Abstract
The ability to separate edible from inedible portions of prey is integral to feeding. However, this is typically overlooked in favour of prey capture as a driving force in the evolution of vertebrate feeding mechanisms. In processing prey, cartilaginous fishes appear handicapped because they lack the pharyngeal jaws of most bony fishes and the muscular tongue and forelimbs of most tetrapods. We argue that the elaborate cranial muscles of some cartilaginous fishes allow complex prey processing in addition to their usual roles in prey capture. The ability to manipulate prey has evolved twice along different mechanical pathways. Batoid chondrichthyans (rays and relatives) use elaborate lower jaw muscles to process armored benthic prey, separating out energetically useless material. In contrast, megacarnivorous carcharhiniform and lamniform sharks use a diversity of upper jaw muscles to control the jaws while gouging, allowing for reduction of prey much larger than the gape. We suggest experimental methods to test these hypotheses empirically.
Keywords: Chondrichthyes, elasmobranch, feeding, functional morphology, asymmetry
1. Introduction
Processing prey is nearly as important as capturing it. Examination of the vertebrate feeding mechanism has been largely limited to studies of the prey capture apparatus, although certainly the head and jaws are also adapted for effective processing. If only edible fractions are to be swallowed, complex prey items (prey with digestible and indigestible portions) must be reduced to remove bones, spines, shells and other energetically useless chaff. Many tetrapods accomplish this task with unilateral mastication and manipulation with the hands and tongue. Though lacking hands and flexible tongue, most bony fishes effectively process prey with bony tongues or pharyngeal jaws. The latter act independently of oral jaws and can tear, crush, winnow and manipulate food (Wainwright 1987; Drucker & Jensen 1991). Teleost fishes also use the movement of water in the oropharyngeal cavity to process and position prey in both sets of jaws (Liem et al. 2001).
Cartilaginous fishes (figure 1) lack hands, a flexible tongue and pharyngeal jaws, yet several lineages process complex prey. The batoids (rays and relatives) and megacarnivores (non-filter feeding lamniform and carcharhiniform sharks) are particularly effective at manipulation, although they differ markedly in prey processing mode. Megacarnivores process prey through an external and mechanical method of reduction; as a result, they are the only sharks to attack prey much larger than their gape (with one bizarre exception, the cookiecutter shark, Isistius brasiliensis). Conversely, batoids are capable of an internal and largely hydrodynamic form of manipulation, permitting exploitation of benthic invertebrates with exoskeletons. We propose that this disparity has developed through differential elaboration of the cranial muscle groups controlling the jaws, resulting in increased flexibility and direct control of the lower jaw in batoids and the upper jaw in megacarnivores.
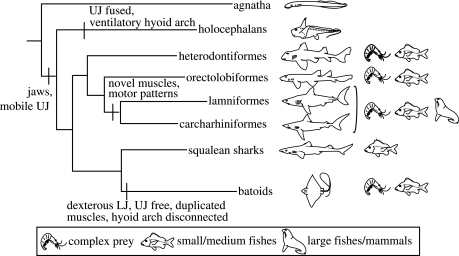
Figure 1.
Phylogeny of elasmobranchs and outgroups (from Shirai 1996). Differences in prey type (represented schematically to the right of the phylogeny) correlate with aspects of jaw mechanics. Increased direct control and mobility of the upper jaw (UJ) has evolved twice and led to increased processing capabilities. Batoids have derived lower jaw (LJ) mobility, allowing them to process complex prey internally, while basal sharks must spit and reingest prey in order to manipulate it. Carcharhiniform and lamniform sharks have evolved finer control of upper jaw protrusion mechanisms, allowing them to reduce large prey externally as compared with squalean sharks that swallow smaller prey whole. Jawless fishes (agnathans) and holocephalans exhibit less specialized musculature for control of the mouth and processing.
