Abstract
The network of movements of cattle between farm holdings is an important determinant of the potential rates and patterns of spread of infectious diseases. Because cattle movements are uni-directional, the network is unusual in that the risks of acquiring infection (by importing cattle) and of passing infection on (by exporting cattle) can be clearly distinguished, and there turns out to be no statistically significant correlation between the two. This means that the high observed degree of heterogeneity in numbers of contacts does not result in an increase in the basic reproduction number, R0, in contrast to findings from studies of other contact networks. Despite this, it is still the case that just 20% of holdings contribute at least 80% of the value of R0.
Keywords: basic reproduction number, heterogeneity, infectious diseases, livestock, movements
1. Introduction
The network of contacts between individuals is a key determinant of the pattern of spread of an infectious disease. Although this has been recognized in principle for some time (e.g. Lajmanovich & Yorke 1976) the role of contact networks in practice was given prominence by empirical and theoretical studies of the transmission of HIV/AIDS, which indicated that small numbers of individuals with very high rates of sexual partner change were disproportionately important for the epidemic spread of this and other sexually transmitted diseases (e.g. Gupta et al. 1989). Later work suggested that this finding was more generally applicable to the spread of infectious diseases in human and non-human populations alike (Woolhouse et al. 1997).
The importance of contact networks can be quantified in terms of their impact on the basic reproduction number, R0, defined as the average number of secondary cases of infection resulting from the introduction of a single primary case into a population of previously unexposed hosts (Anderson & May 1991). Trivially, R0 increases with higher mean rates of contact between individuals but, less obviously, R0 also increases with higher variances in contact rates (and may also be affected by other, ‘higher-order’ properties of the contact network; Woolhouse et al. 1991, 1998). The effects can be substantial: for example, Woolhouse et al. (1997) reported that observed variances in contact rate could increase R0 threefold or more.
An important finding from this body of work is that, in practice, a small fraction of the population contributes disproportionately to R0. This has been expressed as the ‘20–80 rule’ (Woolhouse et al. 1997) which asserts that removing from the contact network the 20% of the population contributing most to R0 will reduce R0 by at least 80%. The 20–80 rule supports the concept of targeted interventions aimed at those individuals making the greatest contribution to R0, noting that a fundamental requirement for a successful control programme is to reduce R0 below 1 (Anderson & May 1991).
One example of a contact network, applicable specifically to infectious diseases of livestock, is the pattern of livestock movements between farms. In this context, an ‘individual’ is a single farm holding and a ‘contact’ is the movement of one or more animals from one holding to another. Such movements are thought to be a risk factor for the spread of various livestock diseases including bovine rhinotrachetis (van Schaik et al. 1998), Neospora caninum (Mainar-Jaime et al. 1999), scrapie (Hoinville et al. 2000), foot-and-mouth disease (Gibbens et al. 2001), Escherichia coli O157 (Schouten et al. 2004) and bovine tuberculosis (Gilbert et al. 2005).
An important feature of livestock movements between farm holdings is that they are uni-directional: movement of an animal from holding i to holding j represents a risk of disease transmission from i to j but not (usually) from j to i. Other mechanisms of disease transmission are usually regarded as bi-directional: for example, sexual contact for HIV/AIDS or mosquito feeding for malaria. For some transmission routes, the relationship between acquiring and passing on infection is more ambiguous: for example, the transmission of human schistosomiasis involves contamination with urine or faeces of water containing intermediate host snails which is subsequently used for bathing or washing; i.e. different activities, but ones which are, in practice, often associated.
Data on livestock movements therefore offer a rare opportunity to investigate the impact of heterogeneities in the contact network in a situation where the risk of acquiring infection and the risk of passing infection on can be clearly distinguished.
2. Data and analysis
Fifty-five holdings were randomly selected for this study from a list of Scottish beef holdings provided by the Scottish Executive Environment and Rural Affairs Department (SEERAD). Cattle movements for these holdings that took place between 1 January 2002 and 31 December 2002 were obtained from a subset of the Department of the Environment, Food and Rural Affairs (DEFRA) Cattle Tracing System (CTS), a record of the movements of all British cattle. The CTS gives individual details of animal ear tag number, birth date, death date, breed, sex, and the time, origin and destination of all movements between holdings.
Here, a ‘movement off’ was defined as an animal moving from one of the holdings examined to another holding (excluding slaughterhouses), and a ‘movement on’ as an animal moving from any holding to one of the holdings examined. For the purposes of this study, we wished to exclude transient movements, so if an animal moved to holding j from holding i via holding k, where it stayed for less than 4 days (as would normally be the case if, for example, k was a market) this was treated as a single movement from i to j. This makes the analysis more immediately relevant to chronic infectious diseases (e.g. bovine tuberculosis) than acute infections (e.g. foot-and-mouth disease).
