Abstract
King and emperor penguins (Aptenodytes patagonicus and Aptenodytes forsteri) are the only species of marine birds so far known to reflect ultraviolet (UV) light from their beaks. Unlike humans, most birds perceive UV light and several species communicate using the near UV spectrum. Indeed, UV reflectance in addition to the colour of songbird feathers has been recognized as an important signal when choosing a mate. The king penguin is endowed with several highly coloured ornaments, notably its beak horn and breast and auricular plumage, but only its beak reflects UV, a property considered to influence its sexual attraction. Because no avian UV-reflecting pigments have yet been identified, the origin of such reflections is probably structural. In an attempt to identify the structures that give rise to UV reflectance, we combined reflectance spectrophotometry and morphological analysis by both light and electron microscopy, after experimental removal of surface layers of the beak horn. Here, we characterize for the first time a multilayer reflector photonic microstructure that produces the UV reflections in the king penguin beak.
Keywords: king penguin, ultraviolet reflectance, photonic structures, beak, birds
1. Introduction
To survive, eat and mate, birds depend heavily on visual signals. In consequence, avian vision is highly developed, with sensitivity in many birds extending into the near ultraviolet (UV), which is invisible to the human eye (Cuthill et al. 2000). Male individuals of various avian species exhibit conspicuous colours on their feathers, which are the product of sexual selection driven by mating preferences (Darwin 1871; Andersson 1994). These often reflect in the UV and it is well documented that UV is employed in several species as an important cue in finding and choosing a mating partner (Finger et al. 1992; Andersson & Amundsen 1997; Bennett et al. 1997; Hunt et al. 2001; Siitari et al. 2002; Hausmann et al. 2003; Pearn et al. 2003).
The king penguin is a marine bird in which both males and females are characterized by yellow-to-orange breast and auricular feathers and an orange beak horn, which in addition to its colour reflects intensely in the UV region (Jouventin et al. 2005). It has been suggested that the biological function of these ornaments is related to choosing a mate, their absence in sexually immature juveniles supporting such a hypothesis (Massaro et al. 2003; Jouventin et al. 2005; B. Dresp, unpublished data). As no avian pigments that reduce or enhance UV reflectance are currently known, this must have a structural basis in the penguin, arising from microstructures that manipulate light within the reflecting tissue. Structural colours are widespread in the natural world and, since bird vision extends into the UV spectrum, UV-reflecting photonic structures in bird feathers have aroused considerable interest in recent years (Hausmann et al. 2003). UV reflectance has also recently been reported in the mouths and flanges of begging passerines (Hunt et al. 2003) and in the skin of certain avian species (Prum & Torres 2003). Here, to identify the UV-reflecting microstructures in the King Penguin and characterize their ultrastructure, we combined spectrophotometry with morphological analysis, after successively removing surface layers of beak horns with a scalpel. This is the first time the morphology of a UV-reflecting photonic structure has been characterized in the beak tissue of any bird.
2. Material and methods
King penguin beak horns moult annually. Specimens were collected after moulting on the Baie du Marin of Possession Island (46°25′ S; 51°45′ E) in the Crozet Archipelago, where large colonies of king penguins gather to breed, and stored at ambient temperature for several months before analysis for UV reflectance and histological structure. Beak horn thickness, measured with a micrometer, varies from 0.3 to 0.4 mm between individuals.
For morphological analysis, beak horns were sectioned laterally into strips approximately 2.5 cm from the end nearest to the eye in situ, the area of maximal UV reflectance. After soaking in water, strips were fixed overnight in phosphate buffered 2.5% glutaraldehyde and post-fixed for 5 h in 2% osmium tetroxide, before dehydration in ethanol and embedding in Spurr epoxy resin. For histology 0.7 μm-thick sections, transverse to the longitudinal axis of the beak horn, were stained with toluidine blue and viewed with bright field optics. For electron microscopy, 60 nm thick sections were post-stained with lead citrate and uranyl acetate and viewed in a Zeiss 900 microscope at 80 kV.
Reflectance spectra were obtained, using an S2000 fibre optic diode array spectrophotometer with a PX-2 pulsed xenon light source (Ocean Optics Inc., Dunedin, Florida, USA). Spectra were measured at least three times, with the probe perpendicular (incident light angle; 90°) to and 2–3 mm away from the reflecting surface for an illuminated field of approximately 3 mm2. A Spectralon diffuse white reference standard was used for calibration and percentage reflectance values were calculated automatically by software provided with the instrument as previously described (Prum et al. 1999). In order to determine the histological structure responsible for the UV reflectance, layers of the beak horn were successively scraped off with a scalpel blade from the upper surface. Reflectance spectra were obtained after each scraping and the amount of beak horn tissue remaining was determined by measuring the thickness with a micrometer.