2. Cranial design
The ecologies, prey capture behaviours and morphologies of cartilaginous fishes are diverse. Chimaeras, some sharks and some rays have independently derived the ability to crush and process hard prey (such as crabs and bivalves). Most species of batoids and some basal sharks are suction feeders, and can separate and remove inedible material through oral cavity manipulation or repeated spitting and reingestion of food. Large megacarnivores can protrude their upper jaws repeatedly during a feeding event to gouge chunks from fast-moving or bulky prey (Motta 2004).
Despite a variety of feeding modes, the basic cranial design is universal: two visceral arches (figure 2), each controlled primarily by two groups of muscles. The mandibular arch consists of an upper and lower jaw, caudally supported to varying degrees by the hyoid arch, which is attached at its dorsal end to the cranium. On the floor of the oropharyngeal cavity, the ventral hyoid and branchial cartilages function as a reduced tongue. Each arch can be pulled dorsally by a series of levator muscles and ventrally by depressor muscles. Depressor muscles may also be involved indirectly in protrusion, if they are active simultaneously with muscles pulling the jaw arch forward. Coordinated contraction and relaxation of these muscle groups creates the arch expansions involved in all feeding modes.
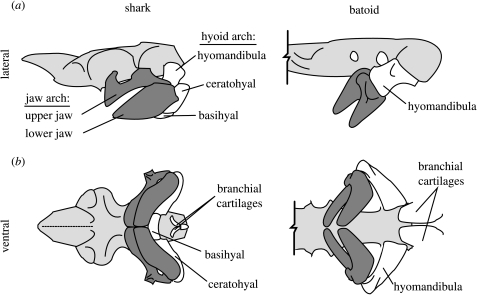
Figure 2.
Schematic cranial anatomy of a generalized shark (left) and batoid (right) in lateral (a) and ventral (b) views. Anterior is to the left and the cranium is light grey in all views. In elasmobranchs, the jaw arch (dark grey) is supported caudally in varying degrees by the hyoid arch. In batoids, only the hyomandibulae provide support and the ventral portions (ceratohyal and basihyal) of the hyoid arch are not associated with the jaws. Additionally, the ventral branchial cartilages may be split anteriorly, allowing independent movement of left and right sides of the branchium.
Protrusion of the upper jaw is fundamental in elasmobranch feeding, with protrusibility limited by the degree of association with the cranium (Wilga et al. 2001). Jaw protrusion may quickly reduce the distance between predator and prey, laterally occlude the gape for suction feeding, reorient the teeth for more effective biting and permit a variety of functional novelties (e.g. chiseling, gouging, excavation; Alexander 1967; Moss 1977; Tricas & McCosker 1984; Frazzetta & Prange 1987). Species with highly protrusible upper jaws either possess a long ligamentous connection between the upper jaw and skull (many sharks) or lack ligamentous attachment (batoids; Compagno 1977; Wilga et al. 2001; Wilga 2002). Mobility of the jaws is also affected by the structure of the hyoid arch. In batoids, only the paired hyomandibular cartilages support the jaws, while the ventral hyoid elements (ceratohyal and basihyal) are lost or have become incorporated with the branchial arches (figure 2). This morphologically and functionally separates the jaws and branchium.
The left and right sides of the visceral arches may be decoupled as well. The majority of elasmobranch species (except hard prey specialists and some planktivores) possess flexible symphyses, functionally dividing the halves of the jaw arch. Further, in some batoids, the ventral branchial cartilages are longitudinally split (figure 2; Miyake & McEachran 1991), allowing autonomy of left and right sides of the throat. Midline flexibility paired with asymmetrical muscle contraction could therefore allow autonomy of left and right sides of the oropharyngeal cavity, permitting precise orientation of flow.
