Abstract
Distribution and abundance patterns at the community and metacommunity scale can result from two distinct mechanisms. Random dispersal followed by non-random, site-specific mortality (species sorting) is the dominant paradigm in community ecology, while habitat selection provides an alternative, largely unexplored, mechanism with different demographic consequences. Rather than differential mortality, habitat selection involves redistribution of individuals among habitat patches based on perceived rather than realized fitness, with perceptions driven by past selection. In particular, habitat preferences based on species composition can create distinct patterns of positive and negative covariance among species, generating more complex linkages among communities than with random dispersal models. In our experiments, the mere presence of predatory fishes, in the absence of any mortality, reduced abundance and species richness of aquatic beetles by up to 80% in comparison with the results from fishless controls. Beetle species' shared habitat preferences generated distinct patterns of species richness, species composition and total abundance, matching large-scale field patterns previously ascribed to random dispersal and differential mortality. Our results indicate that landscape-level patterns of distribution and species diversity can be driven to a large extent by habitat selection behaviour, a critical, but largely overlooked, mechanism of community and metacommunity assembly.
Keywords: community structure, habitat selection, metacommunities, non-lethal effects, species distributions, species interactions
1. Introduction
Identifying the underlying mechanisms that produce patterns of biodiversity is a defining goal of ecology. However, patterns of distribution and diversity may ultimately derive from several alternative mechanisms (Levin 1992). Classic patterns of species segregation between predators and highly vulnerable prey can result from two mechanisms differing in their underlying processes of dispersal and colonization. Random (undirected) dispersal followed by differential mortality (species sorting) is commonly assumed in community and metacommunity models. Immigration rate is largely a function of patch area and distance from sources of colonists (van Baalen & Hochberg 2001; Leibold et al. 2004) and patterns of species distribution are a function of differential mortality among habitat patches. In contrast, habitat selection theory emphasizes the ability of species to disperse and colonize patches with the highest expected fitness (Fretwell & Lucas 1970; Pulliam & Danielson 1991; Rosenzweig 1991; Resetarits 1996; Morris 2003) and resulting patterns of distribution are a function of spatial redistribution of individuals among habitat patches (Resetarits et al. in press). Random dispersal followed by differential mortality is the reigning paradigm in community ecology, while the role of habitat selection at the community (and metacommunity—sensu lato Leibold et al. 2004) level remains largely unexplored.
Habitat selection can affect population growth rate, abundance and persistence for individual species, but community and metacommunity-level consequences depend on its prevalence among dispersing and colonizing species and how niche axes are partitioned by regional species pools (Pulliam & Danielson 1991; Spencer et al. 2002). If habitat selection is prevalent, the very presence of predators, for example, can structure both local communities and regional metacommunities by simultaneously altering the dispersal and colonization of multiple prey species. If a niche axis is distributed (or perceived) as a state variable (e.g. predator presence or absence), performance optima for numerous species can overlap in the same habitat type (Resetarits & Wilbur 1989; Rosenzweig 1991), generating positive and negative covariances among prey species as a result of shared or complementary patterns of avoidance or attraction (Resetarits et al. in press). A critical distinction is that species interactions that have manifested in habitat selection do not require spatial co-occurrence (functioning alternately via behavioural spatial segregation) and thus operate both within communities and at the metacommunity scale (Resetarits 2005).
Multispecies habitat selection generates very different ecological consequences compared with random dispersal and differential mortality because species distributions are based on the redistribution of individuals rather than mortality, and on perceived rather than actual fitness. Perceptions of fitness are presumably driven by habitat interactions in the species' evolutionary past (Resetarits & Wilbur 1989). The distinction between redistribution of individuals versus differential mortality becomes especially critical as metapopulation and metacommunity models move beyond simple patch occupancy to consider variation in abundance. Thus, classic ecological explanations may no longer hold if, for example, immigration and extinction rates are functions of specific habitat characteristics that attract or repel colonists and generate local variation in population size, rather than being simple functions of patch size and distance.
