Abstract
We have employed a novel capillary electrophoresis (CE) approach recently developed in our laboratory, termed ion-interaction-capillary zone electrophoresis (II-CZE), to the resolution of a mixture of 27 synthetic cationic proteomic peptide standards. These peptides were comprised of three groups of nine peptides (with net charges of +1, +2 and +3 for all nine peptides within a group), the hydrophobicity of the nine peptides within a group varying only subtly between adjacent peptides. This bidimensional CE approach achieved excellent resolution of the peptides with high peak capacity by combining the powerful CZE mechanism located in the background electrolyte (BGE) with an hydrophobicity-based mechanism also located in the BGE, the latter consisting of high concentrations (up to 0.4 M) of aqueous perfluorinated acids ( trifluoroacetic acid, pentafluoropropionic acid and heptafluorobutyric acid). Thus, concomitant with a CZE separation of the three differently charged groups of peptides, there is an hydrophobically-mediated separation of the peptides within these groups effected through interaction of the hydrophobic anions of the perfluorinated acids with hydrophobic amino acid side-chains in the peptides. This methodology is dramatically different from other CE methods that have used complexing agents such as micelles or cyclodextrins in MEKC. Overall, the results presented here demonstrate the value of CE as a peptide separative tool in its own right, including its use for proteomic applications, and not merely as a complementary technique to reversed-phase high-performance liquid chromatography (RP-HPLC).
1. Introduction
High peak capacity remains a vital requirement for peptide separations in proteomic applications and is generally accomplished by an instrumental approach consisting of a combination of two (or more) instruments each with their own separative devices (e.g., a chromatography column or a gel). The criterion that such a combination must satisfy is that each separative mechanism introduced by a separative device must be based on a distinct peptide property, e.g., net charge, hydrophobicity or isoelectric point. For example, if charge and hydrophobicity are the two selected peptide properties, then two instruments, e.g., a high-performance liquid chromatography (HPLC) instrument and a capillary electrophoresis (CE) instrument may be combined in-line [1, 2] or out-of-line [3, 4] to perform a bi-dimensional separation. Each instrument is used independently, one for optimization of the separation in terms of peptide hydrophobicity (i.e., separation by reversed-phase HPLC (RP-HPLC) and the other for optimization of the separation in terms of peptide charge [i.e., capillary zone electrophoresis (CZE)]. Such an approach may be viewed either as the best way to exploit the optimum performance of each instrument/technology, or as a limitation due to the fact that both separation mechanisms cannot be run on a single instrument.
Another separation approach is to exploit the aforementioned two peptide properties by two separation devices but using just a single instrument. For example, an ion-exchange column can be combined with a reversed-phase column in a single HPLC instrument to allow a bidimensional, chromatographic separation [5-11]. Thus, a charge-based mechanism (i.e., the ion-exchange mechanism) is implemented in the first dimension and an hydrophobicity-based mechanism (i.e., an hydrophobic interaction mechanism) is used in the second dimension. Another example of such a bidimensional approach is that of two-dimensional (2D) gel electrophoresis, a common approach for proteomic applications [10, 11-13], which combines an isoelectric focusing mechanism (based on protein isoelectric point) with a size-based mechanism [using-SDS (sodium dodecyl sulphate)-gel electrophoresis]. From the examples outlined above, it is apparent that the design of a multi-dimensional separation (characterized by high peak capacity) has several steps or levels of optimization, the first being the selection of analyte properties (e.g., charge and hydrophobicity) that are going to be exploited for separation purposes. Each property may generate more than one separation mechanism. For example, if the selected peptide property is charge, two separation mechanisms utilizing charge could be ion-exchange chromatography or capillary zone electrophoresis. The second step consists of the selection of separation mechanisms to be combined. The way in which such selected mechanisms are combined is dictated by two restrictions: incompatible mechanisms and insufficient selectivity differences provided by each mechanism. Because of these two restrictions, the selected mechanisms must be run individually, one at a time and perhaps in two different physical directions. The third step consists of optimization in terms of physical/chemical parameters controlling each separation mechanism. Although the term “multi-dimensional separation” implies the combination of independent separation mechanisms, there is no fundamental reason that these separation mechanisms need to be independent.
The above short analysis of multi-dimensional separations suggests that a completely different approach is possible if the two restrictions can be lifted. Thus, two compatible separation mechanisms, each based on a different peptide property (e.g., charge and hydrophobicity), that differ greatly in selectivity could be run simultaneously in the same physical direction to provide a peptide separation equivalent to a bi-dimensional separation, a term thus retained for the approach described in the present study. Such a strategy, based on combinations of compatible separation mechanisms, may be termed a mechanistic approach. This approach involves a single instrument with a single separation device and is particularly suitable for capillary electrophoresis, examples of which are open tubular capillary electrochromatography (OT-CEC) and micellar electrokinetic chromatography (MEKC). In both these CE methods (also called modes), two peptide properties (charge and hydrophobicity) are involved. In both methods, the charge-based mechanism (the CZE mechanism, based on peptide charge-to-mass ratio) is located in the background electrolyte (BGE) and is recognized as a very powerful separative mechanism. The hydrophobicity-based mechanism is located at an interface between the BGE and the hydrophobic capillary wall surface in OT-CEC and between the BGE and micelle surface in MEKC in an analogous manner to the chromatographic hydrophobic mechanism located at the interface of the aqueous mobile phase and chemical bonded phase of RP-HPLC. It should be stressed that, when employing micelles or cyclodextrins in MEKC [14], while this approach does involve peptide and micelle or cyclodextrin interactions within the BGE, such interactions are specifically with the surface of the micelles or cyclodextrins and not with the bulk ions of the BGE. In each case, the selected combination of a charge-based mechanism and an hydrophobicity-based mechanism proved to be compatible. However, a weakness of even these effective CE methods lies in the difficulty to optimize fully an essentially chromatographic mechanism under the CE conditions. General opinion appears to point to the necessity of improving the chromatographic hydrophobic interaction mechanism, and great effort has been, and still is, dedicated to this goal. A weak point of this mechanism originates in the unfavourable surface-to-volume ratio. To improve this ratio, capillary wall area has been increased by a factor of 1000 by etching it with ammonium hydrogen difluoride [15]. Alternatively, a functionalized, porous polymer layer was attached to the capillary wall (polymer layer open-tubular (PLOT) column) [16]. A more radical approach of increasing surface to volume ratio is represented by monolithic column technology [17]. These achievements represent a continuation of the chromatographic tradition. Another approach would be to diverge from this tradition by replacing the chromatographic hydrophobic interaction mechanism located at the interface with an hydrophobicity-based mechanism located in the BGE. This view is part of a larger tendency of moving away from previous technologies. For example, in DNA analysis, the chemical gel from gel electrophoresis was replaced by physical gel (polymer solution), then by dilute polymer solution, and more recently by simple BGE [18].