3. Batoids (lower jaw processors)
Batoid fishes have several cranial muscles, not seen in sharks, that control hyoid and lower jaw depression. These depressor muscles are subdivisions of pre-existing muscles or novel derivatives of embryonic muscle plates. Batoids also exhibit many more jaw adductor subdivisions than most sharks (Miyake & McEachran 1991; Miyake et al. 1992; Dean & Motta 2004a). This duplication of muscular pathways (by having similar origins and insertions to existing muscles) functionally increases fine motor control and the flexibility of behaviours (Liem 1973). For instance, Atlantic guitarfishes (Rhinobatos lentiginosus) use one muscle to depress the jaw during prey capture, but another (derived from a different embryonic muscle plate) during processing (Wilga & Motta 1998b). In lesser electric rays (Narcine brasiliensis), numerous muscles attach to the lower jaw, freeing one modified jaw depressor to become involved with jaw protrusion (Dean & Motta 2004a). This precise arrangement of lower jaw musculature is unique to Narcine and relatives (control of the mandible is mediated by a ligamentous sling), however such diversity of lower jaw musculature is the norm for batoids (Miyake 1988; González-Isáis 2003).
Although the batoid jaw suspension results in great upper jaw freedom, there are no articulations or ligaments with the cranium to help guide protrusion. Instead, in some batoids, the upper and lower jaws are mechanically coupled so that protrusion accompanies lower jaw movements. This is accomplished in N. brasiliensis through overlapping ligaments and cartilages, constraining the jaws to protrude simultaneously (Dean & Motta 2004a,b). Myliobatid stingrays possess similar restrictions. Ligaments limit movement of the upper and lower jaws relative to each other, stabilizing the jaws when crushing bivalves (Summers 2000). It is important to note that restricting movement of the jaw arch does not mean that the upper jaw is immobile; the upper–lower jaw coupling allows the elaborate lower jaw musculature to mediate fine control of jaw protrusion (Dean & Motta 2004a,b).
Unique depressor muscles and split anterior branchial cartilages in batoids may also allow differential compression and depression of the left and right sides of the branchial region. The loose symphyses of most batoids extend this flexibility further, permitting independent movement of the left and right sides of the entire ventral head skeleton. Asymmetrical motion of the jaws has been recorded in batoid species (Summers 1995; Dean & Motta 2004b), however it is not known to occur in sharks.
While successful suction capture requires control of water external to the mouth, precise movements of the oral cavity can allow strict internal orientation of water flow, creating a ‘hydrodynamic tongue’ for prey processing (Bemis & Lauder 1986). This and the many muscular degrees of freedom of the batoid mandibular and hyoid arches may explain how suction feeding batoids are capable of delicate food manipulation (such as removal of exoskeletons, mollusc mantle and bivalve shells) completely within the mouth and throat (Wilga & Motta 1998b; Dean & Motta 2004b). All other chondrichthian consumers of complex prey process their food externally. For example, heterodontiform and orectolobiform sharks are suction feeders that must repeatedly spit out and reingest a prey item in order to process and reduce it (Motta 2004). The internal processing mode of batoids may be more precise and therefore would aid in protecting the meal from scavengers
4. Megacarnivorous sharks (upper jaw processors)
In contrast to lower jaw musculature elaboration in batoids, the upper jaw protrusion mechanism becomes more complex in megacarnivorous sharks. Active control of upper jaw protrusion is assisted through loss of ligaments and addition and modification of muscular attachments. Lamniform and carcharhiniform sharks have duplicated muscular pathways through possession of a novel division of a jaw protruder. Additionally, in carcharhinid sharks, an ancestral jaw retractor muscle is realigned with a modified motor pattern to assist upper jaw protrusion (Wilga et al. 2001; Wilga in press).
Megacarnivores possess paired ligaments lateral to the midline connecting the upper jaw to the cranium (Compagno 1977). In derived lamniform sharks, these ligaments are lost and replaced by a single median ligament (Compagno 1977; Wilga in press). Paired with asymmetrical levator muscle contraction, this loss could increase the freedom of movement of the jaw, but would decrease overall stability. However, these same species also acquired a novel insertion of a hyoid retractor muscle onto the jaw joint, thereby permitting more active, direct control of jaw movement.