The distribution of predatory fishes is a critical determinant of the landscape-level distribution and abundance of many aquatic taxa (Wellborn et al. 1996). Common aquatic beetles (Coleoptera) are typically less abundant, and rare species are absent, in habitats that contain fishes. Traditionally, reduced abundance, biomass and richness were attributed to differential mortality (Weir 1972; Healey 1984; Fairchild et al. 2000). However, this pattern can also be generated by multiple species selecting ponds based on the presence or absence of fishes (Resetarits 2001). We conducted two experiments quantifying how the non-lethal presence of predatory fishes affects mean abundance and species richness of colonizing aquatic beetles.
2. Methods
(a) Experiment 1
We crossed the non-lethal presence or absence of one Enneacanthus obesus (Centrarchidae; mean mass: 2.47 g) with the presence or absence of supplemental habitat complexity (artificial substrates). Habitat complexity often reduces predation, thus, we tested beetle responses to a known predator (Graham & Vrijenhoek 1988), preference for complex habitats, and whether preferences were affected by fishes. We established 24 experimental ponds (wading pools: 1.50×0.29 m; 300 l) arranged in six linear blocks (ca 20 m apart) of four pools each (ca 1.3 m apart) that were surrounded by hardwood and pine forest in a field in Chesapeake VA, USA. On 8 July we covered ponds with a tight-fitting fibreglass screen (2 mm2) to prevent premature colonization by insects and filled pools with tap water. Two days later, we added randomized aliquots of 0.4 kg of dried leaf litter and 1.0 l of zooplankton and phytoplankton. Supplemental habitat complexity consisted of 600 plastic cable ties attached to a plastic grid comprising 600 1 cm2 sections. The four treatments (presence or absence of predators×presence or absence of habitat complexity) were randomly assigned to pools within blocks.
On 10 July, fishes were placed under screens, screens pushed underwater and habitat complexity added atop the lids. This eliminated physical interactions between predators and beetles but allowed chemical communication. Treatments lacking habitat complexity had equal amounts of unassembled cable ties and equal numbers of grids placed under the screen. Beetles were collected from the pools on 27 July and preserved in 95% ethanol.
We examined effects of block and treatment on mean beetle abundance (total) and mean species richness. A single MANOVA was performed using Sas for Windows version 8.0 (type III SS, α=0.05) followed by univariate ANOVAs (table 1).
Table 1.
Summary of analyses. num, numerator; den, denominator.
| experiment 1 | |||||
| source | Wilks' λ | F | num. d.f. | den d.f. | p |
| block | 0.098 | 6.16 | 10 | 28 | <0.0001 |
| habitat complexity | 0.754 | 2.28 | 2 | 14 | 0.1384 |
| fishes | 0.184 | 31.11 | 2 | 14 | <0.0001 |
| habitat complexity×fishes | 0.86 | 1.14 | 2 | 14 | 0.3482 |
| source | d.f. | SS | MS | F | p |
| abundance | |||||
| block | 5 | 1339.3 | 267.9 | 9.27 | 0.0004 |
| habitat complexity | 1 | 20.2 | 20.2 | 0.70 | 0.4167 |
| fishes | 1 | 1837.5 | 1837.5 | 63.56 | <0.0001 |
| habitat complexity×fishes | 1 | 32.7 | 32.7 | 1.13 | 0.3046 |
| species richness | |||||
| block | 5 | 35.00 | 7.00 | 6.43 | 0.0022 |
