Abstract
Livestock grazing is a major driver of ecosystem change, and has been associated with significant declines in various bird species worldwide. In Britain, there is particular concern that severe grazing pressure is deleteriously affecting vegetation and birds in upland regions. However, the mechanism by which grazing affects birds is unclear. Here, we report for the first time, to our knowledge, that sheep grazing pressure affects the egg size of a common upland passerine: the meadow pipit Anthus pratensis. We manipulated sheep stocking densities in a replicated field experiment, and found that plots with the highest stocking density contained nests with the smallest eggs, and that plots with low stocking density contained nests with the largest eggs. However, eggs laid in ungrazed plots were also small, suggesting that either too many sheep or their removal from upland areas might have a detrimental effect on pipit egg size. We found no significant effect on fledging success but the reduced post-fledging survival of young from smaller eggs, as seen in other studies, could partly explain declines in upland birds.
Keywords: breeding success, egg size, grazing, passerine
1. Introduction
Livestock grazing is a major driver of land use change, both in Europe (Bardgett et al. 1995; Bignal & McCracken 1996) and North America (Fleischner 1994; Brown & McDonald 1995). Since the 1970s, there has been a large increase in sheep numbers in several European Union countries (Beaufoy et al. 1994). In Britain, sheep numbers more than doubled from 19.7 million to 41.2 million between 1950 and 1990, and the subsequent severe grazing pressure has been implicated in dramatic changes in vegetation and bird abundance in the British uplands (Thompson et al. 1995; Fuller & Gough 1999). Similarly, in North America, the loss and degradation of grassland habitats from grazing has been considered a primary cause of the severe population declines in grassland birds (Askins 1993; Knopf 1994; Vickery et al. 1999). However, the processes by which such declines occur have rarely been investigated and no studies, to our knowledge, have considered a life-history perspective for how livestock grazing affects reproductive performance in birds. In this paper we investigate for the first time the impact of livestock grazing on avian reproductive performance during both the egg and the nestling stage.
There are a number of alternative, non-exclusive hypotheses to account for how increasing grazing pressure could influence reproductive performance. Hypothesis 1 (H1) is that livestock grazing could affect bird reproduction through the direct effects of disturbance and trampling (Paine et al. 1996). In this case, we would expect to see an increase in total nest failures and lower breeding success as grazing pressure increased. Hypothesis 2 (H2) proposes that grazing could indirectly affect birds through altering vegetation structure (Pearce-Higgins & Grant 2002). If so, we would predict a higher incidence of nest failure due to exposure to weather and predators as grazing pressure increases. Hypothesis 3 (H3) suggests that grazing could reduce food abundance and availability (Dennis et al. 2001; Dennis et al. 2002; Hartley et al. 2003), so that increased grazing will limit resources for parents to allocate to eggs and young, resulting in fewer and/or smaller eggs, and lower fledging success, respectively. Alternatively, according to hypothesis 4 (H4), increased grazing could have no effect on reproductive performance, either because there is no negative impact, or because parents can compensate for increased costs.
We tested these hypotheses by experimentally manipulating sheep stocking densities. Our study species was the meadow pipit Anthus pratensis, a ground nesting, generalist insectivore (Cramp 1988) and the most common upland passerine in the UK.
2. Methods
A replicated, randomized-block experiment consisting of six replicates of four treatments was initiated in 2003 at Glen Finglas, in central Scotland (56°16′ N 4°24′W), with baseline data collected in 2002. Glen Finglas is a 4039 ha estate (1ha=104m2) grazed by sheep and cattle and typical of many upland areas of Scotland. Sheep density on the estate prior to the experiment was approximately 0.7 ewes ha−1. Plots were each approximately 3.3 ha in size with altitudes ranging from 200–500 m above sea level.
During the autumn of 2002, the plots were fenced and treatments of [I] 9 ewes per plot (2.72 ewes ha−1), [II] 3 ewes per plot (0.91 ewes ha−1), [III] 2 ewes per plot (0.61 ewes ha−1), and [IV] ungrazed, were randomly allocated to plots within each block. Within each plot, we recorded breeding meadow pipit territories using standard ‘Common Birds Census’ techniques, but restricted to this species (see Bibby et al. 1992) and searched for nests by rope-dragging. We recorded clutch size, and weighed (±0.1 g) and measured (length and breadth±0.1 mm) the eggs. We estimated egg volume (V) using Hoyt's (1979) equation
| (2.1) |
where L is the length (cm), B is the breadth at the equator (cm) and the volume coefficient Kv was 0.51.
