Abstract
Evidence is accumulating that genetic variation within individual hosts can influence their susceptibility to pathogens. However, there have been few opportunities to experimentally test this relationship, particularly within outbred populations of non-domestic vertebrates. We performed a standardized pathogen challenge in house finches (Carpodacus mexicanus) to test whether multilocus heterozygosity across 12 microsatellite loci predicts resistance to a recently emerged strain of the bacterial pathogen, Mycoplasma gallisepticum (MG). We simultaneously tested whether the relationship between heterozygosity and pathogen susceptibility is mediated by differences in cell-mediated or humoral immunocompetence. We inoculated 40 house finches with MG under identical conditions and assayed both humoral and cell-mediated components of the immune response. Heterozygous house finches developed less severe disease when infected with MG, and they mounted stronger cell-mediated immune responses to phytohaemagglutinin. Differences in cell-mediated immunocompetence may, therefore, partly explain why more heterozygous house finches show greater resistance to MG. Overall, our results underscore the importance of multilocus heterozygosity for individual pathogen resistance and immunity.
Keywords: multilocus heterozygosity, pathogen resistance, house finches, Mycoplasma gallisepticum, immunocompetence
1. Introduction
The idea that host genetic variation mediates pathogen susceptibility originated over half a century ago (Haldane 1949), yet evidence from natural populations remains scarce (reviewed in Frankham et al. 2002). Individual (e.g. Acevedo-Whitehouse et al. 2003), population (e.g. Meagher 1999) and species (e.g. Poulin et al. 2000) level correlations between host genetic variation and pathogen prevalence provide indirect evidence that less diverse hosts are more susceptible to many pathogens. However, correlative studies are limited by their inability to detect individuals who have either recovered or died from infection in the wild. Challenge experiments of randomly selected individuals allow more direct inference of causation, and this experimental approach is therefore a powerful means of verifying relationships between genetic variation and pathogen susceptibility.
In this study, we performed a challenge experiment to test whether multilocus heterozygosity in an outbred vertebrate host, the house finch (Carpodacus mexicanus), influences resistance to a recently emerged disease. In 1994, a novel strain of a poultry bacterial pathogen, Mycoplasma gallisepticum (MG), emerged in house finches in eastern North America and quickly reached epidemic levels (Ley et al. 1996; Fischer et al. 1997; Dhondt et al. 1998). MG infections in house finches cause severe conjunctivitis and significantly reduce overwinter survival (Faustino et al. 2004). We experimentally infected 40 house finches under identical captive conditions to test whether multilocus heterozygosity affects MG resistance. We also investigated whether immunocompetence mediates this relationship by simultaneously testing whether multilocus heterozygosity is associated with the strength of immune response to a humoral or cell-mediated assay.
2. Material and methods
(a) Pre-experiment
We trapped 60 juvenile house finches in Tompkins County, NY, USA between June and October in 2001 and 2003 and between July and August 2004 under New York State (LCP 99-039) and US Fish and Wildlife Service (PRT 802829) permits. In 2003 and 2004, we measured tarsus length (0.1 mm) and mass (0.1 g) upon capture. To ensure that all individuals were not previously exposed to MG, we swabbed both conjunctival sacs and submitted samples to North Carolina State University Poultry Health Management Laboratory (D. Ley) for polymerase chain reaction (PCR) analysis of MG presence (Lauerman 1998). We also took blood samples upon capture and tested for the presence of MG-specific antibodies using rapid plate agglutination (Kleven 1998).
We housed birds at constant temperature (21–24 °C) in individual cages (45.7×45.7×76.2 cm) in a biosafety level 2 facility at the College of Veterinary Medicine, Cornell University. We provided clean water and a pelleted diet (Rodebush, Inc., Cameron Park, CA, USA) ad libitum. All experimental procedures were approved by Cornell University's Institutional Animal Care and Use Committee (00-90).
(b) Experimental challenge
We performed two independent challenge experiments in November 2001 and in November 2003. In 2001, we infected 20 individuals (1 : 1 sex ratio) with a single MG dose (3.24×105 colony-forming units (CFUs) per ml). In 2003, we infected 20 individuals (1 : 1 sex ratio) with one of two doses (3.24×103 or 104 CFUs per ml). We inoculated both eyes with 0.05 ml of the assigned dose suspended in Frey's medium (sixth in vitro passage from the original house finch MG isolate, ADRL 7994-1; Ley et al. 1996). Prior to inoculation, no individuals tested positive for MG presence in the conjunctiva or MG antibodies in the plasma, but all 40 individuals tested PCR positive for MG presence by day 7 post-inoculation. We scored the severity of the inflammatory response in each eye on a 0–3 descriptive scale (Kollias et al. 2004) for 70 days post-inoculation and summed these scores as a measure of disease severity for each individual. The single individual that died during the 2001 experiment was excluded from all analyses.
