Abstract
Life-history theory predicts that as organisms approach the end of their life, they should increase their reproductive effort (RE). However, studies on mammals often find that measures of RE do not vary with maternal age. This might be because offspring have some control over energy transfer which may constrain adaptive variation in RE by mothers, particularly in eutherian mammals where placental function is primarily controlled by offspring. However, in marsupials, energy transfer is primarily by lactation and under maternal control, leaving marsupial mothers free to vary RE. Here, we provide the first analysis, to our knowledge, of age-specific RE in a marsupial, the common brushtail possum. RE, measured as the proportion of maternal mass lost during lactation, was strongly correlated with offspring mass as a yearling. Older females had higher RE, gave birth earlier in the season and were more likely to produce two offspring in a year. Females with high RE in one year were lighter at the beginning of the next breeding season. These results provide the clearest support yet for terminal RE in a mammal.
Keywords: age, life history, reproductive effort, residual reproductive value
1. Introduction
Reproduction involves the expenditure of maternal energy, which may have negative effects on a mother's own growth, survival and future reproductive success (Stearns 1992). The level of energy investment in any reproductive attempt should therefore reflect a trade-off between the benefits in terms of offspring quality and costs to reduced potential for future reproduction (Williams 1966). Older females have fewer opportunities for future reproduction and as females age, the trade-off between current and future reproduction should be resolved in favour of high reproductive effort (RE) into current reproduction (Williams 1966; Clutton-Brock 1991; Forslund & Part 1995). If risk of mortality rises abruptly at a particular stage of life, then theory also predicts a large increase in RE, and an associated decrease in resource allocation into somatic reserves, before that age (Pianka 1988; Polak & Starmer 1998).
Although the prediction that RE should increase with maternal age does not hold for all organisms (Charlesworth & Leon 1976), it should apply especially well to mammals, which are iteroparous, undergo terminal senescence (Packer et al. 1998) and because females expend large amounts of energy during lactation (Clutton-Brock 1991). However, RE typically does not increase with age in mammals (Cameron et al. 2000; Coˆté & Festa-Bianchet 2001; Weladji et al. 2002). Offspring of older females sometimes have higher survival rates (Cameron et al. 2000; Clutton-Brock 1984), but this may be a result of maternal experience, rather than greater energy investment (Cameron et al. 2000; McMahon & Bradshaw 2004).
In this paper, we test the prediction that females should show an increase in RE and an associated decline in somatic investment as the probability of surviving to reproduce again declines with increasing age in a marsupial, the common brushtail possum Trichosurus vulpecula.
2. Material and methods
The study was conducted over 4 years on Magnetic Island, North Queensland. Breeding seasonality, sexual dimorphism and density in this population are described in Isaac & Johnson (2003). Patterns of offspring sex allocation are also described elsewhere (Isaac et al. 2005). The degree of sexual dimorphism is low in this population (Isaac & Johnson 2003), and mothers do not bias maternal effort in relation to offspring sex (J. Isaac, unpublished data). Female T. vulpecula begin to reproduce in their first or second year, produce a single offspring and demonstrate a typical marsupial maternal investment strategy, characterized by a short gestation (17 days) and long lactation period (ca 6–9 months; Tyndale-Biscoe & Renfree 1987). On Magnetic Island, the majority of females give birth between April and May (autumn), and a small proportion of females give birth to a second young, after successfully weaning a first, between October and November (spring).
Animals were live trapped monthly; all animals were PIT tagged and weighed and measured at each capture. Individuals were aged using a tooth wear index (Winter 1980), calibrated by reference to females of known age marked during an earlier study (n=8) and those born during this study (n=14). RE was calculated as the percentage of pre-breeding mass (taken the month prior to known parturition) lost during lactation; post-lactation mass was taken the first month a female was caught without a dependent offspring. Mass loss was also calculated for females that gave birth, but lost a pouch young during lactation. Between-years changes in mass for individuals were calculated as absolute change from mass prior to parturition one year, to mass prior to parturition the next.
Between 0 and 40% of females each year gave birth to a second young and for analysis involving annual reproductive effort, the sum of both reproductive events was used for these mothers. Lactation for second offspring occurred during summer, November to March, when all animals increase in mass (J. Isaac, unpublished data). We therefore corrected for a 0.09 kg mass gain (mean summer mass gain for non-lactating adult females).
A date of birth was assigned to all young based on head length (see Isaac & Johnson 2003), expressed as days before/after April 1 in any year. Second offspring born to mothers that double bred were excluded from analysis involving offspring mass at 12 months, as they became yearlings in spring rather than autumn. Male yearlings were also excluded, as the majority of sons dispersed before reaching 12 months of age.
