h at 3°C and 1,600 μmol quanta m−2 h−1 before being incubated under 70 μmol quanta m−2 s−1 white light at 7°C (A), 12°C (B), 17°C (C), 22°C (D), or 27°C (E). The initial rates of recovery, calculated from each graph (see “Materials and Methods”), are plotted in F. Data are averages of 10 samples; vertical bars represent se. The initial Fv/Fm values averaged 0.80 in wild type and 0.81 for the mutant.
DISCUSSION
In most higher plants, PG of the chloroplasts contains an unsaturated 18-carbon fatty acid at the sn-1 position and 16:0 or 16:1,Δ3-trans at the sn-2 position. In contrast, many chilling-sensitive plants accumulate PG in which both the sn-1 and sn-2 positions contain a saturated fatty acid (16:0 or 18:0) or 16:1,Δ3trans (Murata et al., 1982; Roughan, 1985). As a result, the PG molecular species undergo a transition from liquid-crystalline to gel phase at temperatures well above 25°C (Murata and Yamaya, 1984). One hypothesis proposes that the presence of these “high-melting-point” PG results in the formation of gel phase domains at chilling temperatures (5°C–10°C) and that lateral phase separation within chloroplast membranes is the direct cause of chilling sensitivity (Murata et al., 1992; Nishida and Murata, 1996). Typically, plants containing more than 60% high-melting-point fatty acids in PG have been shown to be chilling sensitive (Murata et al., 1982; Roughan, 1985), and experiments with transgenic plants also support this hypothesis (Murata, 1983; Wolter et al., 1992). However, the Arabidopsis fab1 mutant contains 69% high-melting-point fatty acids in PG—a higher percentage than is found in many chilling sensitive plants—but does not show the symptoms of classic chilling sensitivity (Wu and Browse, 1995). Instead, fab1 plants are damaged only by long-term exposure to low temperature. During the first 7 to 10 d after transfer to 2°C, growth and photosynthetic characteristics of fab1 plants remained indistinguishable from wild type, but beyond this time Fv/Fm values for mutant plants declined rapidly and reached values less than 0.1 after 28 d at 2°C. Electron microscopic examination of leaf samples revealed rapid and extensive disruption of thylakoid and chloroplast structure in the mutant. These results were interpreted as a primary disruption of PSII center function that triggers an autophagic response (Wu et al., 1997). Despite the almost complete loss of photosynthetic function and the destruction of photosynthetic machinery, fab1 plants retained a substantial capacity for recovery after transfer to 22°C (Wu et al., 1997).
Moon et al. (1995) used transgenic tobacco plants expressing a squash acyl-ACP:glycerol-3-P acyltransferase (line Rbcs-SQ) to provide a correlation between increased high-melting-point PG and a reduced rate of recovery from photoinhibition at 17°C and 25°C. The possibility that reduced recovery from photoinhibition contributes to plant chilling sensitivity has been debated (Moon et al., 1995; Somerville, 1995). However, transgenic Arabidopsis plants with increased high-melting-point PG (through expression of the Escherichia coli plsB gene; Wolter et al., 1992) were not compromised in their ability to recover from photoinhibition at 22°C (Bruggemann and Wolter, 1995). In agreement with the result of Bruggemann and Wolter (1995), we found no detectable difference between fab1 plants and wild-type Arabidopsis in the extent or kinetics of photoinhibition or in the speed of recovery from photoinhibition at any temperature between 7°C and 27°C. Nevertheless, our results do not contradict those of Moon et al., because the Rbcs-SQ line of tobacco contains very high levels of high-melting-point fatty acids in PG—88% of total PG compared with 69% in fab1 plants. Instead, they suggest that a threshold level of high-melting-point fatty acids in PG may be required before recovery from photoinhibition is compromised.