(c) Immune assays
Following the 2003 challenge experiment, we performed two immune assays when the 20 individuals no longer showed physical symptoms of disease. We also assayed an additional 20 uninfected individuals housed under identical conditions to test of whether MG infection caused immune suppression. For the humoral assay, we intraperitoneally injected individuals with 5×107 sheep red blood cells (SRBCs; MP Biomedicals, Irvine, CA, USA) suspended in 100 μl phosphate-buffered saline (PBS). We took blood samples prior to and 8 days after injection. We quantified antibody titre as the reciprocal of the highest log2 dilution at which an individual's plasma showed positive haemagglutination, subtracting pre-injection titres from post-injection titres. For the cell-mediated immune assay, we quantified T-cell proliferation in response to phytohaemagglutinin (PHA) using a protocol described by Smits et al. (1999). We injected the right patagium (wing web) of each individual with 50 μl of a 0.2 mg ml−1 PHA suspension (Sigma-Aldrich, St Louis, MO, USA) in PBS. We measured patagial width at the site of injection to 0.01 mm using digital callipers prior to and 48 h after injection. We measured each individual three times and averaged measurements to obtain a single pre- and post-injection value, and we divided post-injection width by pre-injection width to obtain a measure of PHA response which standardizes for initial patagial size. We excluded two individuals who showed bruising at the injection site from the PHA analyses.
(d) Genetic and statistical analyses
We quantified multilocus heterozygosity across 12 polymorphic, unlinked microsatellite markers, six that we developed for house finches and six from other species (table 1). All markers had a null allele frequency less than 0.10 in our population. Briefly, we used PCR to amplify dye-labelled microsatellite fragments and quantified fragment size using an ABI 3100 (Applied Biosystems) and the analysis program Genemapper 3.0 (Applied Biosystems). We calculated multilocus heterozygosity as the proportion of markers at which an individual had alleles of different repeat number. To test whether the detected relationships were driven by a single locus, we also calculated multilocus heterozygosity with each locus removed.
Table 1.
Characterization of the 12 microsatellite markers used.
| locus | K | HE | HO | primer reference |
|---|---|---|---|---|
| Hofi 3 | 14 | 0.78 | 0.86 | Hawley (2005) |
| Hofi 5 | 11 | 0.85 | 0.74 | Hawley (2005) |
| Hofi 20 | 11 | 0.86 | 0.76 | Hawley (2005) |
| Hofi 23 | 8 | 0.81 | 0.77 | Hawley (2005) |
| Hofi 24 | 6 | 0.46 | 0.48 | Hawley (2005) |
| Hofi 52 | 9 | 0.83 | 0.83 | Hawley (2005) |
| VeCr 06 | 7 | 0.80 | 0.76 | Stenzler et al. (2004) |
| Lox 2 | 18 | 0.90 | 0.81 | Piertney et al. (1998) |
| Lox 3 | 5 | 0.73 | 0.60 | Piertney et al. (1998) |
| Tc.11B4E | 6 | 0.74 | 0.81 | Tarr et al. (1998) |
| Tc.12B5E | 15 | 0.88 | 0.87 | Tarr et al. (1998) |
| GCSW 10 | 3 | 0.40 | 0.33 | McRae et al. (2005) |
Number of alleles (K), expected heterozygosity (HE) and observed heterozygosity (HO) based on 40 individuals.
We performed all statistical analyses in JMP 5.0 and SAS 8.0 (SAS Institute). We used linear regression to test whether multilocus heterozygosity affects disease severity, and non-parametric Kendall Tau b (Tb) correlations to examine relationships between multilocus heterozygosity and immune response. We square-root transformed disease severity to meet the assumptions of linear regression, and we included a year effect in all analyses to account for the combination of two challenge experiments. We bootstrapped confidence intervals around regression coefficients using 10 000 iterations of sampling with replacement. Since disease severity did not vary across the three MG doses (F2,40=0.33, p=0.72), we pooled doses for all analyses. Brown et al. (1999) provide an analogous example of dosage insensitivity in experimental infections of tortoises with Mycoplasma agassizii.
