Abstract
In this study, we investigated the effects of activation and stretch on the passive force–sarcomere length relationship in skeletal muscle. Single fibres from the lumbrical muscle of frogs were placed at varying sarcomere lengths on the descending limb of the force–sarcomere length relationship, and tetanic contractions, active stretches and passive stretches (amplitudes of ca 10% of fibre length at a speed of 40% fibre length/s) were performed. The passive forces following stretch of an activated fibre were higher than the forces measured after isometric contractions or after stretches of a passive fibre at the corresponding sarcomere length. This effect was more pronounced at increased sarcomere lengths, and the passive force–sarcomere length relationship following active stretch was shifted upwards on the force axis compared with the corresponding relationship obtained following isometric contractions or passive stretches. These results provide strong evidence for an increase in passive force that is mediated by a length-dependent combination of stretch and activation, while activation or stretch alone does not produce this effect. Based on these results and recently published findings of the effects of Ca2+ on titin stiffness, we propose that the observed increase in passive force is caused by the molecular spring titin.
Keywords: titin, Ca2+ activation, stretch, force enhancement, sarcomere length, force
1. Introduction
The passive force–length relationship in skeletal muscle has been investigated for decades, and it shows increasing levels of force at increasing muscle lengths. It has been proposed that most of the passive force in skeletal muscle is produced by the giant protein titin, that spans the half-sarcomere, connecting the A-band to the I-bands and Z-lines (Kellermayer et al. 1997; Rief et al. 1997). Titin consists of proline-glutamate-valine-lysine (PEVK)-rich domains and immunoglobulin-like (Ig) domains, that unfold as titin is stretched (Linke et al. 1998; Trombitas et al. 1998). Recent evidence shows that the PEVK segments of titin bind Ca2+ with high affinity (Tatsumi et al. 2001), and that increasing Ca2+ concentration enhances force produced by titin at a given sarcomere length (Labeit et al. 2003), suggesting a link between Ca2+-induced activation and force produced by titin.
In studies conducted in our laboratory with single-muscle fibres (Rassier et al. 2003; Rassier & Herzog 2004a,b), whole muscles (Herzog & Leonard 2002; Herzog et al. 2003) and human muscles (Lee & Herzog 2002), we observed that passive force is increased when activated muscles are stretched along the descending limb of the force–length relationship, when compared with the passive forces following passive stretches or isometric contractions at the corresponding lengths. We refer to this phenomenon as passive force enhancement (Herzog & Leonard 2002; Herzog et al. 2003; Rassier et al. 2003; Rassier & Herzog 2004a,b). We have suggested that passive force enhancement might be caused by an increase in titin stiffness upon activation and stretch (Herzog & Leonard 2002; Herzog et al. 2003; Rassier & Herzog 2004b). This mechanism would be consistent with the results of Labeit et al. (2003). However, it is still unclear if this phenomenon depends on muscle lengths and, consequently, if the entire passive force–sarcomere length relationship is affected by active muscle stretch. Further, previous experiments looking at passive force enhancement were performed without measurement of sarcomere length, and the optical measurement of fibre/muscle lengths before and after stretch may produce errors in length measurements.
In this paper, we evaluated the passive force–sarcomere length relationship following isometric contractions, passive stretches and active stretches. We observed that the passive force–sarcomere length relationship is shifted upwards on the force axis, when fibres are stretched while simultaneously activated, but activation or passive stretch alone do not produce any shift in the passive force–sarcomere length relationship.
2. Material and methods
(a) Muscle fibre preparation
Experiments were performed with single-muscle fibres (n=6) dissected from lumbrical muscles of the frog Rana Pipiens (ca 2 mm length). Treatment of these animals and all experimental procedures were approved by the University of Calgary committee for the ethical use of animals in research.
The tendons of the dissected fibres were gripped with small pieces of T-shaped aluminium foil close to the tendons. The fibres were mounted in an experimental chamber between a servomotor length controller (Aurora Scientific) and a force transducer (Sensomotor), bathed with a Ringer's solution (NaCl 115 mmol, KCl 3 mmol, CaCl2 3 mmol, NaH2PO4 2 mmol, NaHCO3 20 mmol, pH=7.5, temperature ca 9 °C). Stimulation (Grass S88, Grass Instruments) was given through two platinum wire electrodes placed in the chamber parallel to the muscle fibres, with square-wave pulses (0.4 ms duration) delivered at an amplitude of 25% above the voltage that gave maximal force production (range: 20–45V). The stimulation pattern was set individually for each fibre during 1 s tetanic contractions with the smallest frequency needed to produce a fused tetanic contraction (range in these experiments: 28–38 Hz) to avoid fatigue effects.