Like fab1, the fad3-2 fad7-2 fad8 triple mutant also becomes chlorotic during growth at low temperatures, but it exhibits much less severe reductions in Fv/Fm than fab1 (Wu et al., 1997; Routaboul et al., 2000). Nevertheless, our experiments demonstrate that it is the triple mutant, not fab1, that displays a photoinhibition phenotype. Our data are consistent with a model in which photodamage to the D1 protein occurs at the same rate in the fad3-2 fad7-2 fad8 mutant and wild type, whereas replacement of damaged D1 and recovery from photoinhibition is compromised in the mutant at all temperatures below 27°C (Fig. 6). This model is in general agreement with the conclusion, reached from studies of cyanobacteria, that reduced levels of thylakoid unsaturation prevent the efficient turnover of D1 protein in photodamaged PSII centers (Gombos et al., 1992, 1994; Kanervo et al., 1995, 1997). However, there are important differences between cyanobacteria and Arabidopsis in the relationship between fatty acid composition and the rate of recovery from photoinhibition. In the cyanobacterial model Synechocystis sp. PCC6803, mutations that substantially eliminate trienoic fatty acids were shown to have little or no effect on photoinhibition processes (Gombos et al., 1992), and it was necessary to generate mutants deficient in all polyunsaturated fatty acids Fad6/DesA::Kmr and DesA−/DesD− to observe reduced rates of recovery from photoinhibition (Gombos et al., 1994; Tasaka et al., 1996) and decreased levels of D1 protein at high-light intensities (Kanervo et al., 1995). Despite these photoinhibition phenotypes, the Synechocystis sp. mutants are healthy and viable at normal growth temperatures and moderate light levels. In contrast, a mutant of Arabidopsis lacking polyunsaturated fatty acids, fad2 fad6, is incapable of autotrophic growth and can only be grown on Suc-supplemented media (McConn and Browse, 1998). Because fad2 fad6 plants are robust but severely chlorotic when grown on Suc under low light (80–100 μmol quanta m−2 s−1), we concluded that photosynthesis is the process most severely affected by the elimination of polyunsaturated fatty acids (McConn and Browse, 1998). fad2 fad6 plants rapidly photobleached when exposed to light intensities as low as 150 μmol quanta m−2 s−1 (approximately one-tenth the levels used to induce photoinhibition in the experiments reported here).
In contrast to the fad2 fad6 mutant, the fad3-2 fad7-2 fad8 mutant is largely indistinguishable from wild type in growth and photosynthesis when grown at 22°C and light intensities as high as 250 μmol quanta m−2 s−1 (McConn and Browse, 1996; Routaboul et al., 2000). Instead, effects on photosynthesis are seen in the triple mutant only in environments that are toward the extremes of the physiological range. We have previously characterized the decline in photosynthetic function of triple mutant plants that occurs during growth at 4°C (Routaboul et al., 2000). Similar but less extreme phenotypes have been described for the fad5 and fad6 mutants (Hugly and Somerville, 1992). Now these same three lines are shown to suffer increased photoinhibition (Fig. 1). In fad3-2 fad7-2 fad8 and probably in fad5 and fad6 also, it is the recovery process that is compromised throughout most of the physiological temperature range for this species (Fig. 6), suggesting that lowered thylakoid unsaturation reduces the rate at which damaged D1 protein can be replaced in the PSII complex.
Over the last 15 years, many studies have demonstrated that photodamage to the D1 protein is a major cause of photoinhibition and that recovery requires replacement of the damaged D1 with a newly synthesized D1 molecule. The D1 protein is known to be synthesized on thylakoid-bound ribosomes as a precursor with a 9- to 16-amino acid C-terminal extension. After insertion into the thylakoid, the precursor is processed to its final form by a lumenal protease. PSII is not reactivated until this processing step has occurred (Kanervo et al., 1997; Zhang et al., 1999, 2000). In the mutants of Synechocystis sp. PCC6803 that lack all polyunsaturated fatty acids (Fad6/DesA::Kmr and DesA−/DesD−), the reduced recovery from photoinhibition (Gombos et al., 1994) has been shown to involve a failure to process newly synthesized D1 precursor, which accumulated to considerable levels in mutant but not wild-type cells at low temperatures (Kanervo et al., 1997). In that study, precursor D1 integrated into PSII complexes even at low temperatures, but no activation of photosynthetic O2 evolution occurred. Because the processes of D1 synthesis, assembly into PSII complexes, and protease processing of the C terminus are similar in cyanobacteria and higher plants (Zhang et al., 1999), it is tempting to speculate that a defect in D1 processing is the cause of reduced recovery in the fad3-2 fad7-2 fad8 mutant also. However, experiments indicate that D1 replacement in higher plants may be more complex and more highly regulated than in cyanobacteria. In contrast to cyanobacteria, degradation of D1 in higher plants is regulated by phosphorylation/dephosphorylation processes (Aro et al., 1992; Koivuniemi et al., 1995; Rintamaki et al., 1996). Moreover, degradation of D1 is a highly temperature-sensitive process in higher plants and appears to be a barrier to removal of damaged D1 at low temperatures (Aro et al., 1993). The role of phosphorylation/dephosphorylation in D1 breakdown in higher plants may be to couple protein degradation with insertion of newly synthesized D1 and reassembly of the PSII complex (Koivuniemi et al., 1995). If this is correct, it may be difficult to differentiate between breakdown of photodamaged D1 and synthesis, insertion, or processing of new D1 as the basis of the photoinhibition phenotype in the triple mutant.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Wild-type and mutant Arabidopsis plants were grown in 4-inch pots on a peat-based medium in a controlled environment chamber at 22°C under 120 μmol quanta m−2 s−1 white light provided by cool-white fluorescent lamps. Circular discs 1 cm in diameter excised from fully expanded leaves of 14- to 20-d-old plants were used for experiments.