| habitat complexity | 1 | 0.67 | 0.67 | 0.61 | 0.4461 |
| fishes | 1 | 54.00 | 54.00 | 49.59 | <0.0001 |
| habitat complexity×fishes | 1 | 0.00 | 0.000 | 0.00 | 1.0000 |
| experiment 2 | |||||
| source | Wilks' λ | F | num. d.f. | den d.f. | p |
| block | 0.408 | 0.94 | 6 | 10 | 0.5068 |
| treatment | 0.15 | 3.95 | 4 | 10 | 0.0355 |
| contrast | |||||
| control versus Enneacanthus | 0.161 | 13.03 | 2 | 5 | 0.0104 |
| control versus Aphredoderus | 0.740 | 0.88 | 2 | 5 | 0.4717 |
| Enneacanthus versus Aphredoderus | 0.258 | 7.19 | 2 | 5 | 0.0338 |
| abundance | |||||
| source | d.f. | SS | MS | F | p |
| block | 3 | 376.7 | 125.6 | 0.74 | 0.5648 |
| treatment | 2 | 3004.7 | 1502.3 | 8.88 | 0.0161 |
| contrast | d.f. | contrast SS | MS | F | p |
| control versus Enneacanthus | 1 | 2738 | 2738.0 | 16.18 | 0.0069 |
| control versus Aphredoderus | 1 | 144.5 | 144.5 | 0.85 | 0.3911 |
| Enneacanthus versus Aphredoderus | 1 | 1624.5 | 1624.5 | 9.60 | 0.0212 |
| species richness | |||||
| source | d.f. | SS | MS | F | p |
| block | 3 | 3.67 | 1.22 | 0.57 | 0.6542 |
| treatment | 2 | 71.17 | 35.58 | 16.64 | 0.0036 |
| contrast | d.f. | contrast SS | MS | F | p |
| control versus Enneacanthus | 1 | 66.1 | 66.1 | 30.92 | 0.0014 |
| control versus Aphredoderus | 1 | 4.5 | 4.5 | 2.10 | 0.1971 |
| Enneacanthus versus Aphredoderus | 1 | 36.1 | 36.1 | 16.89 | 0.0063 |
(b) Experiment 2
We used the same methodology to establish 12 experimental ponds in four blocks. On 28 August we randomly assigned three treatments to ponds within blocks: two Enneacanthus gloriosus (mean mass 2.69 g), two Aphredoderus sayanus (Aphredoderidae; 4.06 g) and fishless controls. Aphredoderidae is a monotypic family and was included because it is a known predator of beetles (Sheldon & Meffe 1993) and represents the only species (or family), out of seven species (five families) tested, that is not avoided by ovipositing tree frogs (Resetarits & Wilbur 1989; Binckley & Resetarits 2003). Beetles were collected weekly for three weeks. Analysis consisted of a MANOVA and non-orthogonal multivariate and univariate contrasts to examine differences between treatments.
3. Results
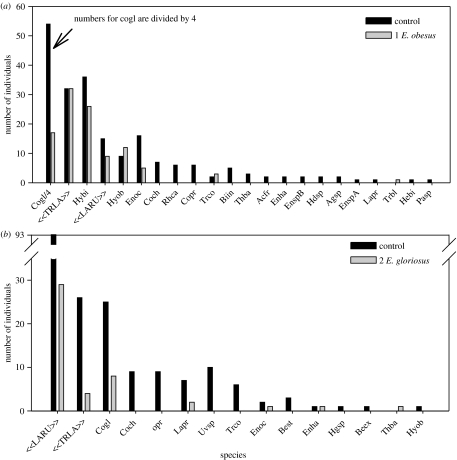
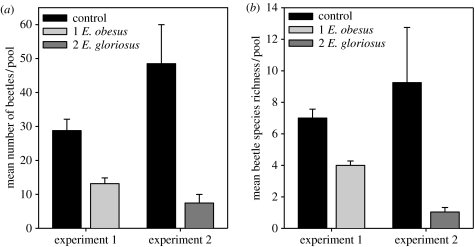
Figure 1 shows the distribution of 783 individuals of 26 beetle species between pools with and without Enneacanthus. The reduction in the abundance of common species and the elimination of rare species was generated behaviourally (figure 1; table 1). Of the 26 species, only nine colonized ponds with Enneacanthus while only a single species (Tropisternus blatchleyi), represented by a single individual, occurred solely with fishes. Both mean species richness/pool and mean abundance/pool were reduced by up to 80% by the non-lethal presence of fishes (figure 2; table 1), with the magnitude of effects increasing in experiment 2.
Figure 1.
Number of beetles (table 2) in controls and experimental ponds containing (a) one caged, Enneacanthus obesus and (b) two caged Enneacanthus gloriosus. Avoidance response of Laccophilus rufus (LARU) and Tropisternus lateralis (TRLA) only in experiment 2 probably indicates a threshold response to fish density (Rieger et al. 2004; see text). Both experiments contained additional, non-significant treatments that were excluded for clarity (see table 1).