This suite of alterations to upper jaw control may be the root of functional shifts in feeding behaviour between basal sharks and megacarnivores. While most elasmobranchs protrude the upper jaw while the mouth is closing, carcharhinid and lamnid sharks are capable of jaw protrusion in both opening and closing phases of prey capture (reviewed in Dean & Motta 2004b). This allows for multiple ‘bites’ per gape cycle, repetitive gouging of the prey as deep as the upper jaw can protrude, as in white sharks (Tricas & McCosker 1984). Coupled with violent head shaking, this mechanism rapidly and externally reduces large, mobile and potentially dangerous prey. Heterodontid sharks possess elaborate jaw adductors, yet these apparently facilitate durophagy and are not used in gouging (Smith 1942). Batoids and squaleans possess less elaborate levator musculature and apparently lack the ability for such modulation of upper jaw protrusion (Wilga & Motta 1998a,b; Dean & Motta 2004a,b; Motta 2004).
5. Conclusions
Capture and processing involve coordinated use of upper and lower jaws. We suggest that batoids possess more precise control of the lower jaw owing to a greater number of muscular insertions, while megacarnivorous sharks take advantage of flexibility in the upper jaw to reduce prey. As a result, the hydrodynamic processing method of batoids is selective, precise and internal, while megacarnivores use an external and purely mechanical form of reduction. These mechanisms possibly arose as a means of differentially exploiting small benthic or oversized midwater prey, respectively.
Muscular modifications for complex processing apparently arose independently within megacarnivores and batoids. Although many squalean sharks are suction feeders, they do not possess the number of muscle insertions for control over upper jaw protrusion and oral cavity manipulation seen in the more derived megacarnivores and batoids, and therefore exhibit less dexterous processing or typically swallow prey whole (Wilga & Motta 1998a; Fouts & Nelson 1999). Outgroups also have less derived processing mechanisms. The upper jaw of chimaeras (holocephalans) is fused to the cranium and therefore akinetic (Wilga 2002). Furthermore, the hyoid arch is intact and bears gills, functioning more in respiration than feeding (Didier 1995). Jawless fishes (agnathans) possess a muscular and cartilaginous pumping organ that may be a precursor to the vertebrate lower jaw, yet the musculature is less elaborate than elasmobranch jaw musculature (Mallatt 1996; Cohn 2002).
We propose a series of experiments to test the hypotheses that batoids process prey via fine control of lower jaw movements and a hydrodynamic tongue, and that carcharhinid and lamnid sharks use primarily upper jaw movements to process prey. Analyses of evolutionary biomechanics require a performance measure. Our hypotheses equate functionality with a flexible behavioural repertoire. Behavioural flexibility should therefore be illustrated through modulation, a different and repeatable muscle pattern in response to different prey types (Wainwright & Friel 2000). We expect the modulatory abilities of batoid lower jaw musculature to exceed those of batoid upper jaw or shark lower jaw musculature. For example, electromyograms (EMGs) of batoids feeding on complex and simple prey (i.e. shelled and unshelled prawn) should show modulation of lower jaw muscle activity during processing of complex prey. In contrast, sharks (regardless of jaw suspension type) presented with the same prey choice will show little change in lower jaw muscle activity.
Control of the hydrodynamic tongue presumably rests in an ability of batoids to exert unilateral force with the visceral arches. Again, using EMGs to compare the activity in the left and right sides of the jaws and branchial arches during processing simple and complex prey should reveal that batoids are capable of this modulation whereas other cartilaginous fishes are not. Finally, comparing the kinematics and EMGs of prey processing in megacarnivores to their sister taxa will reveal whether the former actively control the muscles of the upper jaw during prey processing while the latter do not.