(b) Sarcomere length and force measurements
After the voltage and frequency of stimulation were defined, the fibres were paced for 60 min with twitch contractions (90 s intervals). At the end of this period, fibres were inspected visually for any apparent damage, and were evaluated for a possible decrease in force with 1 s tetanic contractions. If damage was found or force had decreased by 5% or more, the fibres were discarded from analysis. This procedure was repeated several times, including at the end of the experiment.
Sarcomere length was measured with the laser diffraction technique (ter Keurs et al. 1978). A He–Ne laser beam (633 nm wavelength) was projected perpendicular to the axis of the fibre, and the sarcomere length was established in a clear region of the fibre. The position and intensity distribution of the first-order diffraction pattern was reflected through an access port in the microscope on to a photodiode array. An amplifier was used to produce an analogue signal, with a voltage that was proportional to the sarcomere length, based on the median of the intensity profile of the first-order diffraction pattern.
An active (total force−passive force) force–sarcomere length relationship was obtained from isometric tetanic contractions (2 s duration, 5 min intervals). Fibres were then placed at initial lengths varying from 5 to 40% beyond the plateau of the force–sarcomere length relation, and were activated to produce maximal isometric force. At 1000 ms after the onset of activation, fibres were stretched ca 10% of fibre length (ca 0.22 μm per sarcomere) at a speed of 40% fibre length/s, and held isometrically at the final length (total contraction time=4 s). Passive stretches with equivalent stretch amplitude and speeds were performed for comparison. Before and after the stretch contractions, isometric contractions were also performed at corresponding lengths. Forces were measured at 2, 5 and 10 s after deactivation of the fibres (i.e. passive force) for the active and the passive stretches.
3. Results
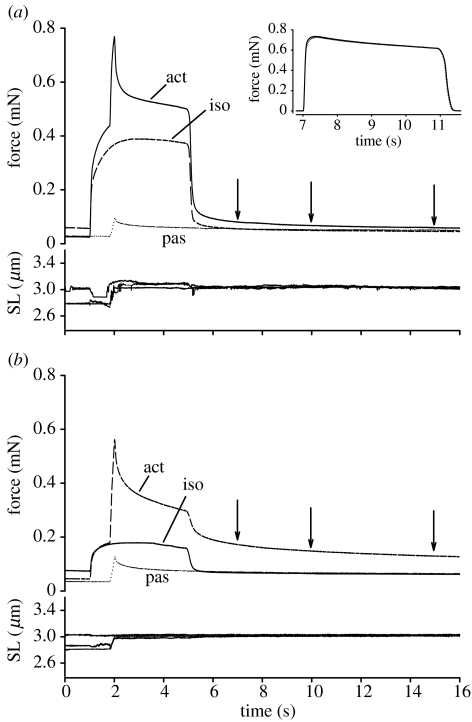
Figure 1(a) shows force–time and sarcomere length–time histories of active stretches, passive stretches, and isometric contractions at corresponding sarcomere lengths (after deactivation of the fibres). The total force after stretch of activated fibres was higher than the force produced during isometric contractions at the corresponding sarcomere length. Such active force enhancement has been observed in several studies by our group (Herzog & Leonard 2002; Herzog et al. 2003; Rassier et al. 2003) and others (Julian & Morgan 1979; Edman et al. 1982; Sugi & Tsuchiya 1988). Passive force after deactivation of the actively stretched fibres was higher than the passive force after a purely isometric contraction, and higher than the force produced when the fibre was stretched passively. Figure 1(b) shows contractions performed with the same fibre as in figure 1(a), but after addition of 5 mM BDM to the Ringer solution. BDM inhibited active force production by biasing cross-bridges towards a weakly-bound state, but did not inhibit the level of passive force enhancement. Isometric reference contractions performed throughout the experiments showed consistent force profiles (figure 1a, inset) indicating that fibres were not damaged.
Figure 1.
Force–time and sarcomere-length time histories of isometric contractions, active stretches and passive stretches in a typical experiment (a) in Ringer solution and (b) after adding 5 mM BDM. The inset shows isometric contractions performed at 2.0 μm at the beginning and at the end of one typical experiment. Note that the isometric force did not decrease throughout the experiment, suggesting that the quality of the fibres was preserved after stretches and BDM treatment. Act: active stretch; Pas: passive stretch. Iso: isometric contraction. Arrows show the times when passive forces were measured.
