Abstract
Although the presence of feathers in the nest is widespread among birds, it has not been previously suggested that feathers can be used as sexual signals. Females of the spotless starling (Sturnus unicolor) regularly carry feathers to their nest, mostly during laying and incubation. We show that the arrangement of these feathers was non-random with respect to the side (obverse or reverse) placed upwards (which can be viewed from the nest entrance). Feathers of the wood pigeon (Columba palumbus) and the spotless starling, which exhibit higher ultraviolet and visible reflectance on their reverse side, were predominantly placed with this side upwards. On the contrary, feathers of the jay (Garrulus glandarius) were predominantly found exhibiting the obverse side, which possesses higher reflectance in this species. Feathers of the azure-winged magpie (Cyanopica cyana), with similar reflectance values on either side, were placed indiscriminately in obverse and reverse positions. The results suggest that feathers are arranged to maximize their conspicuousness within the nest and hence that they might be potentially used as intraspecific signals.
Keywords: nest feathers, female signalling, spectral reflectance, spotless starling
1. Introduction
Theory on the evolution of ornaments as signals of individual quality has focused predominantly on males (Andersson 1994). Females may also exhibit ornaments but they have been commonly viewed as a correlated effect of sexual selection acting on male trait expression (Lande 1980; Halliday & Arnold 1987), which could explain the scarce interest they have received among behavioural ecologists. However, several papers have recently called attention to the fact that females may also exhibit ornaments that, although less elaborated than those of males, may be correlates of their reproductive experience, body condition or genetic quality (Burns 1998; Amundsen 2000; Domb & Pagel 2001; Houde 2001; Roulin et al. 2001; Daunt et al. 2003; Jawor et al. 2004).
In some species of birds, the odd materials carried by males to the nest before the onset of laying are functionally equivalent to morphological ornaments and there are several well documented examples of cases in which they provide reliable signals of mating status or genetic quality (Borgia & Gore 1986; Moreno et al. 1994; Brouwer & Komdeur 2004). However, to our knowledge, there is no published report indicating that ornaments are apportioned to nests once the laying of eggs has started and that such ornaments can be put there by females. In this paper we examine the type and positioning of the feathers found in nests of the spotless starling Sturnus unicolor, items with a potential ornamental function and which are predominantly placed there by females.
We propose that the ultraviolet (UV) radiation as well as the visible (VIS) colours of the feathers carried to the nests by spotless starlings have the potential to constitute a prominent signalling element. Several recent papers have demonstrated the importance of the UV colours of ornamental feathers in mating processes and intrasexual interactions (Sheldon et al. 1999; Keyser & Hill 2000; Pearn et al. 2001; Siitari et al. 2002; Alonso-Alvarez et al. 2004; Limbourg et al. 2004) and it is now known that the UV reflectance of avian plumages is a characteristic of nearly all bird species (Eaton & Lanyon 2003). In the present paper we also show that the spectral properties of the feathers of the four species best represented in the sample taken from nests of the spotless starling, varied between their two surfaces: the obverse and the reverse (see §2). If feathers are carried to the nest with a signalling function instead of providing insulation, as has been classically proposed (Collias & Collias 1984; Hansell 1995, 2000), we expect that (i) feathers carried to the nests are preferentially large flight (wing/tail) feathers and should be placed out of the nest cup in a rather perceptible location (in contrast, the insulation hypothesis predicts that birds use contour feathers and place them in the nest cup); (ii) the UV reflectance of the feathers added to the nest is generally strong (according to the insulation hypothesis we expect a variable, including weak, reflectance); (iii) feathers are oriented to show the surface with maximum reflectance (the insulation hypothesis predicts that they are oriented so that their curvature matches that of the nest cup).
2. Methods
The spotless starling is a medium-sized passerine (mean body mass: 92 g), facultatively polygynous and relatively long lived (Veiga et al. 2002). While males regularly carry green plants to the nests during the courtship period but only occasionally carry feathers, females regularly take feathers of other individuals into their nests during the laying and incubation periods. We studied two colonies located 15 km apart in Madrid province (central Spain). The study was conducted between late March and mid-June in two localities for three consecutive years (2001–2003). From 2 days before the beginning of laying until 5 days after hatching, we recorded the number of feathers present in each of 323 clutches and whether they were contour or flight feathers on a daily basis. Whenever it was possible, we also annotated the avian species to which the feathers belonged. About 90% of feathers found in the nests came from four species: most came from the wood pigeon (Columba palumbus; 59.6%) and the spotless starling (35.0%), and a few came from the jay (Garrulus glandarius; 0.6%), the Iberian azure-winged magpie (Cyanopica cyana; 4.8%) and some other local species. Feathers may be placed with their obverse (concave surface) or their reverse side (convex surface) upwards. Thus, when a feather lies with its reverse side upwards, its curvature matches that of the nest cup. For each clutch, we obtained a composite of the daily positioning of the feathers (on average there were 4.32 feathers per clutch).
