Abstract
Yolk steroids of maternal origin have been proposed to influence genetic sex determination in birds, based on sex differences in yolk steroid concentrations of peafowl eggs incubated for 10 days. More recent reports dispute this proposal, as yolk steroids in eggs incubated for 3 days do not show such sex differences. To date, research examining this phenomenon has only analysed incubated eggs, although sex in avian species is determined before incubation begins. This may be a serious methodological flaw because incubation probably affects yolk steroid concentrations. Therefore, we investigated sex differences in yolk steroid concentrations of unincubated avian eggs. We withdrew yolk for steroid analysis from fresh, unincubated Japanese quail (Coturnix japonica) eggs by biopsy, and then incubated those eggs for 10 days, after which we harvested the embryonic material for genetic sexing and the incubated yolk for further steroid analysis. We found no sex differences in fresh Japanese quail eggs; however, sex differences were apparent in yolk steroids by day 10 of incubation, when female eggs had significantly more oestrogen in relation to androgen than male eggs. Concentrations of all yolk androgens decreased dramatically between laying and day 10 of incubation, whereas oestradiol (E2) concentrations increased marginally. Thus, yolk concentrations of androgens and E2 do not appear critical for avian sex determination.
Keywords: avian yolk steroids, testosterone, oestrogen, sex determination, Coturnix japonica, incubation
1. Introduction
Birds are a favourite model system for tests of Trivers and Willard's seminal sex allocation theory (Trivers & Willard 1973; West & Sheldon 2002). However, the mechanism of maternal manipulation of primary sex ratio in birds remains elusive (Komdeur et al. 2002). Recent research into maternally allocated yolk androgens has offered a potential answer to this question.
Petrie et al. (2001) reported sexually dimorphic sex steroid concentrations in peafowl eggs, and cited this as evidence that yolk steroid levels determine offspring sex. They found higher androstenedione (A4) and testosterone (T) concentrations in male eggs, and higher dihydrotestosterone (DHT) and oestradiol (E2) concentrations in female eggs. However, their study is flawed because the eggs that they assayed had been incubated for 10 days. Sexually dimorphic steroid concentrations in incubated eggs could be attributable to any of at least three sources: (i) a maternal sex-determining mechanism; (ii) sex differences in embryonic production of sex steroids, which ultimately accumulate in the yolk; or (iii) sex differences in sequestration or metabolism of sex steroids. Although Petrie et al. (2001) emphasize the first explanation, avian embryos are known to produce sexually dimorphic levels of E2 (higher in female embryos) and T (higher in male embryos) by 7.5 days of incubation (Galli & Wasserman 1973; Woods et al. 1975; Woods & Brazzill 1981; Ottinger et al. 2001). Steroids are lipophilic and highly yolk-soluble, and easily cross biological membranes. Because the yolk sac is highly vascularized by day 10 of incubation, circulating embryonic steroids probably accumulate in the yolk (see Elf & Fivizzani 2002; Lovern & Wade 2003a). These factors may lead to secondarily sexually dimorphic yolk steroid concentrations during incubation. This explanation is supported by the findings of Petrie et al. (2001) who reported that male eggs had higher concentrations of T, whereas female eggs had higher concentrations of E2. On the other hand, Petrie et al. (2001) found higher levels of DHT in female eggs than in male eggs. This might indicate a role in sex determination, since embryonic DHT production has been reported as undetectable (Woods et al. 1975) or sexually monomorphic (Schumacher et al. 1988).
In a recent study, Eising et al. (2003) did not find sex differences in yolk androgen concentrations between male and female chicken eggs. Müller et al. (2002), also studying chicken eggs, did not find a significant main effect of embryo sex on yolk steroid concentrations, but did find a significant effect of the interaction between maternal social status and offspring sex on yolk T. These studies cast significant doubt on the conclusion that the sex differences reported for peacock eggs, analysed after 10 days of incubation, reflect sex differences at laying. Yet, both Müller et al. (2002) and Eising et al. (2003) used eggs that had been incubated for 3 days, so the concentrations they report may not reflect differences of maternal allocation. Although these studies collected eggs before embryonic steroidogenesis is known, sex differences in sequestration or metabolism of sex steroids could occur before day 3. Furthermore, Lovern & Wade (2003b) have provided some support for the Petrie et al. (2001) hypothesis, reporting sex differences in anole lizard egg yolk before significant development has taken place. Thus, the sex allocation mechanism proposed by Petrie et al. (2001) continues to be influential among behavioural ecologists (e.g. Cook & Monaghan 2004; Rosivall et al. 2004).