Mixed models incorporating random effects (female identity) found no influence of female ID on RE (p=0.25), date of parturition (p=0.56) and probability of double breeding (p=0.1). Female ID was therefore dropped from further analyses, which did not account for random factors. Age-specific survival was calculated as the probability of an individual surviving to reproduce the next year, estimated from live-trapping data, using nominal logistic regression. Only females caught regularly for 2 years or more were included. For some analyses, females were categorized into three age categories; primiparous (1–2 years old; first-time mothers, still growing), middle-aged (3–5 years old; growth completed, high survival) and old (6 or more years old; declining survival).
3. Results
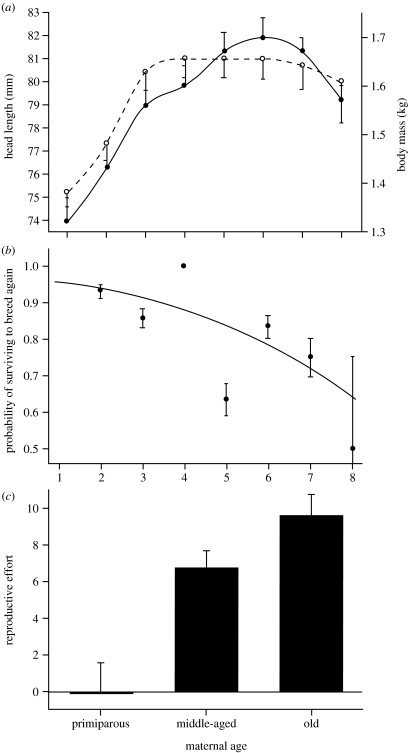
During the study, 36 females produced 101 offspring. Growth in head length was completed by 4 years of age; body mass increased until the age of 6, before declining slightly (figure 1a). Average absolute annual mass gain was significantly higher in primiparous females (, n=18) compared with middle-aged (, n=26) and old (, n=15) females (Tukey–Kramer honestly significant difference (HSD) test; p<0.05). However, annual mass gain did not differ significantly between middle-aged and old females (p>0.05) and mean body mass and head length did not differ significantly between age classes after the age of 3 (Tukey–Kramer HSD; p>0.05 in both cases). Probability of surviving to breed in the next reproductive season declined with age (figure 1b; χ2=4.77, p=0.02, n=86).
Figure 1.
(a) Age-specific changes in mean head length (mm; −s.e.; open circles) and mean body mass (kg; +s.e.; filled circles) in female possums. (b) Age-specific probability of surviving to breed in the next year. The line is that predicted by a nominal logistic regression, filled circles show the actual proportion of females surviving at each age class (±s.e.). (c) Mean reproductive effort (relative mass loss in lactation) in relation to age class, ±s.e., sample sizes shown above columns.
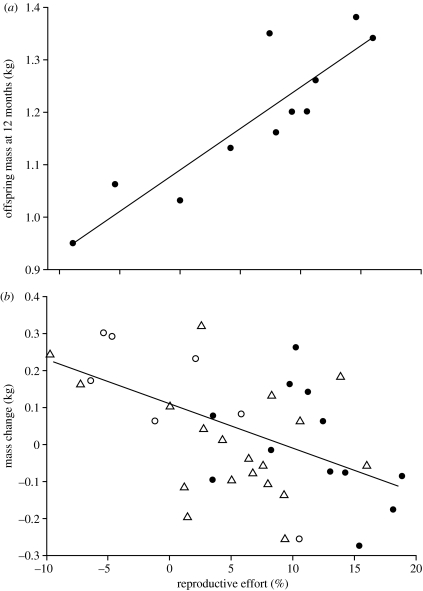
Females lost an average of 6.52±0.79% of their pre-breeding body mass during lactation. Mass loss during lactation increased linearly with maternal age (R2=0.17, p=0.0006, d.f.=63), and differed among the three maternal age groups, being highest in old females (figure 1c; F2,61=10.27, p=0.0001). This relationship remained when second offspring were excluded (F2,61=9.3, p=0.0004), and when absolute mass loss replaced relative mass loss (F2,61=9.99, p=0.0002). The percentage of body mass lost by individual mothers was positively related to offspring mass as a yearling (figure 2a; R2=0.80, p=0.0002, d.f.=10). There was also a negative relationship between relative mass loss in one lactation cycle and a female's mass prior to parturition in the following year (figure 2b; R2=0.26, p=0.0008, d.f.=39).
Figure 2.
(a) The relationship between reproductive effort and offspring mass as a yearling. (b) The relationship between reproductive effort in one reproductive season and change in mass at the start of the following breeding season; primiparous females are denoted by open circles, middle-aged mothers by open triangles and old females by filled circles. Line fit from least-squares linear regression on all data in both cases (see text).