Figure 2.
Effects of habitat selection on (a) mean beetle abundance and (b) mean species richness.
In experiment 1, habitat complexity had no effect and there was no interaction between habitat complexity and the presence or absence of fishes. In experiment 2, responses to A. sayanus did not differ significantly from controls for any response variables. The atypical response to A. sayanus by both beetles and tree frogs (Binckley & Resetarits 2003), which are both readily consumed by this small, benthic fish, is under further investigation.
4. Discussion
Habitat selection theory suggests that, if habitats are hierarchical in suitability, colonization of less suitable habitats occurs only as density increases in preferred sites. Even the least suitable sites are occupied at saturation densities, because the fitness in a poor site exceeds that of a good site where resources are already monopolized (Fretwell & Lucas 1970). This density-dependant process restricts the ability of field patterns alone to establish primary habitat preferences or determine the ultimate processes leading to observed species distributions (but see Morris 2003). Our experimental approach demonstrated that the presence of small predatory fishes significantly reduced the mean abundance and species richness of beetles via habitat selection behaviour alone. Aquatic beetles had clear habitat preferences for fishless ponds and the resulting patterns of species richness and abundance matched those reported by large-scale field surveys (Weir 1972; Healey 1984; Fairchild et al. 2000). Although we did not test specifically for density-dependence habitat choice (sensu the ideal free distribution; Fretwell & Lucas 1970), it is certainly possible that the presence of common species in pools with fishes reflects a response to increased density in preferred (fishless) pools, as suggested by data on tree frogs (Binckley & Resetarits 2003).
Interestingly, we observed decreased abundance of several common beetle species in ponds with fishes only in experiment 2, in which fish identity changed and both density and fish biomass were two times higher. We suspect this, and the stronger overall response, are a consequence of density rather than the relatively subtle change in predator identity, as one of the common beetles, Tropisternus lateralis, shows essentially equivalent responses to two other centrarchid species at similar densities (Resetarits 2001). In addition, we previously identified fish density thresholds that trigger avoidance (Rieger et al. 2004) and established the functional equivalence of fish predators from four families (except A. sayanus) with respect to behavioural avoidance in tree frogs (Binckley & Resetarits 2003). Thus, our experiments, which used low fish densities relative to nature, constitute a conservative estimate of the effects of habitat selection on community structure and substantiate a growing body of work suggesting that habitat selection is a primary mechanism driving the distribution and abundance of species in aquatic systems (Resetarits & Wilbur 1989; Blaustein 1999; Åbjörnsson et al. 2002; Binckley & Resetarits 2003; Kiflawi et al. 2003; Morris 2003; Rieger et al. 2004; Resetarits 2005; Resetarits et al. in press). Our results also affirm a counter-intuitive process of decreasing local mortality with increasing predator abundance for species that detect and avoid fishes (figure 2; Abrams 1993; Rieger et al. 2004).
While both local and larger scale regional processes influence population and community structure, ecologists lack a critical understanding of how processes operating at different scales interact to influence patterns of distribution and diversity (Shurin & Allen 2001; Kneitel & Miller 2003; Leibold et al. 2004; Resetarits 2005). Habitat selection generates such interactions between local and regional processes because rates and magnitudes of dispersal from or into local communities depend on specific habitat characteristics (e.g. predator presence or absence; Resetarits in press). Species interactions thus operate at two distinct spatial scales: locally within communities of spatially co-occurring species and regionally among species residing in spatially discrete local communities. This is strikingly different from the random dispersal/differential mortality paradigm where all interactions occur locally. In our study, two species of Enneacanthus interacted strongly with individuals of numerous beetle species, without co-occurring or causing direct mortality, by invoking a behavioural redistribution among habitats. Such cryptic, phantom interactions are another form of behavioural non-lethal effects that, along with the ‘ecology of fear’ (Brown et al. 1999) and behavioural trait-mediated interactions (Werner & Peacor 2003), constitute a largely missing element in community ecology.