There is a simple reason for using incubated eggs to study sex differences in yolk steroid concentrations. In fresh eggs, there is insufficient embryonic DNA to conduct genetic sexing; there is significant risk of contamination by maternal DNA, causing false reading of ‘female’ embryos owing to sexing of maternal, not embryonic, DNA. However, because the hypothesis is better tested by comparing yolk steroids in freshly laid eggs, we applied a different technique that was first employed by Schwabl (1993). Schwabl (1993) compared yolk T concentrations of canary eggs at laying with the sex of 28–30 day-old chicks, as determined by laparotomy. He found extensive overlap in yolk T concentrations between male and female eggs, and no significant sex difference. We employ a similar method to examine sex differences of yolk steroids in quail eggs, but use larger sample sizes, assays of more steroid hormones (A4, DHT, T and E2) and molecular sexing to ascertain sex during the embryonic period. In addition to examining sex differences in yolk steroids at laying, we also examine sex differences in yolk steroid concentrations after 10 days of incubation, and changes in yolk steroid concentrations between 0 and 10 days of incubation.
2. Methods
Eggs of Japanese quail (Coturnix japonica, a species in the same family as peafowl and chickens) housed in mixed-sex groups were purchased from the quail breeding facilities of Cornell University. Eggs were stored cool (12–15 °C) until biopsies were taken, between 6 and 36 h after laying. Biopsy was conducted with a sterile 23 gauge needle and 1 ml syringe, after sterilizing the eggshell with ethanol. We removed approximately 250 mg of yolk (mean±s.d., 232±33 mg; range, 89–306) and weighed the biopsied sample to the nearest 0.1 mg. We added 500 mg distilled water to the sample, homogenized it by vortexing and stored it at −20 °C until radioimmunoassay. Eggs were sealed with ethyl cyanoacrylate and set to incubate at 38 °C and 60% humidity for 10 days. On day 10, eggs were removed from the incubator and frozen at −20 °C. Approximately one week after freezing, eggs were dissected to harvest yolk as well as embryonic tissue. Embryonic material for genetic sexing was harvested only from eggs with substantial development to ensure collection of samples uncontaminated by maternal DNA. Yolk for steroid assay was collected from eggs showing stage-typical embryonic development.
A methodological problem associated with the use of biopsy to compare yolk steroid concentrations is variation in steroid concentrations across layers of the yolk (Lipar et al. 1999). This problem did not introduce bias in our study because the sex of eggs was not known at the time of biopsy; however, it could increase within-group variation. To ameliorate this problem, we biopsied a large sample of yolk (about 1/16 of the total yolk), which should draw from several layers. Hackl et al. (2003) studied interlayer variation of yolk androgens and progesterone in quail eggs. Androgen concentrations, which showed lower variability among layers than progesterone concentrations, were similar in central- and exterior-most layers, while intermediate layers had higher levels. Lipar et al. (1999) and Möstl et al. (2001) report similar findings for yolk androgens in chicken and passerine eggs. The large biopsy, relatively low interlayer variation, and U-shaped distribution across layers should diminish the effect of layer biopsy on within-group variation in yolk androgens. Lipar et al. (1999) reported that E2 concentrations decreased from the innermost to the outermost layer of yolk in dark-eyed junco eggs, so within-group variation of our E2 data may be elevated.