RE as a middle-aged mother was not related to probability of survival into the old age class (χ2=0.87, p=0.35, d.f.=1, n=22). There was no effect of maternal age on offspring mass at 12 months (p > 0.5).
The proportion of old females that produced two offspring in a year was significantly higher than that predicted for the overall sample (binomial test: p=0.004); this was not the case for the other two age groups (p>0.1 in both cases). Females that double bred did not vary their RE between their first () and second () offspring (paired t-test; t=0.27, p=0.79, d.f.=7). Old females gave birth to their first offspring earlier (, n=26) than both primiparous (, n=16) and middle-aged (, n=26) females (F2,77=8.52, p=0.0005).
4. Discussion
Individual females lost significant mass during lactation, indicating that body fat stores were mobilized to meet the energy requirements of reproduction. The percentage of pre-breeding body mass lost by females during lactation was related to offspring mass at 12 months of age, suggesting that mass change during lactation was an index of transfer of energy from mother to offspring and a useful measure of RE.
Our results also reveal a cost of high RE on somatic condition and body reserves, as females with high RE in one year were lighter at the beginning of the next breeding season. Variation in RE was large and females with the highest rates of mass loss produced yearling offspring more than 50% heavier than females with minimal mass loss. Primiparous females lost little mass during lactation and some gained mass slightly. This is at least partly because they were still growing. However, our results suggest that these females still invested less in reproduction, as they produced offspring that were relatively small as yearlings.
From the age of 6 years, RE increased while the probability of a female surviving to the next breeding season declined. Consistent with increased RE, females tended to lose mass after 6 years of age. These results are consistent with the theoretical expectation that RE should rise sharply in females approaching the end of their life and that increasing RE comes at a cost to somatic investment (Pianka 1988).
Old females gave birth to their first offspring earlier than other mothers did and there is extensive evidence from eutherian mammals that an early date of birth can benefit offspring in both the long and short term (e.g. Green & Rothstein 1993). Older females were also more likely to produce a second young in the spring, thus increasing their RE in two ways—by investing more energy in each offspring and by increasing their annual reproductive output. There was no apparent trade-off between RE and offspring number, as mothers did not vary their RE in first and second offspring. However, non-breeding females gained considerable mass during summer; therefore, older mothers appeared to increase their RE and reproductive output, but sacrificed opportunities to re-build somatic reserves over the summer period, possibly at a cost to future survival.
A predominance of ‘high-quality’ females surviving to old age could provide an alternative explanation for our result. However, we found no evidence that middle-aged females with high RE were more likely to survive to old age. Similarly, age-related breeding experience could also explain our results, but there was no independent influence of maternal age on offspring mass, indicating that age-related RE and not age per se, influenced offspring.
We suggest that differences in the control of maternal investment in marsupials and placental mammals explain why, in contrast to previous studies of mammals, we found increased RE with age in this study. In placental mammals, a high proportion of total energy transfer is via the placenta (Hsu et al. 1999). Because the placenta is derived from foetal tissue and regulated by foetal hormones, offspring have significant control over the rate of energy transfer in gestation (Haig 1993, 1996). Furthermore, an increase in offspring demand can, to some extent, influence milk production and rate of energy transfer via the mammary gland in placental mammals (Park & Jacobson 1993). By contrast, energy transfer in marsupials is predominantly by lactation and the marsupial mammary gland is relatively unresponsive to changes in suckling stimulus: experiments using cross-fostering of pouch young in kangaroos show that if a large pouch young is placed on a mammary gland that has been producing milk for a smaller pouch young, the result is a slowing of the growth rate of the pouch young rather than a compensatory increase in milk production (Findlay & Renfree 1984). In this sense, the marsupial mammary gland is relatively independent of the suckling young, controlled more by maternal factors than offspring demand (Tyndale-Biscoe & Renfree 1987; Trott et al. 2003). Thus, while mother–offspring conflict may constrain adaptive variation in RE by placental mothers, this effect is probably significantly reduced in marsupials.
In conclusion, our study reveals a high degree of variability in age-specific RE in a marsupial, providing an excellent test of a general theory of adaptive variation in maternal RE. Females in the oldest age group combine three strategies to increase their overall maternal effort. RE in each breeding cycle increased late in life; the probability of breeding a second time late in the year increased; and older females also began breeding earlier in the year. Thus, old females increased both their annual reproductive output and their level of RE in individual offspring.
Acknowledgments
The Australian Research Council and James Cook University provided financial support, and Queensland Parks and Wildlife Service provided permits. We thank B. Goodman, M. Festa-Bianchet, D. Fisher, A. Krockenberger, J. Martin, E. Ritchie, S. Robson, D. Salkeld and M. Symonds for comments on previous versions of the manuscript.