Spectral data were taken with an Ocean Optics S2000 fibre-optic spectrometer using a 200 μm fibre-optic probe at a 90° angle to the feather surface with a 2 mm diameter reading area. We also took measurements at a 45° angle, although this only changed the absolute magnitude, not the appearance of reflectance curves. At a 90° angle reflectance values were between 10 and 15% higher than at 45°. Reflectance spectra were generated with a Spectralon white standard (Labsphere, Inc.) as reference. Measurements were made in the proximal, distal and middle parts of the feather. Only the proximal part was used for the analyses because it showed the highest reflectance values on either surface. We captured reflectance curves (250–700 nm) for the two sides of feathers belonging to wood pigeon (n=13), spotless starling (n=13), jay (n=10) and Iberian azure-winged magpie (n=10). We used repeated measures ANOVA to analyse differences in radiant energy in the UV and VIS spectra (first repeated factor) between the reverse and obverse sides (second repeated factor) of feathers belonging to the four species. To estimate the repeatability of feather reflectance we took two measures of reflectance spectra for each side of 30 randomly chosen feathers. The inter-feather variability explained a significant part of the variance and the within-feathers repeatability was very high (repeated measures ANOVA: F29,30=61.80, n=30, r=0.968, p<0.0001), so that we only obtained a reflectance spectra for each feather and side in subsequent analyses.
3. Results
Only 12.5% of the feathers carried to nests were contour feathers (usually less than 3 cm long). The remaining 87.5% were flight (tail or wing) feathers mostly longer than 8 cm (range 6–50 cm; a few vulture (Gyps fulvus) and white stork (Ciconia ciconia) feathers included). In general, flight feathers lay outside the nest cup, without contacting the eggs or the female body during incubation. These feathers were frequently leaned against the nest box walls or piled up between the nest cup and the frontal wall. Out of 468 contour feathers whose position was recorded, 78.3% were found with their reverse side up. This result did not differ very much from the 87.5% of feathers found with this same orientation in a sample of 1000 feathers taken from 10 nests of long-tailed tits (Aegithalos caudatus) (M. H. Hansell, unpublished data). Interestingly, a higher proportion of contour feathers were carried by the starlings to the nest when air temperatures were cooler for first clutches than for second clutches.
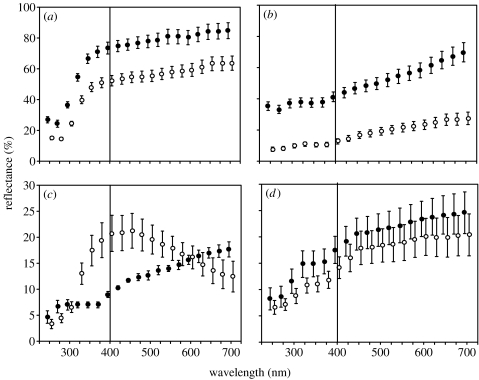
The reflectance of the obverse and reverse sides of flight feathers, both in the UV and VIS spectra, is shown in figure 1 for each of the four species considered. In the spotless starling and the wood pigeon, the reflectance of the reverse side was higher than that of the obverse side (table 1 and figure 1). In contrast, the reflectance of the jay feathers was higher on the obverse side than on the reverse side, both in the UV and VIS spectra. Most feathers of this species found in starlings nests presented conspicuous bright blue bars on their obverse side that possessed a reflectance peak at 450 nm. For the azure-winged magpie, whose feathers present bluish colours only in the obverse side, both sides emitted a similar amount of energy either in the UV or the VIS spectrum.
Figure 1.
Radiance (%) of the feathers of the four species most frequently found in nests of the spotless starling. (a) Wood pigeon, (b) spotless starling, (c) jay and (d) azure-winged magpie. The vertical line separates the UV (left) and VIS (right) spectra. Open circles: obverse side; solid circles: reverse side. Mean and standard error are shown.
Table 1.
Repeated measures ANOVA of the reflectance (%) in the UV and VIS spectra (first repeated factor) of obverse and reverse sides of feathers (second repeated factor) belonging to: jay (n=10), Iberian azure-winged magpie (n=10), spotless starling (n=13) and wood pigeon (n=13).
| dorsal versus ventral | interaction term | |||
|---|---|---|---|---|
| F1,n−1 | p | F1,n−1 | p | |
| jay (Garrulus glandarius) | 53.99 | <0.001 | 2.84 | 0.13 |
| azure-winged magpie (Cyanopica cyana) | 0.32 | 0.58 | 0.53 | 0.48 |
| spotless starling (Sturnus unicolor) | 72.54 | 0.001 | 21.73 | <0.001 |
| wood pigeon (Columba palumbus) | 35.58 | <0.001 | 4.88 | 0.047 |
Differences in radiant energy between obverse and reverse sides and the interaction term between feather side and wavelength range are shown.