The CTS data provide information on two distinct aspects of potential disease transmission: the number of holdings per unit time from which cattle are moved on to a given holding, designated β1; and the number of holdings per unit time to which cattle are moved from a given holding, designated β2. Following Woolhouse et al. (1998), R0 (ignoring other terms) is a function of the means and standard deviations of β1 and β2, and of the linear correlation coefficient between them
where σ(•) represents the standard deviation, r is the linear correlation coefficient, N is the number of farms and the middle term represents the average of the cross-products over all holdings. According to this expression, heterogeneities in contact rates (i.e. positive variance in β1 and/or β2) may increase R0 (if β1 and β2 are positively correlated), have no effect on R0 (if they are uncorrelated or if either has zero variance), or may decrease R0 (if they are negatively correlated).
Statistics were computed using the software package S-Plus (Insightful 2001). The contribution of individual holdings to R0 was assessed by removing holdings (i.e. setting β1=β2=0 for each one removed) in descending order of the value of the product of β1 and β2.
3. Results
During the 12 month period of interest, 4183 cattle were moved from 843 other holdings on to the 55 holdings, and 1924 cattle were moved on to 374 other holdings from the 55 holdings (with negligible numbers of movements within the set of 55). The number of holdings from which cattle were moved to a given holding, β1, had an average value of 16.4, and the number of holdings to which cattle were moved from a given holding, β2, had an average value of 7.6. The distributions of both β1 and β2 were highly overdispersed with the variances (1055.9 and 102.5, respectively) greatly in excess of the means.
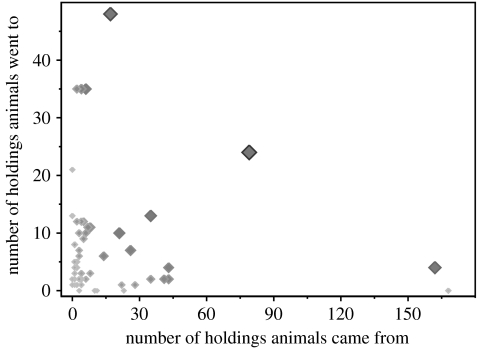
If β1 and β2 were perfectly positively correlated, i.e. r=1, then the observed heterogeneities in β1 and β2 would be expected to have a substantial impact on R0, increasing its value by a factor of 3.64 compared with its value if β1 and β2 had zero variance. However, in practice, β1 and β2 were not significantly correlated (r=−0.06 with 95% confidence limits −0.28 to +0.14; figure 1), and a substantial increase in R0 is therefore not expected (central estimate: 16% decrease; 95% confidence limits: 73% decrease to 37% increase).
Figure 1.
Co-distribution of numbers of different holdings from which cattle were moved on to a given holding (horizontal axis) and of numbers of different holdings to which cattle were moved to from a given holding (vertical axis). The slight negative correlation is not statistically significant. Increased size and darkness of points indicates increased contribution to R0 (see figure 2).
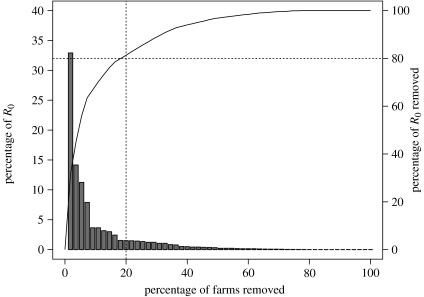
The effects of removing holdings from the contact network, in descending order of the value of β1β2, are shown in figure 2. Removing just 20% of holdings in this fashion decreases R0 by more than 80%.
Figure 2.
Contributions of individual holdings to R0. Histogram shows the distribution of the product β1β2 for individual holdings, arranged in descending order (left vertical axis). Solid line shows the relationship between the percentage of holdings removed from the sample dataset and the cumulative percentage reduction of R0 as a consequence (right vertical axis, see text). Dashed vertical line corresponds to removal of 20% of holdings and the dashed horizontal line to 80% reduction of R0.
4. Discussion
The mean rate of contact between holdings is one determinant of the basic reproduction number, R0, for infectious diseases which can be transmitted by livestock movements. The results presented here indicate that there is a high rate of contact between cattle holdings, with each holding, on average, sending cattle to or receiving cattle from over 20 other holdings per year. However, the contact rate is highly overdispersed, with most holdings making a relatively small number of contacts per year but a few making a much larger number (figure 1). Although previous work (e.g. Woolhouse et al. 1997) has suggested that such heterogeneities in contact rates can substantially increase the absolute value of R0, for this system this appears not to be the case, because the risk of acquiring infection via cattle movement is uncorrelated with the risk of passing infection via the same route. This result is possible in principle for any transmission system where the processes of acquiring infection and of passing infection on are distinct. Nevertheless, individual cattle holdings do make different contributions to R0 (as indicated here by the value of the product of numbers of sources and numbers of destinations) and the population as a whole still obeys the 20–80 rule: 20% of holdings contribute at least 80% of R0 (figure 2).