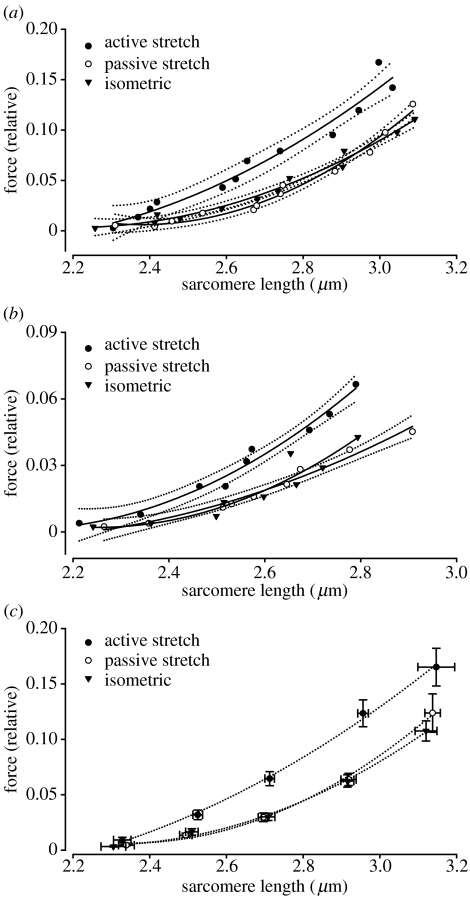
Figure 2 shows the passive force–sarcomere length relationship for two representative fibres (figure 2a,b) and for all fibres following isometric contractions, active stretch and passive stretch (figure 2c). Passive force was increased for the actively stretched fibres. Passive force increased with increasing sarcomere lengths.
Figure 2.
Passive force–sarcomere length relationship after isometric contractions, active stretches and passive stretches. All data were approximated with best-fitting second order polynomial equations. In panels (a) and (b), experiments from two representative fibres are shown, along with the best-fit lines and 95% confidence intervals. Note that the data points for the actively stretched fibres do not overlap with those recorded after isometric contractions or passive stretches. In panel (c), mean (±standard error) values are shown from data that were grouped into intervals of 0.2 μm (range: 2.2–3.2 μm). With the exception of a sarcomere length of 2.2 μm, there is a significant increase (p<0.05) in the passive force following active stretch compared with the passive force following isometric contraction and passive stretch.
4. Discussion
We determined the passive force–sarcomere length relationship of single-muscle fibres for three distinct situations: (i) following isometric contractions; (ii) following passive stretch; and (iii) following active stretch. Passive forces were increased following active stretch, suggesting that stretch and activation combined cause this increase in force, while activation alone (isometric contractions), or stretch alone (passive stretches) did not produce this effect. Passive force enhancement was length-dependent; it increased with increasing sarcomere lengths (figure 2).
Conceptually, passive forces, and the passive force enhancement, could be caused by passive structures or by weakly-bound cross-bridges (Proske & Morgan 1999; Campbell & Moss 2002). Because passive force enhancement is increased with increasing lengths, but filament overlap and the number of available cross-bridges for force production is decreased, it appears that cross-bridge mechanisms are not causing this phenomenon. Furthermore, we observed that muscle fibres treated with 2, 3-butanedione monoxime (BDM), a drug that inhibits cross-bridge force production without altering Ca2+ transients significantly, did not decrease the passive force enhancement (figure 1b), providing further evidence that passive force enhancement is not associated with cross-bridge mechanisms (Rassier & Herzog 2004a,b). Therefore, passive force enhancement is probably caused by a passive structural element in skeletal muscle.
The conclusion that a passive element may be engaged upon activation, and so may cause the passive force enhancement observed here, is strengthened by findings of an instantaneous increase in stiffness in single fibres upon activation that is independent of cross-bridge attachment (Bagni et al. 1994; Bagni et al. 2002). Bagni and colleagues called this observation ‘static tension’, and observed that it increased with increasing sarcomere length and amplitude of stretch, results that are consistent with those observed in this study. Also, Campbell & Moss (2002) showed that initial tension and stiffness, which are greater in activated compared with passive skinned muscle fibres, cannot be explained by cross-bridge structures.
Previously, we suggested that the structure responsible for the increase in passive force following stretch of activated fibres is titin (Herzog & Leonard 2002; Herzog et al. 2003; Rassier & Herzog 2004b). Recent experiments by Labeit et al. (2003) indicate that such an interpretation may be correct. They observed that skinned fibres, in which actomyosin interaction was prevented, showed an upwards shift in the force–sarcomere length relationship when Ca2+ concentration was increased. By performing additional experiments with titin molecules, the authors were able to associate the increase in the passive force with a specific, calcium-dependent conformational change in the PEVK segment of titin. Therefore, the following picture emerges. Upon muscle activation, a rise in Ca2+ triggers actomyosin interactions and increases titin stiffness. When an activated muscle is stretched, titin stiffness is increased, and passive force increases proportionally with the amount of stretch. The length dependence of the phenomenon may possibly be explained by assuming that the increase in fibre stiffness occurs at specific sites, as suggested by Labeit et al. (2003), and that functionally these stiffened sites only become important contributors once titin has reached a critical length.