All of the mutants of Arabidopsis described in this study were isolated from the Columbia wild type. The isolation and characterization of the fab1, fad5, and fad6 mutants as well as the triple mutant fad3-2 fad7-2 fad8 have been described elsewhere (Browse et al., 1989; Kunst et al., 1989; Wu et al., 1994; McConn and Browse, 1996). All of these mutants have exhibited chlorosis or other symptoms of damage after prolonged exposure to chilling temperatures ranging from 2°C to 5°C (Vijayan et al., 1997).
Fluorescence Measurements
Chlorophyll fluorescence from leaf tissue was measured using a PAM Fluorometer (Walz, Effeltrich, Germany). The ratio of variable fluorescence to maximal fluorescence (Fv/Fm)—representing the potential quantum yield of PSII photochemistry—was measured in dark-adapted leaf tissue. Leaf discs were floated on distilled water in brass containers, and dark-adapted at 22°C for 30 min before each Fv/Fm measurement was made. Leaf discs were then equilibrated to the desired temperature in dark before they were exposed to photoinhibitory or recovery treatment.
Measurement of PSII Activity in Isolated Thylakoids
Thylakoids were isolated by grinding Arabidopsis leaves in an ice-cold buffer containing 300 mm sorbitol, 25 mm Tris (pH 7.8), 10 mm NaCl, 5 mm MgCl2, and 5 mm EDTA with a chilled mortar and pestle. The homogenate was then filtered through four layers of miracloth, and the filtrate was centrifuged at 5,000g for 3 min at 4°C. The thylakoid pellet obtained was washed in ice-cold grinding buffer devoid of EDTA, re-suspended in the same buffer, and stored on ice for further use. Total chlorophyll content of the thylakoid suspensions was estimated in 80% (v/v) acetone using the equations of MacKinney (1941). Light-saturated rates of PSII electron transport in isolated thylakoids were measured in an oxygen electrode (Hansatech, King's Lynn, UK) as rates of O2 evolved by thylakoids at 22°C, using water as electron donor and dichlorophenolindophenol (DCPIP) as electron acceptor. The assays were performed in 1 mL of a reaction mixture containing 25 mm Tris (pH 7.4), 100 mm sorbitol, 10 mm NaCl, 5 mm NH4Cl, and 100 μm DCPIP as an electron acceptor and thylakoids containing 10 μg of chlorophyll. The reaction chamber was illuminated at a photon flux density of 800 μmol quanta m−2 s−1 with white light from a slide projector passed through 6 cm of a 0.1 m CuSO4 solution. The reaction mixture was maintained at 22°C by circulating water through a jacket surrounding the reaction chamber. The light-mediated increase in oxygen concentration in the reaction mixture was plotted on a chart recorder, and the rate of oxygen evolution was calculated from the slope of the plot.
Photoinhibition of PSII in thylakoids was induced by illuminating thylakoids with 2,000 μmol m−2 s−1 white light from a projector lamp. Thylakoids were suspended in the reaction buffer at a concentration of 100 μg chlorophyll mL−1 in the absence of DCPIP and NH4Cl in a plexiglass incubation chamber surrounded by a water jacket maintained at 22°C. Aliquots of thylakoid samples were drawn from the chamber at various time intervals and used for measurement of PSII electron transport immediately.