The aim of the present study is to increase the peak capacity of peptide separations in proteomic applications by designing an equivalent CE bi-dimensional separation based on peptide charge and hydrophobicity and applying it to the resolution of 27 synthetic proteomic peptide standards. Optimization follows the mechanistic approach and consists of combining the powerful CZE mechanism located in the BGE with a hydrophobicity-based mechanism also located in the BGE. The existence of such an hydrophobicity-based mechanism in the BGE was suggested by our earlier CE separation of diastereomeric peptide pairs [19].
2. Experimental
2.1. Materials
Trifluoroacetic acid (TFA), pentafluoropropionic acid (PFPA), heptafluorobutyric acid (HFBA), phosphoric acid and lithium hydroxide were purchased from Sigma-Aldrich. HPLC-grade acetonitrile was obtained from EM Science (Gibbstown, NJ, USA).
2.2. Solutions
The background electrolytes (BGE) were prepared from various acids adjusted to pH 2.0 with lithium hydroxide.
2.3. Peptide standards
The proteomic peptide standards were synthesized by standard solid-phase synthesis methodology, purified and characterized as described previously [20].
2.4. Instrumentation and columns
Analytical RP-HPLC runs were carried out on an Agilent 1100 Liquid Chromatograph System (Agilent Technologies, Little Falls Site, DE, USA) using a Zorbax 300SB-C8column (150 mm x 2.1 mm I.D.; 3.5 μm particle size, 300 Å pore size) from Agilent Technologies.
All CE runs were carried out on a Beckman-Coulter P/ACE MDQ Capillary Electrophoresis System (Fullerton, CA, USA) controlled by 32 Karat software (Version 5.0). Uncoated capillaries, 60.2 cm (50 cm) x 50 μm I.D., were provided by Beckman-Coulter. In all cases, capillaries were thermostated at 15°C and used with the shorter aperture (100 x 200 μm). Peptides were detected at 195 nm by UV absorption with photodiode array (DAD). It should be noted that the high currents occasionally generated with the high concentrations of ion-pairing reagents employed in this study (e.g., up to 200 μA with 0.4 M TFA) was well within optimum performance parameters of the Beckman-Coulter instrument.
3. Results and Discussion
3.1. Synthetic peptide standards
The sequences of the synthetic peptide standards together with pertinent properties of mass, charge and relative hydrophobicity are shown in Table 1. From Table 1, the 27 decapeptides making up the peptide mixture possess only two distinct properties with which they may be resolved, i.e., charge and hydrophobicity. The 10-residue length of the peptides was designed to reflect the average size of cleavage fragments from proteolytic digests of proteins. The presence of only basic amino acid residues (lysine), i.e., no acidic, potentially negatively charged residues, and the blocked C-terminus for all peptides makes their effective mobility independent of BGE pH. Also, any potential for acido-basic equilibria was eliminated by working at low pH (pH 2), far below the pKa values of the N-terminal amino group and the lysine side-chain amino group. The absence of negatively charged centres in the peptides also excludes the possibility of conformational differences generated by intrachain electrostatic interactions and a separation mechanism based on such differences. Finally, the random coil nature of the peptides is ensured by the inclusion of helix-disrupting glycine residues [21, 22] throughout the peptide sequences. Thus, as noted above, the sole properties by which a successful separation of the peptide standards may be achieved are differences in positive charge and hydrophobicity.
Table 1.