3. Results
(a) Histology
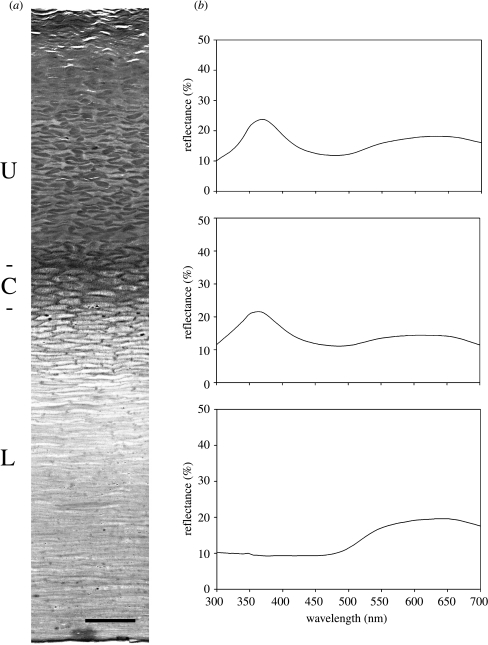
Transverse sections of the beak horn displayed three distinct superposed regions (figure 1a). An upper region, occupying approximately 40% of the depth of the horn, was composed essentially of interconnected elongated dense profiles, most of which were 5–10 μm long and 1–2 μm wide, frequently arranged in regular ‘zigzag’ patterns. The upper part of this region appeared to be poorly preserved and contained numerous large air pockets. Immediately beneath the upper region, a central region could be defined, occupying approximately 10% of the horn thickness, which comprised several layers of juxtaposed cells with central nuclei. Beneath this, occupying about half the depth of the horn, a lower region was constituted of compact clear flattened profiles.
Figure 1.
(a) Toluidine blue stained histological transverse section of beak horn, cut parallel to the short axis, with the outer surface at the top, showing distinct upper (U), central (C) and lower (L) superposed regions. Scale bar, 25 μm. (b) Reflectance spectra obtained on intact and scraped beak horn. The upper spectrum was obtained on intact beak horn; the middle spectrum was obtained on scraped beak horn after partial removal of the upper region; the lower spectrum was obtained when the entire upper region had been removed by scraping, leaving the central and lower regions intact.
(b) Spectral reflectance
Reflectance from the upper surface of the beak horn (figure 1b; upper) was characterized by a pronounced UV peak at 367.5 nm. The percentage reflectance of beak horn specimens collected after moulting was found to be up to 50% lower than that recorded in situ on live penguins. A second very broad reflectance peak in the visible spectrum with a maximum around 630 nm was also observed. In contrast, no UV reflectance was observed from the opposite (under) surface of the beak horn.
The scraping experiments, in which successive layers were removed from the upper surface with a scalpel blade, were performed on a point approximately 2.5 cm from the end nearest the eye in situ. The first scraping, removing approximately 17% of the thickness of the horn, resulted in a slight (10%) reduction in maximum reflectance and a very small shift in the wavelength of maximum reflectance to 361.5 nm (figure 1b; middle). Subsequent successive scrapings were associated with no further significant change in maximum-reflectance wavelength or in percentage reflectance. However, when the horn thickness was reduced by 38%, UV reflectance was abruptly abolished (figure 1b; lower). After processing the beak horn remaining at this stage for histological analysis, the almost total absence of the upper region was confirmed. The central cellular region and the lower region were still present, however, proving that the reflecting structures are situated within the upper region.