We recorded nest location and altitude using a global positioning system receiver with an accuracy of ±5 m. Thereafter, we visited nests every 2–3 days to monitor egg and nestling fate. We calculated the laying date for each nest by subtracting 13 days for incubation (Cramp 1988) from hatching date. We examined the frequency of predation in each treatment using chi-squared tests in Minitab v. 13.32 (Minitab Inc., PA, USA).
We used Genstat v. 7.2 (VSN International Ltd, Hertfordshire, UK) to analyse egg volume and fledging success. Egg volumes were analysed using linear residual maximum likelihood. Fledging success was analysed using generalized linear mixed models with a binomial error structure and a logit link function. We used altitude of nest (metres above sea level), meadow pipit density in the plot (pairs ha−1) and clutch size as covariates in the models. Replicate, and the interaction between replicate and year, were included as categorical fixed effects. Fixed effects were treatment, defined both as a 4-level categorical variable and as a covariate (sheep density), and the linear and quadratic effects of laying date. Nest, plot and the plot×year interaction were included as random effects.
3. Results
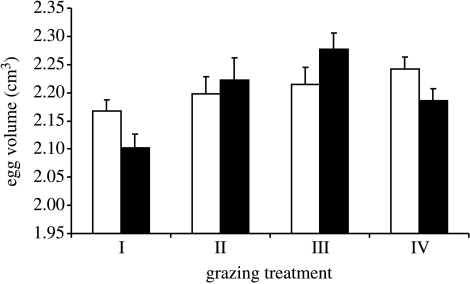
We found 120 nests, from 82 of which we could take egg measurements (the remaining nests contained nestlings only). For 2002 and 2003, total rainfall was 558.5 and 426.5 mm, and average minimum daily temperature was 6.5 (±3.58 s.d.) and 8.7 °C (± 3.96 s.d.) respectively, between 16 April and 26 July. In 2003, there were no statistically significant differences in the proportion of predated nests between treatments (chi-squared test: egg stage χ32=4.36, p=0.225; nestling stage χ32=0.11, p=0.990; table 1). However, egg volume varied significantly between treatments (F1,15=6.81, p=0.02; table 2, figure 1). There was also a significant effect of altitude when adding altitude before replicate in the model, with smaller eggs laid at higher altitudes (F1,15=14.02, p<0.001; table 2). However, this effect was due to differences between the three pairs of replicates, as adding altitude after replicate was not significant (F1,15=0.18, p>0.5). We also found no effect of breeding density, laying date or clutch size on egg volume. We found no effect of egg volume, treatment, or any other factor on the number of eggs that produced a nestling that fledged.
Table 1.
Clutch size and predation (partial and complete) of meadow pipit nests within experimental grazing treatments in 2003.
| egg stage | nestling stage | |||||
|---|---|---|---|---|---|---|
| treatment | n | mean clutch size | s.e. | proportion predated | n | proportion predated |
| I | 16 | 4.25 | 0.14 | 0.18 | 19 | 0.16 |
| II | 7 | 3.86 | 0.34 | 0 | 14 | 0.14 |
| III | 11 | 4.27 | 0.14 | 0.09 | 16 | 0.13 |
| IV | 15 | 3.93 | 0.15 | 0 | 16 | 0.13 |
n, the number of nests; s.e., standard error of the mean.
Table 2.
Models analysing factors affecting egg volume and fledging success in meadow pipits.
| egg size | fledging success | |||||
|---|---|---|---|---|---|---|
| fixed term | W | d.f. | p | W | d.f. | p |
| altitude | 14.02 | 1 | <0.001 | 0.35 | 1 | 0.552 |
| breeding density | 0.24 | 1 | 0.624 | 0.21 | 1 | 0.646 |
| clutch size | 0.67 | 1 | 0.411 | 1.65 | 1 | 0.199 |
| year | 0.42 | 1 | 0.519 | 0.00 | 1 | 0.982 |
| replicate | 1.30 | 5 | 0.262 | 0.38 | 5 | 0.864 |
| year×replicate | 1.14 | 4 | 0.337 | 0.50 | 4 | 0.739 |
| lay date | 2.96 | 1 | 0.085 | 0.06 | 1 | 0.802 |
| (lay date)2 | 0.29 | 1 | 0.588 | 1.19 | 1 | 0.274 |
| sheep density | 6.81 | 1 | <0.01 | 1.82 | 1 | 0.177 |
| treatment | 1.29 | 2 | 0.274 | 1.90 | 2 | 0.150 |
| egg size | — | — | — | 0.07 | 1 | 0.785 |
Egg volume data were fitted to a linear mixed model; fledging success data (i.e. number of eggs that produce a nestling that fledges) were fitted to a generalized linear mixed model, with a binomial distribution and logit link function. Fixed terms were fitted sequentially. The significance of the scaled Wald statistics (W) was assessed using F-tests (with denominator degrees of freedom set at 15 by the experimental design) for altitude, breeding density, year, year×replicate, sheep density and treatment, and using chi-squared tests for clutch size, lay date and egg size effects, as there was considerable replication at the nest level. n=82 nests.