Characteristics of synthetic peptide standards used in this study1
| Peptide2 | Peptide sequence | Mass | Charge | Hydrophobicity3 at pH 2 (min) |
|---|---|---|---|---|
| 3a | GGGG KLGLGK-amide | 843 | +3 | 23.3 |
| 3b | GGAG KLGLGK-amide | 857 | +3 | 25.6 |
| 3c | GGAA KLGLGK-amide | 871 | +3 | 27.4 |
| 3d | GGVG KLGLGK-amide | 885 | +3 | 31.3 |
| 3e | GGVA KLGLGK-amide | 899 | +3 | 32.6 |
| 3f | GGIG KLGLGK-amide | 899 | +3 | 35.5 |
| 3g | GGIA KLGLGK-amide | 813 | +3 | 36.5 |
| 3h | GGIV KLGLGK-amide | 941 | +3 | 40.7 |
| 3i | GGII KLGLGK-amide | 955 | +3 | 44.6 |
| 2a | GGGG GLGLGK-amide | 772 | +2 | 23.3 |
| 2b | GGAG GLGLGK-amide | 786 | +2 | 25.8 |
| 2c | GGAA GLGLGK-amide | 800 | +2 | 27.7 |
| 2d | GGVG GLGLGK-amide | 814 | +2 | 32.4 |
| 2e | GGVA GLGLGK-amide | 828 | +2 | 34.5 |
| 2f | GGIG GLGLGK-amide | 828 | +2 | 36.8 |
| 2g | GGIA GLGLGK-amide | 842 | +2 | 38.9 |
| 2h | GGIV GLGLGK-amide | 870 | +2 | 42.2 |
| 2i | GGII GLGLGK-amide | 884 | +2 | 45.7 |
| 1a | (Ac)-GGGG GLGLGK-amide | 814 | +1 | 26.7 |
| 1b | (Ac)-GGAG GLGLGK-amide | 828 | +1 | 29.4 |
| 1c | (Ac)-GGAA GLGLGK-amide | 842 | +1 | 31.6 |
| 1d | (Ac)-GGVG GLGLGK-amide | 856 | +1 | 35.5 |
| 1e | (Ac)-GGVA GLGLGK-amide | 870 | +1 | 38.2 |
| 1f | (Ac)-GGIG GLGLGK-amide | 870 | +1 | 39.9 |
| 1g | (Ac)-GGIA GLGLGK-amide | 884 | +1 | 42.7 |
| 1h | (Ac)-GGIV GLGLGK-amide | 912 | +1 | 46.9 |
| 1i | (Ac)-GGII GLGLGK-amide | 926 | +1 | 50.7 |
The peptide order in the table is the same as the CE peptide migration order in 0.4 M HFBA (pH 2), with the 3a peptide (2a, 1a) migrating first and the 3i peptide (2i, 1i) migrating last. The substitutions relative to the -GG- sequence are printed in bold letters. Ac denotes Nα-acetyl; amide = Cα-amide.
The number in the peptide denotations refers to nominal positive charge; within each group of identical charged peptides, the letters “a”, “b”, “c”, “d”, “e”, “f”, “g”, “h” and “i” represent peptides with increasing hydrophobicity relative to the -GG- sequence at positions 3 and 4. The e/f pairs produced by VA/IG substitutions have the same charge-to-mass ratio.
Relative hydrophobicity is based on reversed-phase retention time under conditions shown in Fig. 2 for a mobile phase containing 20 mM TFA.
From Table 1, the peptides are divided into three groups of nine peptides with nominal charges of +1 (peptides 1a-1i), +2 (peptides 2a-2i) and +3 (peptides 3a-3i). Within each peptide group, there is only a subtle hydrophobicity variation between adjacent peptides-generally just a single CH2 group change from peptide to peptide-the only exceptions being the 1e/1f, 2e/2f and 3e/3f adjacent peptide pairs which have the same mass and charge. These three peptide pairs of identical charge-to-mass ratio are included in the peptide mixture, not only as a profound separation challenge to CE (their CZE separation is considered impossible by classical physical chemistry) but also to confirm that the CE separation of peptides within each group is due to differences in hydrophobicity and not to the slight variation in charge-to-mass ratio introduced by amino acid substitutions differing by a single CH2 group.
3.2. RP-HPLC of peptides
Traditionally, the high-performance chromatographic mode favoured for peptide separations has been RP-HPLC [23-25] both for unidimensional separations and as the second step of a multidimensional HPLC approach to resolution of complex peptide mixtures [5-11]. Further, the most widely used anionic ion-pairing reagents employed for RP-HPLC peptide separations remain perfluorinated carboxylic acids (trifluoroacetic acid, TFA; pentafluoropropionic acid, PFPA; and heptafluorobutyric acid, HFBA) [23, 25-31], particularly TFA [23-25]. Indeed, standard approaches to peptide separations are still dominated by employment of aq. TFA to TFA/acetonitrile gradients at pH 2.0 [23-25]. Such anionic counterions as TFA-, PFPA- and HFBA- will, of course, interact with the protonated basic groups of a peptide, i.e., lysine, arginine or histidine side-chains or a free N-terminal α-amino group.
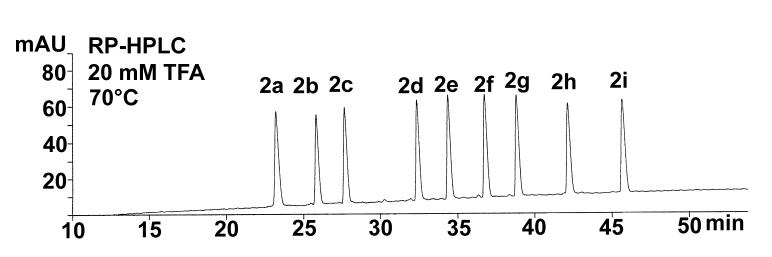
Fig. 1 presents the RP-HPLC separation of the +2 group of peptides shown in Table 1, employing a linear acetonitrile gradient (0.5%/min) in the presence of 20 mM TFA at 70°C. Clearly, an excellent separation of these nine peptide standards was achieved, based on peptide hydrophobicity, in this unidimensional separation. Similar separations were also obtained when running the +1 and +3 peptides as single groups.
Fig. 1.
RP-HPLC separation of representative group (+2) of peptide standards. Conditions: Agilent SB300-C8 column (150 x 2.1 mm I.D.; 3.5 μm particle size 300 Å pore size); linear AB gradient (0.5% acetonitrile/min) at a flow-rate of 0.3 ml/min, where Eluent A is 20 mM aq. TFA and Eluent B is 20 mM TFA in 80% aq. acetonitrile; temperature, 70°C. The sequences of the peptides are shown in Table 1.