These data may have been gathered already in the several studies of feeding in large captive and freeswimming sharks. However, analysis has stopped at prey capture. We argue that the processing phase is equally important and worthy of study. Further, the dichotomy in prey processing abilities in elasmobranchs may be indicative of underlying selective pressures; prey availability may have driven evolution of the feeding mechanism. Perhaps an abundance of large-bodied prey fuelled derivation of upper jaw processing in sharks, while the lower jaw mediated processing of batoids allowed them to exploit a benthic lifestyle where variously armoured invertebrates predominate.
Acknowledgments
Derek Dean, Steve Frank, Jesse Hollister, Dan Huber, Philip Motta, Alpa Patel Wintzer, Nely Pohl and Justin Schaefer provided insights that strengthened this manuscript.
References
- Alexander R.M.N. The functions and mechanisms of the protrusible upper jaws of some acanthopterygian fish. J. Zool. 1967;151:43–64. [Google Scholar]
- Bemis W.E, Lauder G.V. Morphology and function of the feeding apparatus of the lungfish, Lepidosiren paradoxa (Dipnoi) J. Morphol. 1986;187:81–108. doi: 10.1002/jmor.1051870108. [DOI] [PubMed] [Google Scholar]
- Cohn M.J. Lamprey Hox genes and the origin of jaws. Nature. 2002;416:386–387. doi: 10.1038/416386a. [DOI] [PubMed] [Google Scholar]
- Compagno L.J.V. Phyletic relationships of living sharks and rays. Am. Zool. 1977;17:303–322. [Google Scholar]
- Dean M.N, Motta P.J. Anatomy and functional morphology of the feeding apparatus of the lesser electric ray, Narcine brasiliensis (Elasmobranchii: Batoidea) J. Morphol. 2004a;262:462–483. doi: 10.1002/jmor.10245. [DOI] [PubMed] [Google Scholar]
- Dean M.N, Motta P.J. Feeding behavior and kinematics of the lesser electric ray, Narcine brasiliensis. Zoology. 2004b;107:171–189. doi: 10.1016/j.zool.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Didier D.A. Phylogenetic systematics of the extant chimaeroid fishes (Holocephali, Chimaeroidei) Am. Mus. Novit. 1995;3119:1–86. [Google Scholar]
- Drucker E.G, Jensen J.S. Functional analysis of a specialized prey processing behavior: winnowing by surfperches (Teleostei: Embiotocidae) J. Morphol. 1991;210:267–287. doi: 10.1002/jmor.1052100306. [DOI] [PubMed] [Google Scholar]
- Fouts W, Nelson D.R. Prey capture by the Pacific angel shark, Squatina californica: visually mediated strikes and ambush site characteristics. Copeia. 1999;1999:304–312. [Google Scholar]
- Frazzetta T.H, Prange C.D. Movements of cephalic components during feeding in some requiem sharks (Carcharhiniformes: Carcharhinidae) Copeia. 1987;1987:979–993. [Google Scholar]
- González-Isáis M. Anatomical comparison of the cephalic musculature of some members of the superfamily Myliobatoidea (Chondrichthyes): implications for evolutionary understanding. Anat. Rec. A. 2003;271:259–272. doi: 10.1002/ar.a.10031. [DOI] [PubMed] [Google Scholar]
- Liem K.F. Evolutionary strategies and morphological innovations: cichlid pharyngeal jaws. Syst. Zool. 1973;22:425–441. [Google Scholar]
- Liem K.F, Bemis W.E, Walker W.F, Grande L. Brooks Cole; Fremont, CA: 2001. Functional anatomy of the vertebrates: an evolutionary perspective. [Google Scholar]
- Mallatt J. Ventilation and the origin of jawed vertebrates: a new mouth. Zool. J. Linn. Soc. 1996;117:329–404. [Google Scholar]
- Miyake, T. 1988 The systematics of the stingray genus Urotrygon with comments on the interrelationships within Urolophidae (Chondricthyes, Myliobatiformes). Ph.D. thesis, Texas A&M University, College Station, Texas.