References
- Cameron E, Linklater W, Stafford K, Minot E. Aging and improving reproductive success in horses: declining residual reproductive value or just older and wiser? Behav. Ecol. Sociobiol. 2000;47:243–249. [Google Scholar]
- Charlesworth B, Leon J.A. The relation of reproductive effort to age. Am. Nat. 1976;110:449–459. [Google Scholar]
- Clutton-Brock T.H. Reproductive effort and terminal investment in iteroparous animals. Am. Nat. 1984;123:212–229. [Google Scholar]
- Clutton-Brock T. Princeton University Press; 1991. The evolution of parental care. [Google Scholar]
- Côté S.D, Festa-Bianchet M. Reproductive success in female mountain goats: the influence of age and social rank. Anim. Behav. 2001;62:173–181. [Google Scholar]
- Findlay L, Renfree M.B. Growth, development and secretion of the mammary gland of macropodid marsupials. Symp. Zool. Soc. Lond. 1984;51:403–432. [Google Scholar]
- Forslund P, Part T. Age and reproduction in birds—hypotheses and tests. Trends Ecol. Evol. 1995;20:374–378. doi: 10.1016/s0169-5347(00)89141-7. [DOI] [PubMed] [Google Scholar]
- Green A.C.H, Rothstein A. Persistent influences of birth date on dominance, growth and reproductive success in bison. J. Zool. 1993;230:177–186. [Google Scholar]
- Haig D. Genetic conflicts in human-pregnancy. Q. Rev. Biol. 1993;68:495–532. doi: 10.1086/418300. [DOI] [PubMed] [Google Scholar]
- Haig D. Placental hormones, genomic imprinting and maternal–fetal communication. J. Evol. Biol. 1996;9:357–380. [Google Scholar]
- Hsu M.J, Garton D.W, Harder J.D. Energetics of offspring production: a comparison of a marsupial (Monodelphis domestica) and a eutherian (Mesocricetus auratus) J. Comp. Physiol. B. 1999;169:67–76. doi: 10.1007/s003600050195. [DOI] [PubMed] [Google Scholar]
- Isaac J.L, Johnson C.N. Sexual dimorphism and synchrony of breeding: variation in polygyny potential among populations in the common brushtail possum, Trichosurus vulpecula. Behav. Ecol. 2003;14:818–822. [Google Scholar]
- Isaac, J. L., Krockenberger, A. K. & Johnson, C. N. 2005 Adaptive sex allocation in relation to life-history in the common brushtail possum. J. Anim. Ecol.74, 552–558.
- McMahon C.R, Bradshaw C.J.A. Harem choice and breeding experience of female southern elephant seals influence offspring survival. Behav. Ecol. Sociobiol. 2004;55:349–362. [Google Scholar]
- Packer C, Tatar M, Collins A. Reproductive cessation in mammals. Nature. 1998;392:107–111. doi: 10.1038/33910. [DOI] [PubMed] [Google Scholar]
- Park C.S, Jacobson N.L. The mammary gland and lactation. In: Swenson M.J, Reece W.O, editors. Duke's physiology of domestic animals. Comstock Publishing Associates; Ithaca: 1993. [Google Scholar]
- Pianka E.R. Harper Collins; New York: 1988. Evolutionary ecology. [Google Scholar]
- Polak M, Starmer W.T. Parasite-induced risk of mortality elevates reproductive effort in male Drosophila. Proc. R. Soc. B. 1998;265:2197–2201. doi: 10.1098/rspb.1998.0559. doi:10.1098/rspb.1998.0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; New York: 1992. The evolution of life histories. [Google Scholar]
- Trott J.F, Simpson K.J, Moyle R.L.C, Hearn C.M, Shaw G, Nicholas K.R, Renfree M.B. Maternal regulation of milk composition, milk production, and pouch young development during lactation in the tammar wallaby (Macropus eugenii) Biol. Reprod. 2003;68:929–936. doi: 10.1095/biolreprod.102.005934. [DOI] [PubMed] [Google Scholar]
- Tyndale-Biscoe C.H, Renfree M. Cambridge University Press; 1987. Reproductive physiology of marsupials. [Google Scholar]
- Weladji R.B, Mysterud A, Holand O, Lenvik D. Age-related reproductive effort in reindeer (Rangifer tarandus): evidence of senescence. Oecologia. 2002;131:79–82. doi: 10.1007/s00442-001-0864-6. [DOI] [PubMed] [Google Scholar]
- Williams G.C. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 1966;100:687–690. [Google Scholar]
- Winter J.W. Tooth wear as an age index in a population of the brush-tail possum, Trichosurus vulpecula (Kerr) Aust. Wildl. Res. 1980;7:359–363. [Google Scholar]