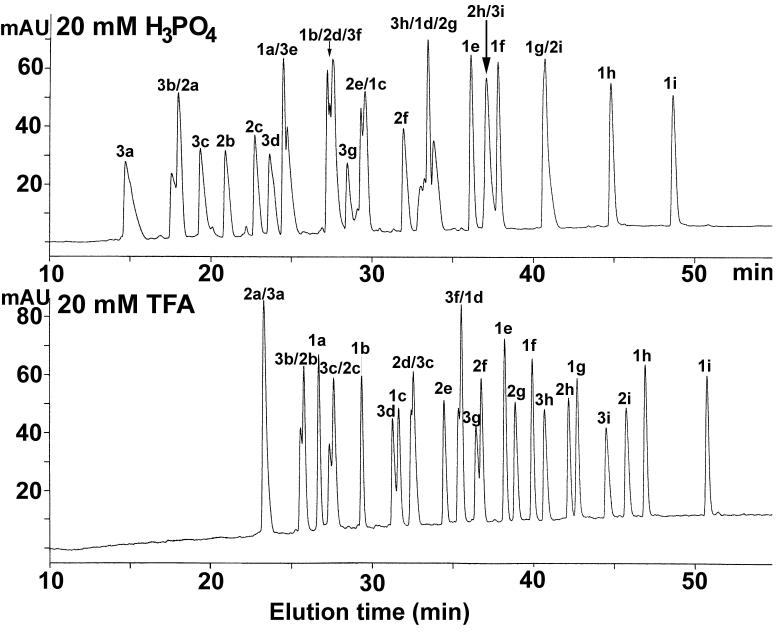
Note that the relative hydrophobicities of the peptides reported in Table 1 are the observed retention times of the peptides obtained under the conditions shown in Fig. 1. From Fig. 2 (bottom panel), when all three peptide groups are run in RP-HPLC as a single mixture, the elution windows of the three groups overlap, resulting in co-elution (peptides 2a/3a) or poor/incomplete resolution (3b/2b, 3c/2c, 3d/1c, 2d/3c, 3f/1d, 3g/2f) of several peptides. In an attempt to improve peptide resolution, a more hydrophilic anionic ion-pairing reagent (H3PO4) and more hydrophobic anionic ion-pairing reagents (PFPA, HFBA) were employed under the same linear gradient conditions. Neither PFPA nor HFBA improved the overall resolution of the peptide mixture (data not shown); indeed, the overlap of the three peptide groups was enhanced with a resulting decrease in overall peak capacity. In contrast, the 20 mM H3PO4 system (Fig. 2, top panel) resulted in a similar, although not improved, peptide separation to that of the 20 mM TFA system (Fig. 2, bottom panel) albeit with selectivity variations-peptides 2h/3i and 1g/2i are now co-eluted whilst 3b/2a, 1a/3e, 1b/2d/3f, 2e/1c and 3h/1d/2g show poor or incomplete resolution. It should be noted that the conditions employed for the separations shown in Figs. 1 and 2 were designed to maximize the effectiveness of RP-HPLC to resolve a complex peptide mixture, i.e., not only through the use of efficient ion-pairing reagents such as H3PO4 and TFA, but also through the use of a high temperature (known generally to improve resolution of adjacent analytes [32-35]), a small particle size (3.5 μm) reversed-phase packing [23,24] and a relatively shallow acetonitrile gradient of 0.5%/min. Although the separations achieved in Fig. 2 are reasonably satisfactory from an analytical point of view (11 and 13 peptides essentially resolved to baseline out of a total of 27 peptides in the H3PO4 and TFA systems, respectively), it is unlikely that this unidimensional approach to the separation of the peptides can be optimized further to achieve complete (or even near complete) resolution of all 27 peptides.
Fig. 2.
RP-HPLC separation of peptide standards. Conditions: column same as Fig. 1; linear AB gradient (0.5% acetonitrile/min) at a flow-rate of 0.3 ml/min, where Eluent A is 20 mM aq. H3PO4 or TFA and Eluent B is 20 mM H3PO4 or TFA, respectively, in 80% aq. acetonitrile; temperature, 70°C. The sequences of the peptides are shown in Table 1.
3.3 CE of peptides
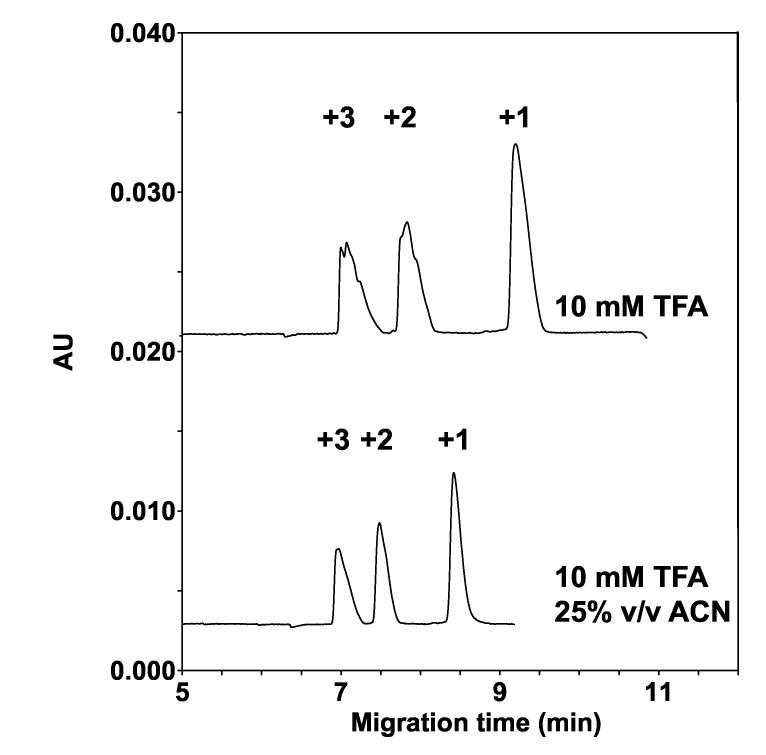
Fig. 3 shows the separation of the 27 peptides by CE in analogous conditions to that of RP-HPLC (i.e., with aq. TFA as the BGE in the absence or presence of acetonitrile), albeit with the absence of an hydrophobic surface (uncoated capillary). Optimum resolution of peptides in RP-HPLC is generally achieved over a range of 15%-40% acetonitrile during a linear gradient [36] and 25% acetonitrile is a compromise between these solvent extremes and was used for CE in Fig. 3 (lower panel). From Fig. 3, both in the absence (top panel) and presence (bottom panel) of 25% acetonitrile, the peptides are separated according to their charge-to-mass ratio (CZE mechanism), each peak representing nine co-migrated peptides. The migration order of the peptide groups is as expected, i.e., the first, second and third peaks correspond to the +3, +2 and +1 peptide groups (Table 1). This is an example of a unidimensional CE separation, where peptide separation is achieved/optimized via a single peptide property (charge) and via a single charge-based mechanism (CZE), with no resolution based on peptide hydrophobicity.