A greater proportion of wood pigeon and starling feathers were found exposing their reverse side (0.79 versus 0.21 exposing the obverse side; Yates corrected χ2=253.25, d.f.=1, p<0.001), that is, they showed the side with higher reflectance. In contrast, feathers of the jay were more frequently found exposing the obverse side, with brilliant blue colours and higher UV reflectance (0.88 versus 0.12, respectively; Yates corrected χ2=18.83, d.f.=1, p<0.001). For the azure-winged magpie feathers with similar reflectance curves on the obverse and reverse sides, both sides were presented equally as often (0.51 versus 0.49, respectively; Yates corrected χ2=0.01, d.f.=1, p=0.91). Thus, for all four of the avian species considered in this study, more feathers were placed by starlings exposing the surface with the highest energy reflectance, either in the UV or the VIS spectra.
4. Discussion
The high proportion of flight feathers, instead of contour feathers, found in spotless starling nests and their non-random placement constitutes robust evidence in favour of the idea that they may function as a signal. Flight feathers are apparently not carried to the nest with a thermoregulatory purpose as they are not put within the nest cup and are not within contact of the eggs or the incubating female. However, the insulation and the signalling hypothesis are not mutually exclusive and our results do not discard the idea that the small proportion of contour feathers found in nests are used by starlings for such an insulating function. Contour feathers were regularly found within the nest cup and predominantly oriented as predicted by the insulation hypothesis, namely, matching the curvature of the nest. Also, the proportion of contour feathers found in our study with this same orientation did not differ very much from that reported in another passerine, the long-tailed tit, which supposedly adds feathers to the nest with a thermoregulatory purpose (Hansell 1995). In addition, contour feathers were apportioned to the nest mainly during low air temperatures; circumstantial evidence that supports the insulation hypothesis.
Flight feathers found in nests were generally exposing the side with the highest reflectance. It has been shown that the obverse side of black and brown feathers, the predominant colours of the spotless starling and the wood pigeon, are the least likely to reflect high levels of UV (Eaton & Lanyon 2003) and the same could happen for the VIS range of the spectra. This could explain why feathers of these two species showed the highest reflectance values in their reverse, hidden side. Also, feathers carried to the nest are ‘used feathers’ that had moulted in order to allow room for new feathers, which suggests that the exposed obverse side is considerably abraded compared with the more protected reverse side. Thus, the reverse side of a feather could preserve better than the obverse side the nanostructure responsible for UV and VIS light reflectance properties (Perrier et al. 2002; Shawkey et al. 2003; Siefferman & Hill 2003). Jay feathers carried to the nest were almost invariably those with brilliant blue patches on their obverse side, which showed higher reflectance than the reverse side throughout most of the range of the UV and VIS spectra. Interestingly, these were the only feathers predominantly placed with the obverse side upwards.
It has been previously discussed that UV radiation contributes strongly to conspicuousness against grey–brown leaf litter and bark backgrounds and that this is enhanced by a soft light effect (Endler 1993; Andersson et al. 1998). Photometer readings used to take nest photographs indicate that the intensity of the light inside nest boxes is 16–32 times lower than that outside. Under this meagre light, the UV radiation of feathers put by starlings into nests could create a strong contrast against the opaque nest material background, mainly consisting of twigs and dry herbs. In fact, we have visualized this effect by dimming photographs of feathers taken under artificial UV illumination.
Spotless starlings are colonial breeders and the frequency of nest intrusions by floater individuals searching for a breeding site is very high (J. P. Veiga and V. Polo, unpublished work). If feathers are communicating some attribute of the nest owner, it is advantageous that they be arranged to maximize their visibility from the nest entrance. Alternatively, feathers could be involved in intersexual communication. Thus, it may also be useful that feathers can be viewed from the nest entrance because male spotless starlings, even if they are monogamous, rarely enter the nest to incubate (Arenas 2000).
It is not evident why feathers may be useful tools in communication. Feathers available to spotless starlings are rather scant in the open fields where they breed and find food (own observations). Females that prolong their stays out of the nest to search for feathers incur the risk of egg chilling and expose the clutch to destruction by potential competitors (Arenas 2000). Thus, the presence of feathers in the nest may be indicating that the female owner is able to search efficiently over her home range, which may signify good condition or long breeding experience. In any case, experimental manipulations in species that carry feathers to their nests and broad comparative approaches are badly needed to clarify what feathers are signalling and who receives the signal.
Acknowledgments
We thank two anonymous referees for their constructive comments. This work was funded by projects BOS2001-0703 (MCYT) and CGL2004-00126/BOS (MEYC) and it was conducted under licence of the Consejería de Medio Ambiente y Desarrollo de la Comunidad de Madrid and the Ayuntamiento de Manzanares el Real de Madrid.