Habitat selection is a critical process in community assembly and is particularly germane to metacommunity dynamics because it elevates the probability and intensity of entirely different sets of local interactions among species forced to spatially co-occur due to shared habitat preferences. Thus, regional landscapes are behaviourally partitioned into different habitat types occurring in unique spatial configurations, resulting in complex patterns of linkage among communities (Resetarits et al. in press). When the important aspects of habitat include other species whose distributions vary in space and time, such as mobile predators, habitat selection may be especially critical and the resulting spatial and temporal dynamics of species distributions and (meta)community structure may be especially volatile (Resetarits & Wilbur 1989; Blaustein 1999; Brown et al. 1999; Resetarits 2005).
Table 2.
Codes and corresponding scientific names used in figure 1.
| code | name | code | name |
|---|---|---|---|
| Cogl | Copelatus glyphicus | Enha | Enochrus hamiltoni |
| Trla | Tropisternus lateralis | EnspB | Enochrus sp. B |
| Hybi | Hydaticus bimarginatus | Hdsp | Hydrochus sp. |
| Laru | Laccophilus rufus | Agsp | Agabus sp. |
| Hyob | Hydrochara obtusata | EnspA | Enochrus sp. A |
| Enoc | Enochrus ochraceus | Lapr | Laccophilus proximus |
| Coch | Copelatus chevrolati | Trbl | Tropisternus blatchleyi |
| Rhca | Rhantus calidus | Hebi | Helocombus bifidus |
| Copr | Copelatus princeps | Pasp | Paracymus sp. |
| Trco | Tropisternus collaris | Uvsp | Uvarus sp. |
| Biin | Bidessonotus inconspicuus | Best | Berosus striatus |
| Thba | Thermonectus basillaris | Hgsp | Hygrotus sp. |
| Acfr | Acilius fraternus | Beex | Berosus exiguus |
Acknowledgments
D. Chalcraft, J. Fauth, J. Knouft, D. Morris, J. Rieger and M. Rosenshield provided helpful comments. J. Rieger helped with set-up and data collection. P. Spangler and W. Steiner (Smithsonian) helped identify beetles. Funded by NSF (DEB-0096051) and EPA-STAR (R825795-01-0).
Footnotes
Present address: Institute of Arctic Biology, PO Box 757000, University of Alaska–Fairbanks, Fairbanks, AK 99775-7000, USA
References
- Åbjörnsson L, Brönmark C, Hansson L. The relative importance of lethal and non-lethal effects of fish on insect colonization of ponds. Freshw. Biol. 2002;47:1489–1495. [Google Scholar]
- Abrams P.A. Why predation rate should not be proportional to predator density. Ecology. 1993;74:726–733. [Google Scholar]
- Binckley C.A, Resetarits W.J., Jr Functional equivalence of non-lethal effects: generalized fish avoidance determines distribution of gray treefrog, Hyla chrysoscelis, larvae. Oikos. 2003;102:623–629. [Google Scholar]
- Blaustein L. Oviposition site selection in response to risk of predation: evidence from aquatic habitats and consequences for population dynamics and community structure. In: Wasser S.P, editor. Evolutionary theory and processes: modern perspectives. Kluwer; Dordrecht: 1999. pp. 441–456. [Google Scholar]
- Brown J.S, Laundré J.W, Gurung M. The ecology of fear: optimal foraging, game theory, and trophic interactions. J. Mammal. 1999;80:385–399. [Google Scholar]
- Fairchild G.W, Faulds A.M, Matta J.F. Beetle assemblages in ponds: effects of habitat and site age. Freshw. Biol. 2000;44:523–534. [Google Scholar]
- Fretwell S.D, Lucas H.L., Jr On territorial behavior and other factors influencing habitat distribution in birds. I. Theoretical development. Acta Biotheor. 1970;19:16–36. [Google Scholar]
- Graham J.H, Vrijenhoek R.C. Detrended correspondence analysis of dietary data. Trans. Am. Fish. Soc. 1988;117:29–36. [Google Scholar]