Final sample sizes included 85 eggs for analysis of yolk steroids at day 0 and 66 eggs for analysis of yolk steroids at day 10. Molecular sex was known for all of these eggs. Two sets of eggs were used in the experiment. The first set of 58 eggs was obtained on 11 November 2001. Forty-eight eggs were biopsied; 32 of those eggs developed sufficiently for DNA harvesting and 23 developed to a stage typical for day 10. The second set of eggs was obtained on 18 December 2002 and included 88 eggs, all of which were biopsied; 53 eggs developed sufficiently for DNA harvesting and 36 for day 10 yolk steroid analysis. Thus, 43% of biopsied eggs developed until day 10, which is typical for biopsied quail eggs (Daisley et al. 2005; K. M. Pilz, personal observation). Fertilization and hatching success in domestic Japanese quail are typically low; hatching success in this quail line is normally 65% (H. Redder, personal communication). To estimate the developmental success of unbiopsied eggs in our experiment, 10 of the eggs obtained on 11 November were incubated to day 10 without biopsy. Seven of the unbiopsied eggs (70%) developed to day 10.
Approximately 120 mg of yolk were used for steroid extraction and assay. Methods are described in Pilz et al. (2003). Recoveries averaged 53% for A4, 34% for DHT, 52% for T and 49% for E2. Intra-assay variation was 7.8% for A4, 9.6% for DHT and 8.1% for T. Owing to its extremely low concentrations, E2 was run singly to maximize sample volume. E2 values below the minimum sensitivity of the assay (0.2 pg mg−1) were entered as 0 s; excluding these data from the dataset did not change the results. All samples were run in a single assay.
Molecular sexing was conducted by amplifying conserved regions of the CHD-W and CHD-Z genes using primers 2550 F and 2718 R, following the protocol of Fridolfsson & Ellegren (1999). Samples were divided into four sets of PCR reactions and DNA from an adult male and female quail was included in all PCRs to validate the results. Six embryonic samples were PCR-amplified twice, for which the results always agreed.
3. Results
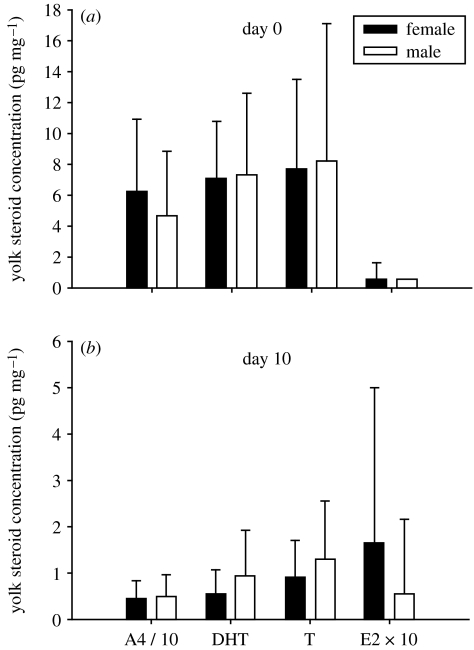
Yolk steroid concentrations did not differ between male and female eggs prior to incubation (MANOVA, F4,79=1.22, p=0.31; n=47 females, 37 males; figure 1a). However, male and female eggs did differ significantly in yolk steroid concentrations after 10 days of incubation (MANOVA, F4,66=1.07, p<0.05; n=34 females, 32 males; figure 1b). Post hoc comparisons revealed only marginal or non-significant sex differences for individual steroids on day 10 (Fisher's protected least significant difference test, E2: p=0.056; DHT: p=0.12; T: p=0.21; A4: p=0.86), but the sex difference in the ratio of E2 to total androgen was highly significant (t64=2.68; p=0.0094). At both stages, yolk steroid concentrations of the two sexes overlapped extensively (compare standard deviations in figure 1).
Figure 1.
Sex steroid concentrations (means±s.d.) in yolks of male and female quail eggs. (a) Freshly laid quail eggs did not show sexually dimorphic yolk sex steroid levels (MANOVA, F4,79=1.22, p=0.31). (b) Yolk sex steroid levels were significantly sexually dimorphic after 10 days of incubation (MANOVA, F4,66=1.07, p<0.05; note difference in y-axis scale). Standard deviations are presented to illustrate overlap in yolk steroid concentrations between the sexes. Note that, for graphical presentation, A4 concentrations were divided by 10 and E2 concentrations were multiplied by 10.