Fig. 3.
Effect of TFA and acetonitrile on CE separation of peptide standards. Conditions: capillary, uncoated 60.2 cm (50 cm) x 50 μm I.D.; background electrolyte (BGE), 10 mM aq. TFA, adjusted to pH 2.0 with lithium hydroxide, with or without 25% (v/v) acetonitrile; applied voltage, 25 kV (direct polarity) with 5-min voltage ramp; temperature, 15°C; UV absorption at 195 nm. The sequences of the peptides are shown in Table 1.
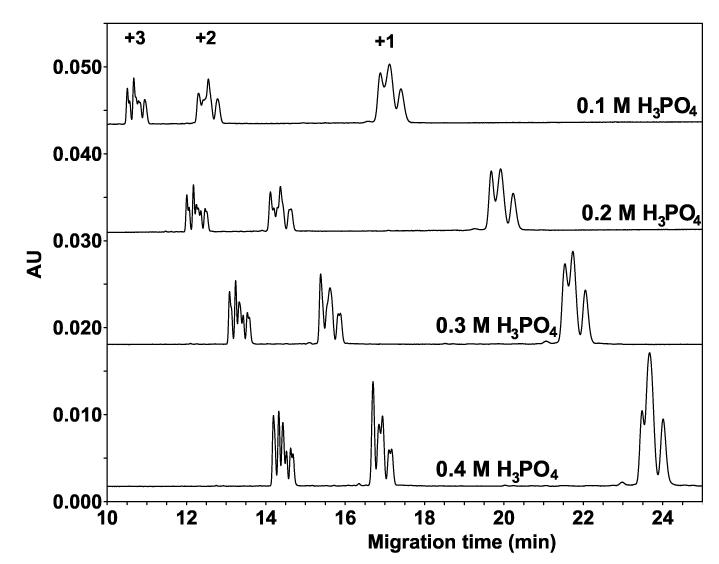
In a previous paper, we reported the existence of an hydrophobicity-based separation mechanism in phosphate buffer at high concentration (100 mM NaH2PO4) [19]. Figure 4 now shows that, in phosphate buffer at high concentrations, the CZE mechanism is again present (and, indeed, dominant, i.e., the three groups of peptides are well separated based on charge-to-mass ratio) but in addition, and in spite of the absence of an hydrophobic surface, a clear partial separation according to peptide hydrophobicity is now observed within each peptide group. In addition, this separation improves as the concentration of the ion-pairing reagent increases from 0.1 M to 0.4 M H3PO4, with concomitant increasing peptide migration times. These results suggest that the lack of peptide separation within the three groups of peptides in Fig. 3 may have been due to the low acid concentration (just 10 mM TFA).
Fig. 4.
Effect of hydrophilic anionic ion-pairing reagent (H3PO4) concentration on CE separation of peptide standards. Conditions: capillary, uncoated 60.2 cm (50 cm) x 50 μm I.D.; BGE, 0.1 M, 0.2 M, 0.3 M, or 0.4 M aq. H3PO4, adjusted to pH 2.0 with lithium hydroxide. Other conditions as in Fig. 3. The sequences of the peptides are shown in Table 1.
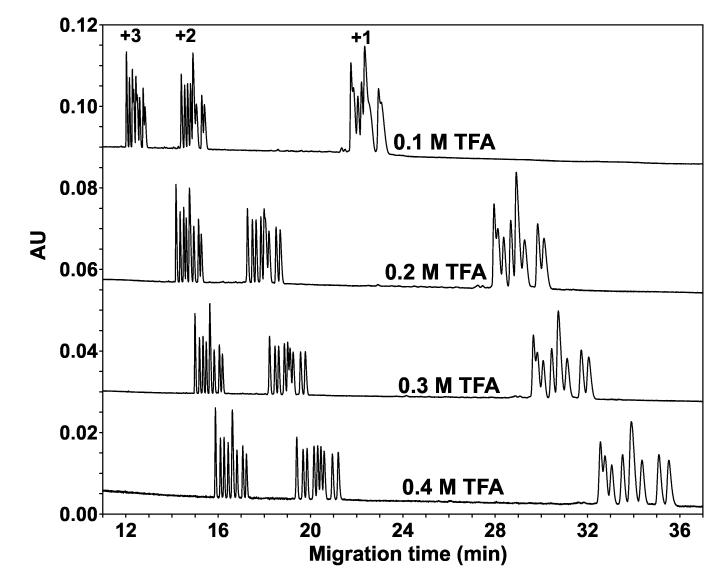
Fig. 5 now illustrates the effect of increasing TFA concentration (0.1 M TFA-0.4 M TFA) on the CE separation of the peptides. Compared to Fig. 3 (10 mM TFA), increasing the TFA concentration in the BGE resulted in an excellent separation within each peptide group based on peptide hydrophobicity, with the most hydrophilic peptide in each group (1a, 2a, 3a; Table 1) migrating earliest and the most hydrophobic peptide (1i, 2i, 3i; Table 1) migrating last. In addition, as seen in Fig. 4, the migration times of the peptides are increasing with increasing acid concentration. Among the three groups, the separation within the +1 group is the most difficult to achieve. Comparison with Fig. 4 suggests that for the hydrophobicity-based separation within each group, the nature of the anionic ion-pairing reagent (H3PO4 versus TFA) is even more important than its concentration.