Photoinhibition and Recovery of PSII in Leaf Discs
Photoinhibition of PSII was induced in a temperature-controlled chamber by illuminating the leaf discs under 1,200 or 1,600 μmol quanta m−2 s−1 white light from a 1,000-W metal halide lamp (Philips, Somerset, NJ), passed through a 4-inch heat filter consisting of 0.1 m CuSO4 solution. Ten to 12 randomly selected leaves were sampled after different time intervals and dark-adapted for 30 min at 22°C before Fv/Fm was measured as described above.
Recovery of PSII from photoinhibition was measured in leaf discs previously photoinhibited at 3°C for 3 h. Fv/Fm was then measured in the photoinhibited leaf discs after dark incubation at 22°C for 30 min. Samples of leaf discs were subsequently equilibrated at one of five temperatures (7°C, 12°C, 17°C, 22°C, or 27°C) in the dark before being shifted to a weakly illuminated (70 μmol quanta m−2 s−1) chamber at the same temperature for recovery. Ten to 12 discs each were removed at different time intervals and incubated in the dark for 30 min at 22°C before measurement of Fv/Fm.
Lincomycin Treatment
Petioles of wild-type and mutant Arabidopsis leaves freshly excised under water were immersed in 1 mm aqueous solution of the prokaryotic protein synthesis inhibitor lincomycin and incubated for 3 h at 22°C in a chamber under dim white light (15 μmol quanta m−2 s−1). Petioles of control leaves were immersed in distilled water and incubated under similar conditions. The lincomycin-treated leaves did not recover from photoinhibition after 6 h in low light, whereas the control leaves exhibited recovery comparable with untreated leaves, indicating that lincomycin treatment effectively inhibited D1 synthesis.
Footnotes
This work was supported by the U.S. National Science Foundation (grant no. IBN–0084329) and by the Agricultural Research Center, Washington State University.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.004341.
LITERATURE CITED
- Aro EM, Kettunen P, Tyystjärvi E. ATP and light regulate D1 protein modification and degradation: role of D1* in photoinhibition. FEBS Lett. 1992;2971:29–33. doi: 10.1016/0014-5793(92)80320-g. [DOI] [PubMed] [Google Scholar]
- Aro EM, McCaffery S, Anderson JM. Recovery from photoinhibition in peas (Pisum sativum L.) acclimated to varying growth irradiances: role of D1 protein-turnover. Plant Physiol. 1994;104:1033–1041. doi: 10.1104/pp.104.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro EM, Virgin I, Andersson B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Browse J, Kunst L, Anderson S, Hugly S, Somerville CR. A mutant of Arabidopsis deficient in the chloroplast 16:1/18:1 desaturase. Plant Physiol. 1989;90:522–529. doi: 10.1104/pp.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse JA, McCourt PJ, Somerville CR. A mutant of Arabidopsis lacking a chloroplast-specific lipid. Science. 1985;227:763–765. doi: 10.1126/science.227.4688.763. [DOI] [PubMed] [Google Scholar]
- Bruggemann W, Wolter FP. Decrease of energy-dependent quenching, but no major changes of photosynthesis parameters in Arabidopsis thaliana with genetically-engineered phosphatidylglycerol composition. Plant Sci. 1995;108:13–21. [Google Scholar]
- Gombos Z, Wada H, Murata N. Unsaturation of fatty acids in membrane lipids enhances tolerance of the cyanobacterium Synechocystis PCC6803 to low-temperature photoinhibition. Proc Natl Acad Sci USA. 1992;89:9959–9963. doi: 10.1073/pnas.89.20.9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos Z, Wada H, Murata N. The recovery of photosynthesis from low-temperature photoinhibition is accelerated by the unsaturation of membrane lipids: a mechanism of chilling tolerance. Proc Natl Acad Sci USA. 1994;91:8787–8791. doi: 10.1073/pnas.91.19.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer DH, Ottander C, Oquist G. Photoinhibition and recovery of photosynthesis in intact barley leaves at 5 and 20-degrees-C. Physiol Plant. 1991;81:203–210. [Google Scholar]