References
- Bagni M.A, Cecchi G, Colombini B, Colomo F. A non-cross-bridge stiffness in activated frog muscle fibers. Biophys. J. 2002;82:3118–3127. doi: 10.1016/S0006-3495(02)75653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni M.A, Cecchi G, Colomo F, Garzella P. Development of stiffness precedes cross-bridge attachment during the early tension rise in single frog muscle fibres. J. Physiol. 1994;481:273–278. doi: 10.1113/jphysiol.1994.sp020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K.S, Moss R.L. History-dependent mechanical properties of permeabilized rat soleus muscle fibers. Biophys. J. 2002;82:929–943. doi: 10.1016/S0006-3495(02)75454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K.A, Elzinga G, Noble M.I. Residual force enhancement after stretch of contracting frog single muscle fibers. J. Gen. Physiol. 1982;80:769–784. doi: 10.1085/jgp.80.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog W, Leonard T.R. Force enhancement following stretching of skeletal muscle: a new mechanism. J. Exp. Biol. 2002;205:1275–1283. doi: 10.1242/jeb.205.9.1275. [DOI] [PubMed] [Google Scholar]
- Herzog W, Schachar R, Leonard T.R. Characterization of the passive component of force enhancement following active stretching of skeletal muscle. J. Exp. Biol. 2003;206:3635–3643. doi: 10.1242/jeb.00645. [DOI] [PubMed] [Google Scholar]
- Julian F.J, Morgan D.L. The effect on tension of non-uniform distribution of length changes applied to frog muscle fibres. J. Physiol. 1979;293:379–392. doi: 10.1113/jphysiol.1979.sp012895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermayer M.S, Smith S.B, Granzier H.L, Bustamante C. Folding–unfolding transitions in single titin molecules characterized with laser tweezers. Science. 1997;276:1112–1116. doi: 10.1126/science.276.5315.1112. [DOI] [PubMed] [Google Scholar]
- Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H. Calcium-dependent molecular spring elements in the giant protein titin. Proc. Natl. Acad. Sci. USA. 2003;100:13716–13721. doi: 10.1073/pnas.2235652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.D, Herzog W. Force enhancement following muscle stretch of electrically stimulated and voluntarily activated human adductor pollicis. J. Physiol. 2002;545:321–330. doi: 10.1113/jphysiol.2002.018010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke W.A, Ivemeyer M, Mundel P, Stockmeier M.R, Kolmerer B. Nature of PEVK-titin elasticity in skeletal muscle. Proc. Natl. Acad. Sci. USA. 1998;95:8052–8057. doi: 10.1073/pnas.95.14.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Morgan D.L. Do cross-bridges contribute to the tension during stretch of passive muscle? J. Muscle Res. Cell Motil. 1999;20:433–442. doi: 10.1023/a:1005573625675. [DOI] [PubMed] [Google Scholar]
- Rassier D.E, Herzog W. Active force inhibition and stretch-induced force enhancement in frog muscle treated with BDM. J. Appl. Physiol. 2004a;97:1395–1400. doi: 10.1152/japplphysiol.00377.2004. [DOI] [PubMed] [Google Scholar]
- Rassier D.E, Herzog W. Effects of shortening on stretch-induced force enhancement in single skeletal muscle fibers. J. Biomech. 2004b;37:1305–1312. doi: 10.1016/j.jbiomech.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Rassier D.E, Herzog W, Wakeling J, Syme D.A. Stretch-induced, steady-state force enhancement in single skeletal muscle fibers exceeds the isometric force at optimum fiber length. J. Biomech. 2003;36:1309–1316. doi: 10.1016/s0021-9290(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Rief M, Gautel M, Oesterhelt F, Fernandez J.M, Gaub H.E. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science. 1997;276:1109–1112. doi: 10.1126/science.276.5315.1109. [DOI] [PubMed] [Google Scholar]
- Sugi H, Tsuchiya T. Stiffness changes during enhancement and deficit of isometric force by slow length changes in frog skeletal muscle fibres. J. Physiol. 1988;407:215–229. doi: 10.1113/jphysiol.1988.sp017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi R, Maeda K, Hattori A, Takahashi K. Calcium binding to an elastic portion of connectin/titin filaments. J. Muscle Res. Cell Motil. 2001;22:149–162. doi: 10.1023/a:1010349416723. [DOI] [PubMed] [Google Scholar]
- ter Keurs H.E, Iwazumi T, Pollack G.H. The sarcomere length-tension relation in skeletal muscle. J. Gen. Physiol. 1978;72:565–592. doi: 10.1085/jgp.72.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombitas K, Greaser M, Labeit S, Jin J.P, Kellermayer M, Helmes M, Granzier H. Titin extensibility in situ: entropic elasticity of permanently folded and permanently unfolded molecular segments. J. Cell Biol. 1998;140:853–859. doi: 10.1083/jcb.140.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]