Fig. 5.
Effect of hydrophobic anionic ion-pairing reagent (TFA) concentration on CE separation of peptide standards. Conditions: capillary, uncoated 60.2 cm (50 cm) x 50 μm I.D.; BGE, 0.1 M, 0.2 M, 0.3 M, or 0.4 M aq. TFA, adjusted to pH 2.0 with lithium hydroxide. Other conditions as in Fig. 3. The sequences of the peptides are shown in Table 1.
It is hypothesized that within each group of peptides, the hydrophobicity of the anionic ion-pairing reagent is responsible for the huge differences in observed separations, i.e., compare the much improved separation in Fig. 5 with the hydrophobic TFA reagent over that of Fig. 4 with the hydrophilic H3PO4 reagent. Such an hypothesis is validated in Fig. 6 where increasing hydrophobicity of the ion-pairing reagent (TFA < PFPA < HFBA) resulted in an improvement in resolution of all 27 peptides. Thus, at 0.4 M HFBA, all but one peptide pair are separated. Of the three pairs having the same charge-to-mass ratio (3e/3f, 2e/2f and 1e/1f), only the pair with the lowest charge (1e/1f) remains completely unresolved. Note that, in an analogous manner to the effect of acid concentration (Figs. 4 and 5), migration times of the peptides increase with increasing hydrophobicity of the acidic counterion (TFA- < PFPA- < HFBA-).The separations at these high levels of perfluorinated acid concentrations represent bidimensional separations: (i) peptides of the same length but of different nominal charge are separated in order of decreasing charge (i.e., +3 peptides migrate the earliest and +1 peptides migrate the last) according to a CZE mechanism; (ii) within each group of peptides, the peptides are separated in order of increasing hydrophobicity according to an hydrophobically-mediated mechanism introduced by the anionic ion-pairing reagent. The method which achieves this bidimensional separation is referred to as Ion-Interaction-Capillary Zone Electrophoresis (II-CZE) [30,37,38]. Clearly, for this mixture of 27 peptide standards, RP-HPLC (based solely/mainly on overall peptide hydrophobicity) in the presence of 20 mM H3PO4 or 20 mM TFA (typical RP-HPLC conditions for peptide separations) (Fig. 2) could not match the overall peak capacity achieved by CE (specifically, II-CZE) in the presence of 0.4 M TFA, PFPA or HFBA (Fig. 6), with separations based on both peptide charge and hydrophobicity. As shown in Fig. 6, we are suggesting that the gap between the +2 and +3 groups of peptides ( 20 min) could be occupied by +2 peptides of greater hydrophobicity and/or lower charge-to-mass ratio in comparison to peptides used in the present study (note that our synthetic peptide standards have a very narrow charge-to-mass ratio, since they are all ten residues in length; Table 1). For example, tryptic digests of native proteins produce, in general, +2 peptides with a wide range of peptide hydrophobicity and chain length ( typically 6-30 residues) which, in turn, will be characterized by an aforementioned wide range of charge-to-mass ratios. Concomitantly, the peak capacity of the present CE approach will be much higher than that of RP-HPLC.
Fig. 6.

Effect of increasing hydrophobicity of hydrophobic anionic ion-pairing reagents (TFA < PFPA < HFBA) on CE separation of peptide standards. Conditions: capillary, uncoated 60.2 cm (50 cm) x 50 μm I.D.; BGE, 0.4 M aq. TFA, PFPA or HFBA, adjusted to pH 2.0 with lithium hydroxide. Other conditions as in Fig. 3. The sequences of the peptides are shown in Table 1. The peptide peak denoted 1e/1f represents the co-migrated +1 peptide pair with identical charge-to-mass ratio.
3.3.1. Speculations concerning the separation mechanism
The term “ion-interaction CZE” implies that the ion-pairing property of the acids with the positively charged peptide groups is a critical factor in the successful peptide separations by this method. Such an ion-pairing effect in RP-HPLC has, of course, been well documented [23-31]. However, it is perhaps difficult to explain how the excellent separations within each charged peptide group, in order of increasing peptide hydrophobicity with increasing migration time (Figs. 5 and 6), would be based only on ion-pairing of the ion-pairing reagent to the positively charged peptide side-chains since the substitution sites where all nine peptides differ in hydrophobicity are five to six residues away from the Lys side-chain in the +1 charged group (Table 1). A likely explanation for this hydrophobicity-based separation is interaction of the peptides with the bulk acid in the BGE [37], specifically through interaction of the hydrophobic side-chains in the peptides with the hydrophobic moiety of the anionic ion-pairing regents.