(c) Electron microscopy
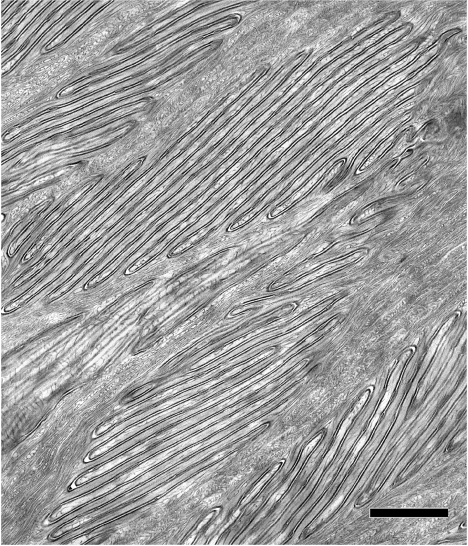
Transmission electron microscopy of transverse sections cut perpendicular to the long axis of the beak horn showed the upper region of the horn to be filled with vast numbers of interconnected, folded-membrane microstructures (figure 2). Such microstructures differed in size, from 1.9 to 12.5 μm long; mean 5.8±2.7 (s.d.) μm and from 0.8 to 6.6 μm wide; mean 2.0±1.2 (s.d.) μm, and in the number of folded sheets they contained. The smallest consisted of sixfolded lamellae, while the biggest comprised up to 40 folds. The space between successive folds, measured on prints of electron micrographs at a final magnification of ×80 000, was found to be approximately 130 nm. Each folded membrane consisted of two dense membranes separated by a narrow clear space in which elongated dense profiles were occasionally observed. Irregular bundles of filaments were interspersed between these microstructures. Transverse sections of the beak horn cut in a plane parallel to its long axis also showed identical microstructures with similar dimensions. Thus, these elements consist of three-dimensional stacks of 6–40 layers of superposed flat lamellar folds. No cell nuclei were observed within this region, suggesting that these folded structures are extracellular. The central beak horn region immediately beneath the upper region contained cells with elongated nuclei. Elaborate folded membrane networks were present in their cytoplasm, probably representing the developmental forerunners of the membrane systems concentrated in the region above.
Figure 2.
Transmission electron micrograph of the upper region of the beak horn, showing it to be packed with microstructures constituted of multilayer folded membrane stacks. Scale bar, 1.0 μm.
4. Discussion
In contrast with colour produced by pigments, so-called ‘structural colour’ results from selected reflectance of incident light due to the physical nature of the reflecting material. Mechanisms based on random scattering from air-filled matrices, or based on coherent scattering from single or multiple thin-layer reflectors or from surface diffraction gratings have been evoked to explain structural colour production (Dyck 1976; Parker 1998; Prum et al. 1999; Vukusic et al. 2001). Nature has evolved an extraordinary diversity of highly specialized structures capable of reflecting different colours or UV (Parker 2000; Vukusic & Sambles 2003) and they are well documented in insects, fishes, butterflies, birds and plants. Multilayer reflectors, which produce the most intense structural colours (Parker et al. 2001), are relatively common. Photonic structures produce coloured or UV reflections in avian plumage, and it has been estimated that skin colour is also produced structurally, by ordered collagen arrays, in more than 2.5% of all avian species (Prum & Torres 2003). In addition, ordered collagen arrays have been reported to produce UV reflectance in the facial skin of certain birds (Prum et al. 1999). Although the mouths and flanges of begging passerines have been reported to reflect in the ultraviolet (Hunt et al. 2003), this is the first time that the nature of the UV-reflecting microstructures has been characterized in beak tissue of any bird. The ultrastructure of the photonic microstructures found in the present study differs radically from that of those previously described in either bird feathers or skin. The regular multilayer membrane arrays found in the beak horn microstructures closely approximate to two dimensional crystal lattices, strongly suggesting that UV reflectance here is produced by interference between incident light and that reflected from successive folds in these microstructures (Prum & Torres 2003). Using Bragg's law (Bragg & Bragg 1915) to calculate the wavelength of light reflected from such a structure, assuming a refractive index of 1.4, the predicted maximum wavelength of reflectance is 364 nm. Given the possible sources of error in Bragg analysis (Prum et al. 1999) and the uncertainty in estimating the refractive index, the predicted value is remarkably close to the observed wavelength.
Several biological functions of the visual signals produced by structural reflectors have been suggested, but one of the most important is probably conspecific recognition such as during courtship aggregation (Parker 1995). As has been shown in other avian species (Hausmann et al. 2003), visual signals based on UV contrast are probably especially important in the king penguin during courtship displays. Interestingly, during courtship displays, king penguins flaunt their UV-reflecting beak ornament to potential partners in a face-to-face encounter. Moreover, this ornament is displayed against the black background of the penguin beak. This would engender a contrast effect, augmenting the perception of the signal, and could thus be functionally significant in attracting a partner. In addition, the orientation of the reflecting layers in different microstructures varies with respect to the beak surface, which would produce a spread in the wavelength of reflected light and result in reflection over a broad viewing angle, thus generating a more readily detectable signal. In summary, the present data demonstrate that the king penguin has evolved a highly specialized structure capable of reflecting UV, presumably to optimize visual signals produced by its ornaments. Moreover, this is found not in the feathers as described previously in other birds, but on the most prominent part of its anatomy, the beak.
Acknowledgments
We gratefully acknowledge the financial support of the Institut Polaire Français (IPEV).