Examining changes of yolk steroid concentrations over time, there was not a significant day by sex interaction (unpaired MANOVA using all data, F4,143=1.48, p=0.21; repeated-measures MANOVA only using eggs with data from both day 0 and day 10, F1,57=1.73; p=0.19). We therefore analysed the effect of day without the interaction. Yolk steroid concentrations changed dramatically from day 0 to day 10 (MANOVA, day 0 versus day 10, F4,143=146, p<0.0001), decreasing significantly for each androgen (Fisher's PLSD, p<0.0001 for A4, for DHT and for T). E2 showed a marginal increase (Fisher's PLSD, p=0.063).
4. Discussion
We report here the first study examining the relationship between embryonic sex and avian egg yolk steroids at laying. We found no difference in yolk steroid concentrations between male and female eggs at laying. However, by day 10 of incubation, yolk steroid concentrations were sexually dimorphic. On day 10, males had qualitatively higher concentrations of all androgens but lower concentrations of E2 than females, and the ratio of oestradiol : androgen was significantly different between the sexes. Concentrations of all androgens decreased significantly between day 0 and day 10, whereas the concentration of E2 increased marginally. Although the interaction between sex and day in the full MANOVA was not significant, further studies with greater sample sizes may reveal a significant relationship. There are two reasonable explanations as to why avian eggs might show sex differences in yolk steroid concentrations late in incubation but not at laying. The first is that female embryos sequester/metabolize androgens from the yolk more rapidly than males, but that males sequester/metabolize E2 from the yolk more rapidly. We know of no research that has explicitly examined such processes. The second explanation is that maternal yolk steroids are sequestered/metabolized equally by both sexes (causing an overall decrease in yolk steroid concentrations; Elf & Fivizzani 2002; K. M. Pilz, unpublished data), but that the yolk accumulates embryonic steroids and male embryos produce more androgen while female embryos produce more E2. There is good evidence that embryonic sex steroid production is sexually dimorphic by day 10 of incubation, and that males produce more T whereas females produce more E2 at this stage (see §1). While there is currently no direct evidence that embryonic steroids accumulate in the yolk, such a scenario is probable since steroids are highly yolk soluble and because the embryonic vasculature is in intimate contact with the yolk. Accumulation of embryonic steroids in the yolk is supported by observations of increasing yolk steroid concentrations late in incubation (Elf & Fivizzani 2002; Lovern & Wade 2003a).
Our results from day 0 match closely with those of Müller et al. (2002) and Eising et al. (2003). These authors examined A4 and T concentrations in male and female chicken eggs after 3 days of incubation. Neither found significant main effects of sex on yolk androgen concentrations. These similarities in results allay fears that our day 0 results may have resulted from increased within-sex variation due to the biopsy technique. The sex differences in yolk steroid concentrations that we found at day 10 resembled those reported by Petrie et al. (2001). The only disparate result between our study and theirs is that yolk DHT concentrations were higher in female than male peafowl eggs, whereas we found higher DHT levels in male than in female quail eggs at day 10. Thus, the sex differences reported by Petrie et al. (2001) are better explained by incubation than by sex allocation. In further support of this conclusion, von Engelhardt et al. (2004) found no effect of treating female zebra finches (Taeniopygia guttata) with E2 on primary sex ratio (but see Veiga et al. 2004). However, yolk steroid allocation may vary in a species-specific manner, and females of some species may differentially allocate yolk steroids to male and female eggs even if sex steroids do not influence sex determination (Müller et al. 2002; Rutstein et al. 2005).
Our study cannot disprove that yolk steroids play some role in avian chromosomal sex determination. Sex determination could be affected by local concentrations of yolk steroids near the embryo (not reflected in whole yolk or large biopsies), by steroids not measured here (see Correa et al. 2005), or might only be mildly influenced by yolk androgens or oestrogens. Nevertheless, the great overlap in steroid concentrations of the two sexes makes it unlikely that the yolk steroids examined here are a critical component. Thus, the mechanism by which birds manipulate the sex of their offspring remains a mystery. Sex steroids do alter sex determination in reptiles with temperature-dependent sex determination (Crews 1996; Godwin & Crews 2002). Yolk steroids might play an important natural role in sex determination in those species (Janzen et al. 1998; Bowden et al. 2000), and possibly in reptiles with genetic sex determination as well (Lovern & Wade 2003b).