3. Results
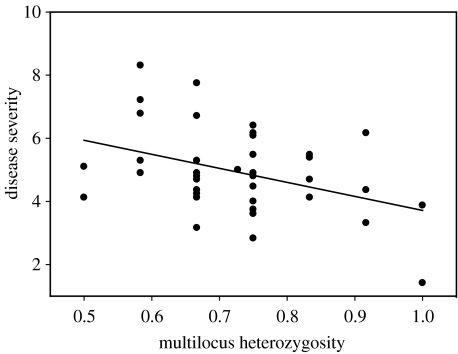
Multilocus heterozygosity predicted disease severity across individuals (figure 1; bHet=−4.31; r2=0.19, F1,36=6.50, p=0.015). Bootstrapped confidence limits around bHet were −8.33 and −0.70, with a median of −4.38. When we removed each locus from our calculations of heterozygosity, bHet ranged from −3.52 to −4.92, and F-tests ranged in significance from 0.005 to 0.065. Multilocus heterozygosity did not predict individual condition (mass/tarsus; r2=0.02, F1,36=0.60, p=0.44).
Figure 1.
Multilocus heterozygosity predicts disease severity in response to infection with Mycoplasma gallisepticum. We scored the severity of inflammatory symptoms for 70 days post-inoculation and summed these scores as a measure of disease severity. Disease severity was square-root transformed prior to analysis.
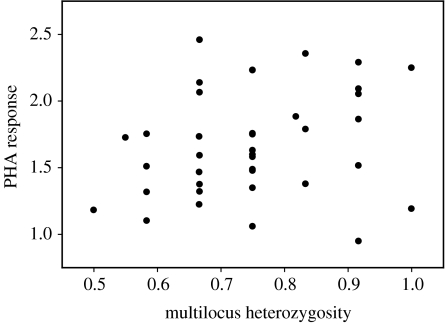
Multilocus heterozygosity was positively related to PHA response (figure 2; Tb=0.23, n=38, p=0.05) but not to SRBC antibody titre (Tb=0.08, n=40, p=0.50). PHA and SRBC responses were not correlated across individuals (Tb=−0.04, n=38, p=0.70). Furthermore, PHA response did not significantly correlate with disease severity across the 18 individuals for which we had all three measures (Tb=−0.20, n=18, p=0.25). Immune responses of the infected and uninfected individuals were comparable for both assays (unequal variances: t39<0.75, p>0.46), indicating that MG infection did not compromise the legitimacy of either assay.
Figure 2.
Multilocus heterozygosity correlates with T-lymphocyte proliferation in response to phytohaemagglutinin (PHA) injection. We calculated PHA response as the ratio of post-injection (48 h) patagial width to pre-injection width.
4. Discussion
We found that more heterozygous house finches show less severe disease expression in response to experimental inoculation with MG, a recently emerged pathogen in this wild bird species. Although previous studies have found similar relationships between individual genetic variation and pathogen intensity (Coltman et al. 1999; Cassinello et al. 2001; Acevedo-Whitehouse et al. 2003), our study is the first, to our knowledge, to show this relationship in an outbred vertebrate using standardized inoculation in controlled conditions. Our experimental approach eliminates confounding social and environmental factors that may explain the higher pathogen prevalence of more homozygous individuals in the wild.
Multilocus heterozygosity does not directly influence pathogen susceptibility when the measured loci are selectively neutral, as is the case for microsatellites. Instead, multilocus heterozygosity acts via associative overdominance; that is, apparent heterozygote advantage due to correlations between heterozygosity at neutral loci and loci under selection (reviewed in Hansson & Westerberg 2002). The extent of the genome across which these correlations occur is a topic of recent interest, and in some cases, it appears that multilocus heterozygosity best reflects ‘local effects’ or apparent heterozygote advantage at closely linked genes, rather than the entire genome (Hansson & Westerberg 2002). Owing to the recent rapid expansion of the eastern house finch population (Hill 1993), a demographic process that increases linkage disequilibrium, the ‘local effects’ hypothesis is an important candidate mechanism for the heterozygosity–fitness correlations observed here. However, our post hoc test for local effects, whereby we calculated multilocus heterozygosity with each of the 12 loci removed, provides evidence against strong single-locus effects in this study.
Results from the immune assays suggest that differences in cell-mediated immunity partly underlie the relationship between multilocus heterozygosity and disease severity; heterozygosity was related to PHA response but not SRBC response, an index of humoral immunity. That our results differed between assays may reflect the limitations of using single immune assays to draw inference about generalized humoral or cell-mediated immunocompetence (Adamo 2004). Alternatively, the results may indicate meaningful differences in the way that multilocus heterozygosity influences each component of the adaptive immune response. For example, multilocus heterozygosity may correlate with co-dominant expression at major histocompatibility complex genes, which code for receptors on the T lymphoctyes that are stimulated during a PHA response (Goto et al. 1978; Roitt 1997). Interestingly, the only other study that has examined genetic variation and cell-mediated immunity in a wild bird found that inbreeding levels strongly influenced the strength of PHA response (Reid et al. 2003).