- Hugly S, Somerville C. A role for membrane lipid polyunsaturation in chloroplast biogenesis at low temperature. Plant Physiol. 1992;99:197–202. doi: 10.1104/pp.99.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanervo E, Aro E-M, Murata N. Low unsaturation level of thylakoid membrane lipids limits turnover of the D1 protein of photosystem II at high irradiance. FEBS Lett. 1995;364:239–242. doi: 10.1016/0014-5793(95)00404-w. [DOI] [PubMed] [Google Scholar]
- Kanervo E, Tasaka Y, Murata N, Aro EM. Membrane lipid unsaturation modulates processing of the photosystem II reaction-center protein D1 at low temperatures. Plant Physiol. 1997;114:841–849. doi: 10.1104/pp.114.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen R, Pursiheimo S, Rintamaki E, van Wijk KJ, Aro EM. Transcriptional and translational adjustments of psbA gene expression in mature chloroplasts during photoinhibition and subsequent repair of photosystem II. Eur J Biochem. 1997;247:441–448. doi: 10.1111/j.1432-1033.1997.00441.x. [DOI] [PubMed] [Google Scholar]
- Koivuniemi A, Aro EM, Andersson B. Degradation of the D1- and D2-proteins of photosystem II in higher plants is regulated by reversible phosphorylation. Biochemistry. 1995;34:16022–16029. doi: 10.1021/bi00049a016. [DOI] [PubMed] [Google Scholar]
- Kunst L, Browse J, Somerville C. A mutant of Arabidopsis deficient in desaturation of palmitic acid in leaf lipids. Plant Physiol. 1989;90:943–947. doi: 10.1104/pp.90.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinney G. Absorption of light by chlorophyll solutions. J Biol Chem. 1941;140:315–322. [Google Scholar]
- McConn M, Browse J. The critical requirement for linolenic acid is for pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell. 1996;8:403–416. doi: 10.1105/tpc.8.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M, Browse J. Polyunsaturated membranes are required for photosynthetic competence in a mutant of Arabidopsis. Plant J. 1998;15:521–530. doi: 10.1046/j.1365-313x.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- Miquel M, James D, Dooner H, Browse J. Arabidopsis requires polyunsaturated lipids for low temperature survival. Proc Natl Acad Sci USA. 1993;90:6208–6212. doi: 10.1073/pnas.90.13.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon BY, Higashi S-I, Gombos Z, Murata N. Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. Proc Natl Acad Sci USA. 1995;92:6219–6223. doi: 10.1073/pnas.92.14.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N. Molecular species composition of phosphatidylglycerols from chilling-sensitive and chilling-resistant plants. Plant Cell Physiol. 1983;24:81–86. [Google Scholar]
- Murata N, Ishizaki-Nishizawa O, Higashi S, Hayashi H, Tasaka Y, Nishida I. Genetically engineered alteration in the chilling sensitivity of plants. Nature. 1992;356:313–326. [Google Scholar]
- Murata N, Sato N, Takahashi N, Hamazaki Y. Compositions and positional distributions of fatty acids in phospholipids from leaves of chilling-sensitive and chilling-resistant plants. Plant Cell Physiol. 1982;23:1071–1079. [Google Scholar]
- Murata N, Yamaya J. Temperature-dependent phase behavior of phosphatidylglycerols from chilling-sensitive and chilling-resistant plants. Plant Physiol. 1984;74:1016–1024. doi: 10.1104/pp.74.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida I, Murata N. Chilling sensitivity in plants and cyanobacteria: the crucial contribution of membrane lipids. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:541–568. doi: 10.1146/annurev.arplant.47.1.541. [DOI] [PubMed] [Google Scholar]
- Rintamaki E, Kettunen R, Aro EM. Differential D1 dephosphorylation in functional and photodamaged photosystem II centers: dephosphorylation is a prerequisite for degradation of damaged D1. J Biol Chem. 1996;271:14870–14875. doi: 10.1074/jbc.271.25.14870. [DOI] [PubMed] [Google Scholar]