It is well documented that the nonspecific interaction of hydrophobic groups in an aqueous environment is entropically favourable, with the layer of water surrounding a dissolved hydrophobic group being less mobile than bulk water [39]. Indeed, hydrophobic interactions are among the strongest interactions among amino acids in water due to a drive toward desolvation of non-polar groups [40]. Further, it is also well known that anions can have a dramatic effect on the structure of water surrounding the polypeptide chain where such a solvent additive may result in preferential binding of this additive to the polypeptide or preferential hydration of the polypeptide [41,42].
From above, not only can anions affect the degree of hydration about a peptide or protein molecule but it is also well understood that hydrophobic side-chains are more easily desolvated than polar or charged side-chains. Differential desolvation of the hydrophobic side-chains, on interaction of such side-chains with the hydrophobic group on the perfluorinated acid, then becomes a critical aspect of our proposed separation mechanism. It would be easier to desolvate a considerably more hydrophobic side-chain such as that of isoleucine, for example, with concomitant subsequent interaction of this side-chain with the acid hydrophobic moiety, than a moderately hydrophobic side-chain such as that of alanine. Thus, the ratio of anions in close contact to the peptide increases with increasing hydrophobicity of the substituting side-chains and this equilibrium of peptide-associated anions and anions in the BGE could affect the overall positive charge on the peptide. Taking this one step further, it would then be expected that the more hydrophobic a peptide within a peptide group (+1, +2, +3; Table 1), the greater would be its migration time, which was indeed observed in our results (Figs. 5 and 6). Interestingly, this implies that a CZE mechanism not only separated the three peptide groups but also separated the peptides within these peptide groups, albeit by a charged and hydrophobically-mediated peptide-anion interaction, hence “ion-interaction CZE” as our term of choice. Also, it would be expected that the magnitude of peptide interactions based on hydrophobicity with the BGE would be greater the more concentrated or more hydrophobic (TFA < PFPA < HFBA) the anionic ion-pairing reagent, translating into longer migration times as were clearly observed in Fig. 5 (effect of TFA concentration) and Fig. 6 (effect of ion-pairing reagent hydrophobicity).
4. Conclusions
A novel bi-dimensional peptide separation characterized by high peak capacity has been designed following a mechanistic approach, and tested with a mixture of 27 cationic random coil peptide standards. Selected peptide properties to achieve this separation were charge and hydrophobicity, and the corresponding separation mechanisms were the CZE mechanism and a novel hydrophobicity-based mechanism referred to as ion-interaction (II). The method resulting from the combination of these two mechanisms is referred to as II-CZE. The ion-interaction mechanism is located in the BGE (like the CZE mechanism) and is based on the (cationic) peptide interaction with the counterions provided by the BGE. The optimization is accomplished easily in terms of counterion properties (e.g., hydrophobicity) and concentration using commercially available CE instruments.
In comparison with other CE methods, the II-CZE method represents a break from the chromatographic tradition of CE: the (chromatographic) hydrophobic interaction mechanism located at an interface (e.g., BGE-capillary wall in OT-CEC or BGE-micelle in MEKC) is replaced by an hydrophobicity-based mechanism (e.g., II) located within the BGE. This has major practical and theoretical implications: from a practical point of view, high peak capacity separations are demonstrated to be feasible for proteomics-related applications independent of the chromatographic mechanism. Our approach opens the field of systematic investigations of the specificity of the interaction of the ionic analyte (here a cationic peptide) with the counterion provided by the BGE.
Acknowledgement
This work was supported by an NIH grant (RO1GM61855) to R.S.H.
References
- [1].Moore AB, Jorgenson JW. Anal. Chem. 1995;67:3448. doi: 10.1021/ac00115a013. [DOI] [PubMed] [Google Scholar]
- [2].Moore AB, Jorgenson JW. Anal. Chem. 1995;67:3456. doi: 10.1021/ac00115a014. [DOI] [PubMed] [Google Scholar]
- [3].Issaq HJ, Khan KC, Janini GM, Muschik GM. Electrophoresis. 1999;20:1533. doi: 10.1002/(SICI)1522-2683(19990601)20:7<1533::AID-ELPS1533>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- [4].Issaq HJ, Khan KC, Liu C-S, Li Q. Electrophoresis. 2001;22:1133. doi: 10.1002/1522-2683()22:6<1133::AID-ELPS1133>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- [5].Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR, Garvik BM, Yates JR., III Nat. Biotechnol. 1999;17:676. doi: 10.1038/10890. [DOI] [PubMed] [Google Scholar]