Acknowledgments
We appreciate the assistance of Stephanie Correa and Cary Leung in the laboratory. Stephanie Correa and Marion Petrie provided valuable comments on the manuscript. K.M.P. was supported by a predoctoral research fellowship from the Howard Hughes Medical Institute and a postdoctoral International Research Fellowship from NSF. Research funding was provided to E.A.-R. by NSF grants IBN-9514088 and IBN-0130986 and to K.M.P. by an NSF Doctoral Dissertation Improvement Grant (IBN-0104907).
Footnotes
Present address: Department of Evolutionary Ecology, Museo Nacional de Ciencias Naturales, José Gutiérrez Abascal, 2, Madrid 28006, Spain
References
- Bowden R.M. Environmental sex determination in a reptile varies seasonally and with yolk hormones. Proc. R. Soc. B. 2000;267:1745–1749. doi: 10.1098/rspb.2000.1205. doi:10.1098/rspb.2000.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M.I, Monaghan P. Sex differences in embryo development periods and effects on avian hatching patterns. Behav. Ecol. 2004;15:205–209. [Google Scholar]
- Correa S.M, Adkins-Regan E, Johnson P.A. High progesterone during avian meiosis biases sex ratios toward females. Biol. Lett. 2005;1:215–218. doi: 10.1098/rsbl.2004.0283. doi:10.1098/rsbl.2004.0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D. Temperature-dependent sex determination: the interplay of steroid hormones and temperature. Zool. Sci. 1996;13:1–13. doi: 10.2108/zsj.13.1. [DOI] [PubMed] [Google Scholar]
- Daisley J.N, Bromundt V, Möstl E, Kotrschal K. Enhanced yolk testosterone influences behavioral phenotype independent of sex in Japanese quail chicks. Coturnix japonica. Horm. Behav. 2005;47:185–194. doi: 10.1016/j.yhbeh.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Eising C.M, Müller W, Dijkstra C, Groothuis T.G. Maternal androgens in egg yolks: relation with sex, incubation time and embryonic growth. Gen. Comp. Endocrinol. 2003;132:241–247. doi: 10.1016/s0016-6480(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Elf P.K, Fivizzani A.J. Changes in sex steroid levels in yolks of the leghorn chicken, Gallus domesticus, during embryonic development. J. Exp. Zool. 2002;293:594–600. doi: 10.1002/jez.10169. [DOI] [PubMed] [Google Scholar]
- Fridolfsson A.-K, Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999;30:116–121. [Google Scholar]
- Galli F.E, Wassermann G.F. Steroid biosynthesis by gonads of 7-day-old and 10-day-old chick embryos. Gen. Comp. Endocrinol. 1973;21:77–83. doi: 10.1016/0016-6480(73)90157-3. [DOI] [PubMed] [Google Scholar]
- Godwin J, Crews D. Hormones, brain and behavior in reptiles. In: Pfaff D.W, et al., editors. Hormones, brain and behavior. Academic Press; San Diego: 2002. pp. 545–585. [Google Scholar]
- Hackl R, Bromundt V, Daisley J, Kotrschal K, Mostl E. Distribution and origin of steroid hormones in the yolk of Japanese quail eggs (Coturnix coturnix japonica) J. Comp. Physiol. B. 2003;173:327–331. doi: 10.1007/s00360-003-0339-7. [DOI] [PubMed] [Google Scholar]
- Janzen F.J. Endogenous yolk steroid hormones in turtles with different sex-determining mechanisms. Gen. Comp. Endocrinol. 1998;111:306–317. doi: 10.1006/gcen.1998.7115. [DOI] [PubMed] [Google Scholar]
- Komdeur J, Magrath M.J, Krackow S. Pre-ovulation control of hatchling sex ratio in the Seychelles warbler. Proc. R. Soc. B. 2002;269:1067–1072. doi: 10.1098/rspb.2002.1965. doi:10.1098/rspb.2002.1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipar J.L, Ketterson E.D, Nolan V.J, Casto J.M. Egg yolk layers vary in the concentration of steroid hormones in two avian species. Gen. Comp. Endocrinol. 1999;115:220–227. doi: 10.1006/gcen.1999.7296. [DOI] [PubMed] [Google Scholar]