The pattern of contacts between cattle holdings has been described here in terms simply of whether or not any cattle are moved from one holding to another or vice versa during a 12 month period: the analysis ignores differences in the strength of the contacts, which might reflect the numbers of cattle or batches of cattle moved between holdings, though these could be incorporated in principle. The pattern of contacts observed reflects the structure of the British cattle farming industry, and would not necessarily be similar for other livestock species in Britain, or for livestock farming in other regions of the world.
The results have a number of implications for reducing the potential for the spread of infectious diseases through cattle movements. First, reducing the mean rate of movement will act to reduce R0, and this remains an important option for disease control and prevention. Second, because there is considerable heterogeneity in how many contacts a given holding has with other holdings, targeting particular holdings or categories of holding is likely to be an efficient intervention strategy. The holdings to be targeted are those which contribute most to R0, as indicated by the product of the number of sources from which they receive cattle and the number of destinations to which they send cattle (figures 1 and 2).
More generally, the results illustrate the need for studies of the relationship between the contact network and the potential spread of infectious diseases in order to consider explicitly the extent to which the risk of acquiring infection and of the risk of passing infection on are correlated.
Acknowledgments
The authors thank the Wellcome Trust for funding, DEFRA and SEERAD for access to data, Nicola Batchelor and Robert Webster for technical assistance and Loeske Kruuk for helpful discussions.
References
- Anderson R.M, May R.M. Oxford Scientific Press; 1991. Infectious diseases of humans: dynamics and control. [Google Scholar]
- Gibbens J.C, Sharpe C.E, Wilesmith J.W, Mansley L.M, Michalopoulou E, Ryan J.B.M, Hudson M. Descriptive epidemiology of the 2001 foot-and-mouth disease epidemic in Great Britain: the first five months. Vet. Rec. 2001;149:729–743. [PubMed] [Google Scholar]
- Gilbert M, Mitchell A, Bourn D, Mawdsley J, Clifton-Hadley R, Wint W. Cattle movements and bovine tuberculosis in Great Britain. Nature. 2005;435:491–496. doi: 10.1038/nature03548. [DOI] [PubMed] [Google Scholar]
- Gupta S, Anderson R.M, May R.M. Networks of sexual contacts: implications for the patterns of spread of HIV. AIDS. 1989;3:807–817. [PubMed] [Google Scholar]
- Hoinville L.J, Hoek A, Gravenor M.B, McLean A.R. Descriptive epidemiology of scrapie in Great Britain: results of a postal survey. Vet. Rec. 2000;146:455–461. doi: 10.1136/vr.146.16.455. [DOI] [PubMed] [Google Scholar]
- Lajmanovich A, Yorke J.A. A deterministic model for gonorrhea in a nonhomogeneous population. Math. Biosci. 1976;72:83–111. [Google Scholar]
- Mainar-Jaime R.C, Thurmond M.C, Berzal-Herranz B, Hietala S.K. Seroprevalence of Neospora caninum and abortion in dairy cows in northern Spain. Vet. Rec. 1999;145:72–75. doi: 10.1136/vr.145.3.72. [DOI] [PubMed] [Google Scholar]
- Schouten J.M, Bouwknegt M, van de Giessen A.W, Frankena K, De Jong M.C.M, Graat E.A.M. Prevalence estimation and risk factors for Escherichia coli O157 on Dutch dairy farms. Prev. Vet. Med. 2004;64:49–61. doi: 10.1016/j.prevetmed.2004.03.004. [DOI] [PubMed] [Google Scholar]
- van Schaik G, Dijkhuizen A.A, Huirne R.B.M, Schukken Y.H, Nielen M, Hage H.J. Risk factors for existence of bovine herpes virus 1 antibodies on nonvaccinating Dutch dairy farms. Prev. Vet. Med. 1998;34:125–136. doi: 10.1016/s0167-5877(97)00085-8. [DOI] [PubMed] [Google Scholar]
- Woolhouse M.E.J, Watts C.H, Chandiwana S.K. Heterogeneities in rates of transmission and the epidemiology of schistosome infection. Proc. R. Soc. B. 1991;245:109–114. doi: 10.1098/rspb.1991.0095. [DOI] [PubMed] [Google Scholar]
- Woolhouse M.E.J, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programmes. Proc. Natl Acad. Sci. USA. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse M.E.J, Etard J.-F, Dietz K, Ndhlovu P.D, Chandiwana S.K. Heterogeneities in schistosome transmission dynamics and control. Parasitology. 1998;117:475–482. doi: 10.1017/s003118209800331x. [DOI] [PubMed] [Google Scholar]