- Miyake T, McEachran J.D. The morphology and evolution of the ventral gill arch skeleton in batoid fishes (Chondricthyes: Batoidea) Zool. J. Linn. Soc. 1991;102:75–100. [Google Scholar]
- Miyake T, McEachran J.D, Hall B.K. Edgeworth's legacy of cranial muscle development with an analysis of muscles in the ventral gill arch region of batoid fishes (Chondrichthyes: Batoidea) J. Morphol. 1992;212:213–256. doi: 10.1002/jmor.1052120304. [DOI] [PubMed] [Google Scholar]
- Moss S.A. Feeding mechanisms in sharks. Am. Zool. 1977;17:355–364. [Google Scholar]
- Motta P.J. Prey capture behavior and feeding mechanics of elasmobranchs. In: Carrier J.C, Musick J.A, Heithaus M.R, editors. Biology of sharks and the relatives. CRC Press; Boca Raton: 2004. pp. 139–164. [Google Scholar]
- Shirai S. Phylogenetic interrelationships of neoselachians (Chondricthyes: Euselachii) In: Stiassny M.L.J, Parenti L.R, Johnson G.D, editors. Interrelationships of fishes. Academic Press; New York: 1996. pp. 9–34. [Google Scholar]
- Smith B.G. The heterodontid sharks: their natural history, and the external development of Heterodontus japonicus based on notes and drawings by Bashford Dean. In: Gudger E.W, editor. The Bashford Dean memorial volume archaic fishes. American Museum of Natural History; New York: 1942. pp. 710–711. [Google Scholar]
- Summers A.P. Is there really bilateral asynchrony in muscle activation patterns in batoids? Copeia. 1995;1995:1036. [Google Scholar]
- Summers A.P. Stiffening the stingray skeleton—an investigation of durophagy in myliobatid stingrays (Chondrichthyes, Batoidea, Myliobatidae) J. Morphol. 2000;243:113–126. doi: 10.1002/(SICI)1097-4687(200002)243:2<113::AID-JMOR1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tricas T.C, McCosker J.E. Predatory behavior of the white shark (Carcharodon carcharias), with notes on its biology. Proc. Calif. Acad. Sci. 1984;43:221–238. [Google Scholar]
- Wainwright P.C. Biomechanical limits to ecological performance: mollusc-crushing by the Caribbean hogfish, Lachnolaimus maximus (labridae) J. Zool. Lond. 1987;213:283–297. [Google Scholar]
- Wainwright P.C, Friel J.P. Effects of prey type on motor pattern in tetraodontiform fishes. J. Exp. Zool. 2000;286:563–571. doi: 10.1002/(sici)1097-010x(20000501)286:6<563::aid-jez3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Wilga C.D. A functional analysis of jaw suspension in elasmobranchs. Biol. J. Linn. Soc. 2002;75:483–502. [Google Scholar]
- Wilga, C. D. In press. Morphology and evolution of the jaw suspension in lamniform sharks. J. Morphol. Published online 6 May 2005. (doi:10.1002/jmor.10342) [DOI] [PubMed]
- Wilga C.D, Motta P.J. Conservation and variation in the feeding mechanism of the spiny dogfish Squalus acanthias. J. Exp. Biol. 1998a;201:1345–1358. doi: 10.1242/jeb.201.9.1345. [DOI] [PubMed] [Google Scholar]
- Wilga C.D, Motta P.J. Feeding mechanism of the Atlantic guitarfish Rhinobatos lentiginosus: modulation of kinematic and motor activity. J. Exp. Biol. 1998b;201:3167–3184. doi: 10.1242/jeb.201.23.3167. [DOI] [PubMed] [Google Scholar]
- Wilga C.D, Hueter R.E, Wainwright P.C, Motta P.J. Evolution of upper jaw protrusion mechanisms in elasmobranchs. Am. Zool. 2001;41:1248–1257. [Google Scholar]