References
- Andersson M. Princeton University Press; 1994. Sexual selection. [Google Scholar]
- Andersson S, Amundsen T. Ultraviolet colour vision and ornamentation in bluethroats. Proc. R. Soc. B. 1997;264:1587–1591. doi:10.1098/rspb.1997.0221 [Google Scholar]
- Bennett A.T, Cuthill I.C, Partridge J.C, Lunau K. Ultraviolet plumage colors predict mate preferences in starlings. Proc. Natl Acad. Sci. USA. 1997;94:8618–8621. doi: 10.1073/pnas.94.16.8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg W.H, Bragg W.L. G. Bell; London: 1915. X-rays and crystal structure. [Google Scholar]
- Cuthill I.C, Partridge J.C, Bennett A.T.D, Church S.C, Hart N.S, Hunt S. Ultraviolet vision in birds. Adv. Study Behav. 2000;29:159–214. [Google Scholar]
- Darwin C. Murray; London: 1871. The descent of man and selection in relation to sex. [Google Scholar]
- Dyck J. Structural colours. Proc. Int. Ornithol. Congr. 1976;16:426–437. [Google Scholar]
- Finger E, Burkhardt D, Dyck J. Avian plumage colors: origin of UV reflection in a black parrot. Naturwissenschaften. 1992;79:187–188. [Google Scholar]
- Hausmann F, Arnold K.E, Marshall N.J, Owens I.P.F. Ultraviolet signals in birds are special. Proc. R. Soc. B. 2003;270:61–67. doi: 10.1098/rspb.2002.2200. doi:10.1098/rspb.2002.2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S, Cuthill I.C, Bennett A.T, Church S.C, Partridge J.C. Is the ultraviolet waveband a special communication channel in avian mate choice? J. Exp. Biol. 2001;204:2499–2507. doi: 10.1242/jeb.204.14.2499. [DOI] [PubMed] [Google Scholar]
- Hunt S, Kilner R.M, Langmore N.E, Benett A.T. Conspicuous, ultraviolet-rich mouth colours in begging chicks. Proc. R. Soc. B. 2003;270(Suppl. 1):S25–S28. doi: 10.1098/rsbl.2003.0009. doi:10.1098/rsbl.2003.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouventin P, Nolan P.M, Ornborg J, Dobson F.S. Ultraviolet beak spots in king and emperor penguins. Condor. 2005;107:144–150. [Google Scholar]
- Massaro M, Lloyd S.D, Darby J.Y. Carotenoid-derived ornaments reflect parental quality in male and female yellow-eyed penguins Megadyptes antipodes. Behav. Ecol. Sociobiol. 2003;55:169–175. [Google Scholar]
- Parker A.R. The diversity and implications of animal structural colours. J. Exp. Biol. 1998;201:2343–2347. doi: 10.1242/jeb.201.16.2343. [DOI] [PubMed] [Google Scholar]
- Parker A.R. 515 million years of structural colour. J.Opt. A: Pure Appl. Opt. 2000;2:R15–R28. [Google Scholar]
- Parker A.R, McPhedran R.C, McKenzie D.R, Botten L.C, Nicorovici N.A. Photonic engineering. Aphrodite's iridescence. Nature. 2001;409:36–37. doi: 10.1038/35051168. [DOI] [PubMed] [Google Scholar]
- Pearn S.M, Bennett A.T, Cuthill I.C. The role of ultraviolet-A reflectance and ultraviolet-A-induced fluorescence in budgerigar mate choice. Ethology. 2003;109:961–970. [Google Scholar]
- Prum R.O, Torres R. Structural colouration of avian skin: convergent evolution of coherently scattering dermal collagen arrays. J. Exp. Biol. 2003;206:2409–2429. doi: 10.1242/jeb.00431. [DOI] [PubMed] [Google Scholar]
- Prum R.O, Torres R, Kovach C, Williamson S, Goodman S.M. Coherent light scattering by nanostructured collagen arrays in the caruncles of the malagasy asities (Eurylaimidae: aves) J. Exp. Biol. 1999;202:3507–3522. doi: 10.1242/jeb.202.24.3507. [DOI] [PubMed] [Google Scholar]
- Siitari H, Honhavaara J, Huhta E, Viitala J. Ultraviolet reflection and female mate choice in the pied flycatcher Ficedula hypoleuca. Anim. Behav. 2002;63:97–102. [Google Scholar]
- Vukusic P, Sambles J.R, Lawrence C.R, Wootton R.J. Structural colour: now you see it—now you don't. Nature. 2001;410:36. doi: 10.1038/35065161. [DOI] [PubMed] [Google Scholar]
- Vukusic P, Sambles J.R. Photonic structures in biology. Nature. 2003;424:852–855. doi: 10.1038/nature01941. [DOI] [PubMed] [Google Scholar]