- Lovern M.B, Wade J. Sex steroids in green anoles (Anolis carolinensis): uncoupled maternal plasma and yolking follicle concentrations, potential embryonic steroidogenesis, and evolutionary implications. Gen. Comp. Endocrinol. 2003a;134:109–115. doi: 10.1016/s0016-6480(03)00240-5. [DOI] [PubMed] [Google Scholar]
- Lovern M.B, Wade J. Yolk testosterone varies with sex in eggs of the lizard, Anolis carolinensis. J. Exp. Zool. A. 2003b;295:206–210. doi: 10.1002/jez.a.10225. [DOI] [PubMed] [Google Scholar]
- Möstl E, Spendier H, Kotrschal K. Concentration of immunoreactive progesterone and androgens in the yolk of hens' eggs (Gallus domesticus) Wien. Tierarztl. Monatsschr. 2001;88:62–65. [Google Scholar]
- Müller W, Eising C.M, Dijkstra C, Groothuis T. Sex differences in yolk hormones depend on maternal social status in Leghorn chickens (Gallus gallus domesticus) Proc. R. Soc. B. 2002;269:2249–2255. doi: 10.1098/rspb.2002.2159. doi:10.1098/rspb.2002.2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottinger M.A. Steroid hormones during embryonic development in Japanese quail: plasma, gonadal, and adrenal levels. Poult. Sci. 2001;80:795–799. doi: 10.1093/ps/80.6.795. [DOI] [PubMed] [Google Scholar]
- Petrie M, Schwabl H, Brande-Lavridsen N, Burke T. Sex differences in avian yolk hormone levels. Nature. 2001;412:498–499. doi: 10.1038/35087652. [DOI] [PubMed] [Google Scholar]
- Pilz K.M, Smith H.G, Sandell M, Schwabl H. Inter-female variation in egg yolk androgen allocation in the European starling: do high quality females invest more? Anim. Behav. 2003;65:841–850. [Google Scholar]
- Rosivall B, Torok J, Hasselquist D, Bensch S. Brood sex ratio adjustment in collared flycatchers (Ficedula albicollis): results differ between populations. Behav. Ecol. Sociobiol. 2004;56:346–351. [Google Scholar]
- Rutstein A.N, Gilbert L, Slater P.J.B, Graves J.A. Sex-specific patterns of yolk androgen allocation depend on maternal diet in the zebra finch. Behav. Ecol. 2005;16:62–69. [Google Scholar]
- Schumacher M, Sulon J, Balthazart J. Changes in serum concentrations of steroids during embryonic and post-hatching development of male and female Japanese quail (Coturnix coturnix japonica) J. Endrocinol. 1988;118:127–134. doi: 10.1677/joe.0.1180127. [DOI] [PubMed] [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA. 1993;90:11446–11450. doi: 10.1073/pnas.90.24.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R.L, Willard D.E. Natural-selection of parental ability to vary sex-ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Veiga J.P, Viñuela J, Cordero P.J, Aparicio J.M, Polo V. Experimentally increased testosterone affects social rank and primary sex ratio in the spotless starling. Horm. Behav. 2004;46:47–53. doi: 10.1016/j.yhbeh.2004.01.007. [DOI] [PubMed] [Google Scholar]
- von Engelhardt N, Dijkstra C, Daan S, Groothuis T.G.G. Effects of 17-beta-estradiol treatment of female zebra finches on offspring sex ratio and survival. Horm. Behav. 2004;45:306–313. doi: 10.1016/j.yhbeh.2003.12.009. [DOI] [PubMed] [Google Scholar]
- West S.A, Sheldon B.C. Constraints in the evolution of sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. [DOI] [PubMed] [Google Scholar]
- Woods J.E, Brazzill D.M. Plasma 17 beta-estradiol levels in the chick embryo. Gen. Comp. Endocrinol. 1981;44:37–43. doi: 10.1016/0016-6480(81)90353-1. [DOI] [PubMed] [Google Scholar]
- Woods J.E, Simpson R.M, Moore P.L. Plasma testosterone levels in the chick embryo. Gen. Comp. Endocrinol. 1975;27:543–547. doi: 10.1016/0016-6480(75)90076-3. [DOI] [PubMed] [Google Scholar]