Overall, we found that multilocus heterozygosity in house finches predicts resistance to a recently emerged bacterial disease under controlled conditions. Furthermore, multilocus heterozygosity was related to cell-mediated immunocompetence, suggesting that the relationship between heterozygosity and susceptibility may extend to the many classes of pathogens that stimulate cellular components of resistance. The effects of genetic variation on pathogen resistance in our study system may be particularly severe owing to the fact that eastern North American house finches are the descendants of a small number of birds introduced around 1940 (Elliott & Arbib 1953). The extent to which population-level genetic diversity affects MG resistance in house finches is an exciting avenue for future research, particularly because the MG pathogen recently spread to native house finch populations in western North America (Duckworth et al. 2003).
Acknowledgments
We thank W. Hochachka for statistical assistance, D. Ley for inoculum, T. Muscato and E. Swarthout for assistance with bird capture, S. McCrae and L. Stenzler for laboratory help and I. Lovette, K. Schat, D. Ardia, T. Coulson and several anonymous reviewers for useful comments on the manuscript. Our work was supported by NSF Doctoral Dissertation Improvement grant DEB-0407543 to D.M.H. and NSF grant DEB-0094456 to A.A.D. under the Ecology of Infectious Diseases program.
References
- Acevedo-Whitehouse K, Gulland F, Grieg D, Amos W. Disease susceptibility in California sea lions. Nature. 2003;422:35. doi: 10.1038/422035a. [DOI] [PubMed] [Google Scholar]
- Adamo S.A. How should behavioural ecologists interpret measures of immunity? Anim. Behav. 2004;68:1443–1449. [Google Scholar]
- Brown M.B, McLaughlin G.S, Klein P.A, Crenshaw B.C, Schumacher I.M, Brown D.R, Jacobson E.R. Upper respiratory tract disease in the gopher tortoise is caused by Mycoplasma agassizii. J. Clin. Micobiol. 1999;37:2262–2269. doi: 10.1128/jcm.37.7.2262-2269.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassinello J, Gomendio M, Roldan E.R.S. Relationship between coefficient of inbreeding and parasite burden in endangered gazelles. Conserv. Biol. 2001;15:1171–1174. [Google Scholar]
- Coltman D.W, Pilkington J.G, Smith J.A, Pemberton J.M. Parasite-mediated selection against inbred Soay sheep in a free-living, island population. Evolution. 1999;53:1259–1267. doi: 10.1111/j.1558-5646.1999.tb04538.x. [DOI] [PubMed] [Google Scholar]
- Dhondt A.A, Tessaglia D.L, Slothower R.L. Epidemic mycoplasmal conjunctivitis in house finches from eastern North America. J. Wildl. Dis. 1998;34:265–280. doi: 10.7589/0090-3558-34.2.265. [DOI] [PubMed] [Google Scholar]
- Duckworth R.A, Badyaev A.V, Farmer K.L, Hill G.E, Roberts S.R. First case of Mycoplasma gallisepticum infection in the western range of the house finch (Carpodacus mexicanus) Auk. 2003;120:528–530. [Google Scholar]
- Elliott J.J, Arbib R.S. Origin and status of the house finch in the eastern United States. Auk. 1953;70:31–37. [Google Scholar]
- Faustino C.R, Jennelle C.S, Connolly V, Davis A.K, Swarthout E.C, Dhondt A.A, Cooch E.G. Mycoplasma gallisepticum infection dynamics in a house finch population: empirical analysis of seasonal variation in survival, encounter and transmission rate. J. Anim. Ecol. 2004;73:651–669. [Google Scholar]
- Fischer J.R, Stallknecht D.E, Luttrell M.P, Dhondt A.A, Converse K.A. Mycoplasmal conjunctivitis in wild songbirds: the spread of a new contagious disease in a mobile host population. Emerging Infect. Dis. 1997;3:69–72. doi: 10.3201/eid0301.970110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankham R, Ballou J.D, Brisco D.A. Cambridge University Press; 2002. Introduction to conservation genetics. [Google Scholar]
- Goto N, Kodama H, Okada K, Fujimoto Y. Suppression of phytohemagglutinin skin-response in thymectomized chickens. Poult. Sci. 1978;52:246–250. doi: 10.3382/ps.0570246. [DOI] [PubMed] [Google Scholar]
- Haldane J.B.S. Disease and evolution. La Ricerca Scientifica. 1949;19(Suppl. a La Anno):68–75. [Google Scholar]
- Hansson B, Westerberg L. On the correlation between heterozygosity and fitness in natural populations. Mol. Ecol. 2002;11:2467–2474. doi: 10.1046/j.1365-294x.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- Hawley, D. M. 2005 Isolation and characterization of eight microsatellite loci from the house finch (Carpodacus mexicanus). Mol. Ecol. Notes5, 443–445.