Figure 1.
The effects of sheep grazing intensity on pipit egg volume (means+s.e. calculated after initially averaging over eggs within each plot by year combination) pre-treatment (2002, white) and post-treatment (2003, black) commencement at Glen Finglas, Scotland. (Treatment I=9 ewes per plot; treatment II=3 ewes per plot; treatment III=2 ewes per plot; treatment IV=ungrazed.)
4. Discussion
We have demonstrated for the first time that sheep grazing pressure affects the egg size of a common upland passerine. Intensively grazed plots (i.e. high stocking density) contained nests with the smallest meadow pipit eggs, and extensively grazed plots (i.e. low stocking density) contained nests with the largest eggs. Interestingly, ungrazed plots contained nests with smaller eggs than lightly grazed plots, demonstrating that both too many sheep and the complete removal of sheep in the uplands adversely affect meadow pipit egg size. We could find no effect of egg size or grazing treatments on fledging success. It is possible that meadow pipits were able to compensate for the negative effects of small eggs through greater parental care (Ricklefs 1984), but equally, such effects might not become apparent until later in life (e.g. post-fledging survival). These results support the hypothesis that grazing pressure affects food availability and hence limits the amount of resources that parents can allocate to egg production (H3). We can reject the hypothesis that increased grazing has no effect on reproductive performance (H4). Also, our results provide little support for the hypotheses that the adverse effects of grazing pressure act primarily through disturbance (H1) or changes in vegetation structure (H2).
Mechanisms determining pipit egg size remain unclear. It is not known to what extent livestock grazing affects pipit territory, and hence parental quality, through altering food abundance or vegetation structure. While our results support the hypothesis that food availability was affected by grazing, the hypotheses are not independent. Indeed, a plausible explanation for our treatment effect is that food availability is a function of both food abundance and vegetation structure (Smith & Rotenberry 1990). Temperature and nest microclimate could also be important factors that may influence egg size. Further research is necessary to explain the relative importance of each of these factors.
The amount of effort allocated to egg production and incubation may have an important role in determining parental fitness (Monaghan & Nager 1997). Larger eggs are typically considered advantageous as egg size is positively correlated with hatchling size, nestling growth and survival in a range of species (see Martin 1987 for a review). However, egg size may be a characteristic of individual females and most studies have not controlled for the effects of parental traits (Williams 1994; Christians 2002). Yet the female traits that determine egg size are not clear. We do not yet have the data to examine the effect of livestock grazing on parental condition, how female traits affect egg size, nor the longer-term consequences of hatching from small eggs. However, it follows from other work that generally there are disadvantages of hatching from a smaller egg, and these could ultimately have an adverse effect on offspring fitness. Potentially therefore, our study could give a partial explanation for the observed link between increased grazing pressure and declines in grassland birds in both North America and Europe.
Acknowledgments
We thank Adam Wallace and Jane Begg of the Woodland Trust. The research was conducted as part of a collaborative project funded by the Scottish Executive Environment and Rural Affairs Department (GRUB).