- [6].Wagner K, Racaityte K, Unger KK, Miliotis T, Edholm LE, Bischoff R, Marko-Varga G. J. Chromatogr. A. 2000;893:293. doi: 10.1016/s0021-9673(00)00736-6. [DOI] [PubMed] [Google Scholar]
- [7].Regnier F, Amini A, Chakraborty A, Geng M, Ji J, Riggs L, Sioma C, Wang S, Zhang X. LC.GC. 2001;19:200. [Google Scholar]
- [8].Washburn MP, Wolters D, Yates JR., III Nat. Biotechnol. 2001;19:242. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- [9].Wagner K, Miliotis T, Marko-Varga G, Bischoff R, Unger KK. Anal. Chem. 2002;74:809. doi: 10.1021/ac010627f. [DOI] [PubMed] [Google Scholar]
- [10].Issaq H, Conrads TP, Janini GM, Veenstra TD. Electrophoresis. 2002;23:3048. doi: 10.1002/1522-2683(200209)23:17<3048::AID-ELPS3048>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- [11].Wang H, Hanash S. J. Chromatogr. B. 2003;787:11. doi: 10.1016/s1570-0232(02)00335-5. [DOI] [PubMed] [Google Scholar]
- [12].Hanash SM, Bobek MP, Rickman DS, Williams T, Rouillard JM, Kuick R, Puravs E. Proteomics. 2002;2:69. [PubMed] [Google Scholar]
- [13].Liao X, Ying T, Wang H, Wang J, Shi Z, Feng E, Wei K, Wang Y, Zhang X, Huang L, Su G, Huang P. Electrophoresis. 2003;24:2864. doi: 10.1002/elps.200305519. [DOI] [PubMed] [Google Scholar]
- [14].Liu J, Cobb KA, Novotny M. J. Chromatogr. 1990;519:189. doi: 10.1016/0021-9673(90)85147-n. [DOI] [PubMed] [Google Scholar]
- [15].Pesek JJ, Matyska MT. J. Sep. Sci. 2004;27:1285. doi: 10.1002/jssc.200401907. [DOI] [PubMed] [Google Scholar]
- [16].Svec F. J. Sep. Sci. 2004;27:1255. doi: 10.1002/jssc.200401906. [DOI] [PubMed] [Google Scholar]
- [17].Svec F, Tennikova TB, Deyl Z, editors. Monolithic Materials: Preparation, Properties and Applications. Elsevier, Amsterdam: 2003. [Google Scholar]
- [18].Meagher RJ, Won J-I, McCormick LC, Nedeleu S, Bertrand MM, Bertram JL, Drouin G, Barron AE, Slater GW. Electrophoresis. 2005;26:331. doi: 10.1002/elps.200410219. [DOI] [PubMed] [Google Scholar]
- [19].Popa TV, Mant CT, Hodges RS. Electrophoresis. 2004;25:94. doi: 10.1002/elps.200305654. [DOI] [PubMed] [Google Scholar]
- [20].Sereda TJ, Mant CT, Quinn AM, Hodges RS. J. Chromatogr. 1993;646:17. doi: 10.1016/s0021-9673(99)87003-4. [DOI] [PubMed] [Google Scholar]
- [21].Zhou NE, Monera OD, Kay CM, Hodges RS. Protein Peptide Lett. 1994;1:114. [Google Scholar]
- [22].Monera OD, Sereda TJ, Zhou NE, Kay CM, Hodges RS. J. Peptide Sci. 1995;1:319. doi: 10.1002/psc.310010507. [DOI] [PubMed] [Google Scholar]
- [23].Mant CT, Hodges RS, editors. High-Performance Liquid Chromatography of Peptides and Proteins: Separation, Analysis and Conformation. CRC Press; Boca Raton, FL: 1991. [Google Scholar]
- [24].Cunico RL, Gooding KM, Wehr T, editors. HPLC and CE of Biomolecules, Bay Bioanalytical Laboratory. Richmond, VA: 1998. [Google Scholar]
- [25].Mant CT, Hodges RS. In: HPLC of Biological Macromolecules. Gooding KM, Regnier FE, editors. Marcel Dekker; New York: 2002. p. 433. [Google Scholar]
- [26].Bennett HPJ, Browne CA, Solomon S. J. Liq. Chromatogr. 1980;3:1353. [Google Scholar]
- [27].Schaaper WMM, Voskamp D, Olieman C. J. Chromatogr. 1980;195:181. [Google Scholar]
- [28].Harding DRK, Bishop CA, Tarttellin MF, Hancock WS. Int. J. Peptide Protein Res. 1981;18:314. doi: 10.1111/j.1399-3011.1981.tb02060.x. [DOI] [PubMed] [Google Scholar]
- [29].Guo D, Mant CT, Hodges RS. J. Chromatogr. 1987;386:205. doi: 10.1016/s0021-9673(01)94598-4. [DOI] [PubMed] [Google Scholar]
- [30].Popa TV, Mant CT, Hodges RS. Electrophoresis. 2004;25:1219. doi: 10.1002/elps.200305889. [DOI] [PubMed] [Google Scholar]
- [31].Chen Y, Mehok AR, Mant CT, Hodges RS. J. Chromatogr. A. 2004;1043:9. doi: 10.1016/j.chroma.2004.03.070. [DOI] [PubMed] [Google Scholar]
- [32].Antia FD, Horváth CS. J. Chromatogr. 1988;435:1. [Google Scholar]
- [33].Boyes BE, Kirkland JJ. Pept. Res. 1993;6:249. [PubMed] [Google Scholar]
- [34].Li JW, Carr PW. Anal. Chem. 1997;69:2202. doi: 10.1021/ac9608681. [DOI] [PubMed] [Google Scholar]
- [35].Li JW, Carr PW. Anal. Chem. 1997;69:2550. doi: 10.1021/ac961170q. [DOI] [PubMed] [Google Scholar]
- [36].Hermodson M, Mahoney WC. Methods Enzymol. 1983;91:352. doi: 10.1016/s0076-6879(83)91032-7. [DOI] [PubMed] [Google Scholar]
- [37].Popa TV, Mant CT, Chen Y, Hodges RS. J. Chromatogr. A. 2004;1043:113. doi: 10.1016/j.chroma.2004.04.029. [DOI] [PubMed] [Google Scholar]
- [38].Popa TV, Mant CT, Hodges RS. Electrophoresis. 2003;24:4197. doi: 10.1002/elps.200305576. [DOI] [PubMed] [Google Scholar]
- [39].Hoppe W, Lohmann W, Markl H, Ziegler H, editors. Biophysics. Springer; Berlin: 1982. [Google Scholar]
- [40].Dill KA. Protein Sci. 1999;8:1166. doi: 10.1110/ps.8.6.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Creighton TE, editor. Protein Structure: A Practical Approach. IRL Press; Oxford, UK: 1989. [Google Scholar]
- [42].Washabaugh MW, Collins KD. J. Biol. Chem. 1986;261:12477. [PubMed] [Google Scholar]