- Healey M. Fish predation on aquatic insects. In: Resh V.H, Rosenberg D.M, editors. Ecology of aquatic insects. Praeger; New York: 1984. pp. 255–288. [Google Scholar]
- Kiflawi M, Blaustein L, Mangel M. Predation-dependent oviposition habitat selection by the mosquito Culiseta longiareolata: a test of competing hypotheses. Ecol. Lett. 2003;6:35–40. [Google Scholar]
- Kneitel J.M, Miller T.E. Dispersal rates affect species composition in metacommunities of Sarracenia purpurea inquilines. Am. Nat. 2003;162:165–171. doi: 10.1086/376585. [DOI] [PubMed] [Google Scholar]
- Leibold M.A, et al. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 2004;7:601–613. [Google Scholar]
- Levin S.A. The problem of pattern and scale in ecology. Ecology. 1992;73:1943–1967. [Google Scholar]
- Morris D.W. Toward an ecological synthesis: a case for habitat selection. Oecologia. 2003;136:1–13. doi: 10.1007/s00442-003-1241-4. [DOI] [PubMed] [Google Scholar]
- Pulliam R.H, Danielson B.J. Sources, sinks, and habitat selection: a landscape perspective on population dynamics. Am. Nat. 1991;137:S51–S66. [Google Scholar]
- Resetarits W.J., Jr Oviposition site choice and life history evolution. Am. Zool. 1996;36:205–215. [Google Scholar]
- Resetarits W.J., Jr Experimental evidence that past predation affects community assembly: fish avoidance in a colonizing/ovipositing aquatic beetle. Oecologia. 2001;129:155–160. doi: 10.1007/s004420100704. [DOI] [PubMed] [Google Scholar]
- Resetarits, Jr, W. J. 2005 Habitat selection links local and regional scales in aquatic systems. Ecol. Lett.8, 480–486. [DOI] [PubMed]
- Resetarits W.J, Jr, Wilbur H.M. Choice of oviposition site by Hyla chrysoscelis: role of predators and competitors. Ecology. 1989;70:220–228. [Google Scholar]
- Resetarits, Jr, W. J., Binckley, C. A. & Chalcraft, D. R. In press. Habitat selection, species interactions, and processes of community assembly in complex landscapes: a metacommunity perspective. In Metacommunities: spatial dynamics and ecological communities (ed. M. Holyoak, M. A. Leibold & R. D. Holt). University of Chicago Press.
- Rieger J.F, Binckley C.A, Resetarits W.J., Jr Female oviposition site preference mirrors larval performance in a treefrog, Hyla femoralis. Ecology. 2004;85:2094–2099. [Google Scholar]
- Rosenzweig M.L. Habitat selection and population interactions: the search for mechanism. Am. Nat. 1991;137:S5–S28. [Google Scholar]
- Sheldon A.L, Meffe G.K. Multivariate analysis of feeding relationships of fishes in blackwater streams. Environ. Biol. Fish. 1993;37:161–171. [Google Scholar]
- Shurin J.B, Allen E.G. Effects of competition, predation, and dispersal on species richness at local and regional scales. Am. Nat. 2001;158:624–637. doi: 10.1086/323589. [DOI] [PubMed] [Google Scholar]
- Spencer M.L, Blaustein L, Cohen J.E. Oviposition habitat selection by mosquitoes Culiseta longiareolata and consequences for population size. Ecology. 2002;83:669–679. [Google Scholar]
- van Baalen M, Hochberg M.E. Dispersal in antagonistic interactions. In: Colbert J, Danchin E, Dhondt A.A, Nichols J.D, editors. Dispersal. Oxford University Press; 2001. pp. 299–310. [Google Scholar]
- Weir J.S. Diversity and abundance of aquatic insects reduced by introduction of the fish Clarias gariepinus to pools in central Africa. Biol. Conserv. 1972;4:169–175. [Google Scholar]
- Wellborn G.A, Werner E.E, Skelly D.K. Mechanisms creating community structure across a freshwater habitat gradient. Annu. Rev. Ecol. Syst. 1996;27:337–363. [Google Scholar]
- Werner E.E, Peacor S.D. A review of trait-mediated indirect interactions. Ecology. 2003;84:1083–1100. [Google Scholar]