- Roughan PG. Phosphatidylglycerol and chilling sensitivity in plants. Plant Physiol. 1985;77:740–746. doi: 10.1104/pp.77.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routaboul J-M, Fischer S, Browse J. Trienoic fatty acids are required for photosynthesis at low temperatures. Plant Physiol. 2000;124:1697–1705. doi: 10.1104/pp.124.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AW, Critchley C, Robinson SA, Franklin LA, Seaton GGR, Chow WS, Anderson JM, Osmond CB. Photosystem II regulation and dynamics of the chloroplast D1 protein in Arabidopsis leaves during photosynthesis and photoinhibition. Plant Physiol. 1995;107:943–952. doi: 10.1104/pp.107.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler PA, Murata N. Lipids in Photosynthesis: Structure Function and Genetics. Dordrecht, The Netherlands: Kluwer Academic Press; 1998. [Google Scholar]
- Sippola K, Kanervo E, Murata N, Aro EM. A genetically engineered increase in fatty acid unsaturation in Synechococcus sp. PCC 7942 allows exchange of D1 protein forms and sustenance of photosystem II activity at low temperature. Eur J Biochem. 1998;251:641–648. doi: 10.1046/j.1432-1327.1998.2510641.x. [DOI] [PubMed] [Google Scholar]
- Somerville C. Direct tests of the role of membrane lipid composition in low-temperature-induced photoinhibition and chilling sensitivity in plants and cyanobacteria. Proc Natl Acad Sci USA. 1995;92:6215–6218. doi: 10.1073/pnas.92.14.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaka Y, Gombos Z, Nishiyama Y, Mohnaty P, Ohba T, Ohki K, Murata N. Targeted mutagenesis of acyl-lipid desaturases in Synechocystis: evidence for the important roles of polyunsaturated membrane lipids in growth, respiration and photosynthesis. EMBO J. 1996;15:6416–6425. [PMC free article] [PubMed] [Google Scholar]
- Tyystjärvi E, Aro E-M. The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci USA. 1996;93:2213–2218. doi: 10.1073/pnas.93.5.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk KJ, Roobol-Boza M, Kettunen R, Andersson B, Aro EM. Synthesis and assembly of the D1 protein into photosystem II: processing of the C-terminus and identification of the initial assembly partners and complexes during photosystem II repair. Biochemistry. 1997;36:6178–6186. doi: 10.1021/bi962921l. [DOI] [PubMed] [Google Scholar]
- Vijayan P, Routaboul J-M, Browse J. A trienoic fatty acid deficient mutant of Arabidopsis defective in recovery from photoinhibition at low temperatures. In: Williams JP, Khan MU, Lem NW, editors. Physiology, Biochemistry and Molecular Biology of Plant Lipids. Dordrecht, The Netherlands: Kluwer Academic Press; 1997. pp. 203–205. [Google Scholar]
- Wolter FP, Schmidt R, Heinz E. Chilling sensitivity of Arabidopsis thaliana with genetically engineering membrane lipids. EMBO J. 1992;11:4685–4692. doi: 10.1002/j.1460-2075.1992.tb05573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Browse J. Elevated levels of high-melting-point phosphatidylglycerols do not induce chilling sensitivity in a mutant of Arabidopsis. Plant Cell. 1995;7:17–27. doi: 10.1105/tpc.7.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, James DW, Jr, Dooner HK, Browse J. A mutant of Arabidopsis deficient in the elongation of palmitic acid. Plant Physiol. 1994;106:143–150. doi: 10.1104/pp.106.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lightner J, Warwick N, Browse J. Low-temperature damage and subsequent recovery of fab1 mutant Arabidopsis exposed to 2°C. Plant Physiol. 1997;113:347–356. doi: 10.1104/pp.113.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunschmann G, Brand JJ. Rapid turnover of a component required for photosynthesis explains temperature-dependence and kinetics of photoinhibition in a cyanobacterium, Synechococcus-6301. Planta. 1992;186:426–433. doi: 10.1007/BF00195324. [DOI] [PubMed] [Google Scholar]
- Zhang L, Paakkarinen V, van Wijk KJ, Aro EM. Co-translational assembly of the D1 protein into photosystem II. J Biol Chem. 1999;274:16062–16067. doi: 10.1074/jbc.274.23.16062. [DOI] [PubMed] [Google Scholar]
- Zhang L, Paakarinen V, van Wijk KJ, Aro EM. Biogenesis of the chloroplast-encoded D1 protein: regulation of translation elongation, insertion, and assembly into photosystem II. Plant Cell. 2000;12:1769–1782. doi: 10.1105/tpc.12.9.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]