References
- Askins R.A. Population trends in grassland, shrubland, and forest birds in eastern North America. Curr. Ornithol. 1993;11:1–34. [Google Scholar]
- Bardgett R.D, Marsden J.H, Howard D.C. The extent and condition of heather on moorland in the uplands of England and Wales. Biol. Conserv. 1995;71:155–161. [Google Scholar]
- Beaufoy G, Baldock D, Clark J. Institute for European Environmental Policy; London: 1994. The nature of farming: low intensity farming in nine European countries. [Google Scholar]
- Bibby C.J, Burgess N.D, Hill D.A. Academic Press; London: 1992. Bird census techniques. [Google Scholar]
- Bignal E.M, McCracken D.I. Low-intensity farming systems in the conservation of the countryside. J. Appl. Ecol. 1996;33:413–424. [Google Scholar]
- Brown J.H, McDonald W. Livestock grazing and conservation on southwestern rangelands. Conserv. Biol. 1995;9:1644–1647. [Google Scholar]
- Christians J.K. Avian egg size: variation within species and inflexibility within individuals. Biol. Rev. Camb. Phil. Soc. 2002;77:1–26. doi: 10.1017/s1464793101005784. [DOI] [PubMed] [Google Scholar]
- Cramp S. Oxford University Press; Oxford, UK: 1988. Handbook of the birds of Europe, the Middle East and North Africa: the birds of the western Palearctic. [Google Scholar]
- Dennis P, Young M.R, Bentley C. The effects of varied grazing management on epigeal spiders, harvestmen and pseudoscorpions of Nardus stricta grassland in upland Scotland. Agric. Ecosyst. Environ. 2001;86 [Google Scholar]
- Dennis P, Aspinall R.J, Gordon I.J. Spatial distribution of upland beetles in relation to landform, vegetation and grazing management. Basic Appl. Ecol. 2002;3:183–193. [Google Scholar]
- Fleischner T.L. Ecological costs of livestock grazing in western North America. Conserv. Biol. 1994;8:629–644. [Google Scholar]
- Fuller R.J, Gough S.J. Changes in sheep numbers in Britain: implications for bird populations. Biol. Conserv. 1999;91:73–89. [Google Scholar]
- Hartley S.E, Gardner S.M, Mitchell R.J. Indirect effects of grazing and nutrient addition on the hemipteran community of heather moorlands. J. Appl. Ecol. 2003;40:793–803. [Google Scholar]
- Hoyt D.F. Practical methods of estimating volume and fresh weight of bird eggs. Auk. 1979;96:73–77. [Google Scholar]
- Knopf F.L. Avian assemblages on altered grasslands. Stud. Avian Biol. 1994;15:247–257. [Google Scholar]
- Martin T.E. Food as a limit on breeding birds: a life-history perspective. Annu. Rev. Ecol. Syst. 1987;18:453–487. [Google Scholar]
- Monaghan P, Nager R.G. Why don't birds lay more eggs? Trends Ecol. Evol. 1997;12:270–274. doi: 10.1016/s0169-5347(97)01094-x. [DOI] [PubMed] [Google Scholar]
- Paine L, Undersander D.J, Sample D.W, Bartelt G.A, Schatteman T.A. Cattle trampling of simulated ground nests in rotationally grazed pastures. J. Range Manag. 1996;49:294–300. [Google Scholar]
- Pearce-Higgins J.W, Grant M.C. The effects of grazing-related variation in habitat on the distribution of moorland skylarks Alauda arvensis and meadow pipits Anthus pratensis. Asp. Appl. Biol. 2002;67:155–163. [Google Scholar]
- Ricklefs R.E. Components of variance in measurements of nestling European starlings (Sturnus vulgaris) in southeastern Pennsylvania. Auk. 1984;101:319–333. [Google Scholar]
- Smith K.G, Rotenberry J.T. Quantifying food resources in avian studies: present problems and future needs. In: Morrison M.L, Ralph C.J, Verner J, Jehl J.R Jr, editors. Avian foraging: theory, methodology and applications. vol. 13. Cooper Ornithological Society; California: 1990. pp. 3–5. [Google Scholar]
- Thompson D.B.A, MacDonald A.J, Hudson P.J. Upland heaths and moors. In: Sutherland W.J, Hill D.A, editors. Managing habitats for conservation. Cambridge University Press; Cambridge, UK: 1995. pp. 292–326. [Google Scholar]
- Vickery P.D, Tubaro P.L, da Silva J.M.C, Peterjohn P.G, Herkert J.R, Calvalcanti R.B. Conservation of grassland birds in the western hemisphere. In: Vickery P.D, Herkert J.R, editors. Ecology and Conservation of Grassland Birds of the Western Hemisphere, 1999. Vol. 19. Cooper Ornithological Society; Camarillo, CA: 1999. pp. 2–26. Studies in Avian Biology. [Google Scholar]
- Williams T.D. Intraspecific variation in egg size and egg composition in birds—effects on offspring fitness. Biol. Rev. Camb. Phil. Soc. 1994;68:35–59. doi: 10.1111/j.1469-185x.1994.tb01485.x. [DOI] [PubMed] [Google Scholar]