- Hill G.E. House finch (Carpodacus mexicanus) In: Poole A, Gill F, editors. The birds of North America, No. 46. The Academy of Natural Sciences; Philadelphia, PA: 1993. [Google Scholar]
- Kleven S.H. Mycoplasmosis. In: Swayne D.E, Glisson J.R, Jackwood M.W, Pearson J.E, Reed W.M, editors. A laboratory manual for the isolation and identification of avian pathogens. American Association of Veterinary Laboratory Diagnosticians; Turlock, CA: 1998. pp. 74–80. [Google Scholar]
- Kollias G.V, Sydenstricker K.V, Kollias H.W, Ley D.H, Hosseini P.R, Connolly V, Dhondt A.A. Experimental infection of house finches with Mycoplasma gallisepticum. J. Wildl. Dis. 2004;40:79–86. doi: 10.7589/0090-3558-40.1.79. [DOI] [PubMed] [Google Scholar]
- Lauerman L.H. Mycoplasma PCR assays. In: Lauerman L.H, editor. Nucleic acid amplification assays for diagnosis of animal diseases. American Association of Veterinary Laboratory Diagnosticians; Turlock, CA: 1998. pp. 41–42. [Google Scholar]
- Ley D.H, Berkhoff J.E, McLaren J.M. Mycoplasma gallisepticum isolated from house finches (Carpodacus mexicanus) with conjunctivitis. Avian Dis. 1996;40:480–483. [PubMed] [Google Scholar]
- McRae S.B, Emlen S.T, Rubenstein D.R, Bogdanowicz S.M. Polymorphic microsatellite loci in a plural bleeder, the grey-capped social weaver (Pseudonigrita arnaudi), isolated with an improved enrichment protocol using fragment size selection. Mol. Ecol. Notes. 2005;5:16–20. doi:10.1111/j.1471-8286.2004.00816 [Google Scholar]
- Meagher S. Genetic diversity and Capillaria hepatica (Nematoda) prevalence in Michigan deer mouse populations. Evolution. 1999;53:1318–1324. doi: 10.1111/j.1558-5646.1999.tb04547.x. [DOI] [PubMed] [Google Scholar]
- Piertney S.B, Marguiss M, Summers R. Characterization of tetranucleotide microsatellite markers in the Scottish crossbill (Loxia scotica) Mol. Ecol. 1998;7:1261–1263. [PubMed] [Google Scholar]
- Poulin R, Marshall L.J, Spencer H.G. Metazoan parasite species richness and genetic variation among freshwater fish species: cause or consequence? Int. J. Parasitol. 2000;30:697–703. doi: 10.1016/s0020-7519(00)00047-3. [DOI] [PubMed] [Google Scholar]
- Reid J.M, Arcese P, Keller L.F. Inbreeding depresses immune response in song sparrows (Melospiza melodia): direct and inter-generational effects. Proc. R. Soc. B. 2003;270:2151–2157. doi: 10.1098/rspb.2003.2480. doi:10.1098/rspb.2003.2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitt I. Blackwell Publishing; London: 1997. Essential immunology. [Google Scholar]
- Smits J.E, Bortolotti G.R, Tella J.L. Simplifying the phytohaemagglutinin skin-testing technique in studies of avian immunocompetence. Funct. Ecol. 1999;13:567–572. [Google Scholar]
- Stenzler L.M, Fraser R, Lovette I.J. Isolation and characterization of 12 microsatellite loci from golden-winged warblers (Vermivora chrysoptera) with broad cross-taxon utility in emberizine songbirds. Mol. Ecol. Notes. 2004;4:602–604. [Google Scholar]
- Tarr C.L, Conant S, Fleischer R.C. Founder events and variation at microsatellite loci in an insular passerine bird, the Laysan finch (Telespiza cantans) Mol. Ecol. 1998;7:719–731. [Google Scholar]