References
- Alonso-Alvarez C, Doutrelant C, Sorci G. Ultraviolet reflectance affects male–male interactions in the blue tit (Parus caeruleus ultramarinus) Behav. Ecol. 2004;15:805–809. [Google Scholar]
- Amundsen T. Why are female birds ornamented? Trends Ecol. Evol. 2000;15:149–155. doi: 10.1016/s0169-5347(99)01800-5. [DOI] [PubMed] [Google Scholar]
- Andersson M. Princeton University Press; 1994. Sexual selection. [Google Scholar]
- Andersson S, Örnborg J, Andersson M. Ultraviolet sexual dimorphism and assortative mating in blue tits. Proc. R. Soc. B. 1998;265:445–450. doi:10.1098/rspb.1998.0315 [Google Scholar]
- Arenas M. Tesina de licenciatura. Universidad Complutense; Madrid: 2000. Inversión parental, estatus de emparejamiento e interferencia social en el estornino negro, Sturnus unicolor. Importancia del conflicto sexual en el desarrollo de estrategias de reproducción. [Google Scholar]
- Borgia G, Gore M.A. Feather stealing in the satin bowerbird (Ptilonorhynchus violaceus); mate competition and the quality of display. Anim. Behav. 1986;34:727–738. [Google Scholar]
- Brouwer L, Komdeur J. Green nesting material has a function in mate attraction in the European starling. Anim. Behav. 2004;67:539–548. [Google Scholar]
- Burns K.J. A phylogenetic perspective on the evolution of sexual dichromatism in tanagers (Thraupidae): the role of female versus male plumage. Evolution. 1998;52:1219–1224. doi: 10.1111/j.1558-5646.1998.tb01849.x. [DOI] [PubMed] [Google Scholar]
- Collias N.E, Collias E.C. Princeton University Press; 1984. Nest building and bird behavior. [Google Scholar]
- Daunt F, Monaghan P, Wanless S, Harris M.P. Sexual ornament size and breeding performance in female and male European Shags Phalacrocorax aristotelis. Ibis. 2003;145:54–60. [Google Scholar]
- Domb L.G, Pagel M. Sexual swellings advertise female quality in wild baboons. Nature. 2001;410:204–206. doi: 10.1038/35065597. [DOI] [PubMed] [Google Scholar]
- Eaton M.D, Lanyon S.M. The ubiquity of avian ultraviolet plumage reflectance. Proc. R. Soc. B. 2003;270:1721–1726. doi: 10.1098/rspb.2003.2431. doi:10.1098/rspb.2003.2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler J.A. The colour of light in forests and its implications. Ecol. Monogr. 1993;63:1–27. [Google Scholar]
- Halliday T.R, Arnold S.J. Multiple mating by females: a perspective from quantitative genetics. Anim. Behav. 1987;35:939–941. [Google Scholar]
- Hansell M.H. The demand for feathers as building material by woodland nesting birds. Bird Study. 1995;42:240–245. [Google Scholar]
- Hansell M.H. Cambridge University Press; 2000. Bird nests and construction behaviour. [Google Scholar]
- Houde A.E. Sex roles, ornaments, and evolutionary explanation. Proc. Natl Acad. Sci. USA. 2001;98:12 857–12 859. doi: 10.1073/pnas.241503598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawor J.M, Gray N, Beall S.M, Breitwisch R. Multiple ornaments correlate with aspects of condition and behaviour in female northern cardinals, Cardinalis cardinalis. Anim. Behav. 2004;67:875–882. [Google Scholar]
- Keyser A.J, Hill G.E. Structurally based plumage coloration is an honest signal of quality in male blue grosbeaks. Behav. Ecol. 2000;11:202–209. [Google Scholar]
- Lande R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution. 1980;34:292–305. doi: 10.1111/j.1558-5646.1980.tb04817.x. [DOI] [PubMed] [Google Scholar]
- Limbourg T, Mateman A.C, Andersson S, Lessers C.M. Female blue tits adjust parental effort to manipulated male UV attractiveness. Proc. R. Soc. B. 2004;271:1903–1908. doi: 10.1098/rspb.2004.2825. doi:10.1098/rspb.2004.2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J, Soler M, Møller A.P, Lindén M. The function of stone carrying in the black wheatear, Oenante leucura. Anim. Behav. 1994;47:1297–1309. [Google Scholar]
- Pearn S.M, Bennet A.T.D, Cuthill I.C. Ultraviolet vision, fluorescence and mate choice in a parrot, the budgerigar Melopsittacus undulates. Proc. R. Soc. B. 2001;268:2273–2279. doi: 10.1098/rspb.2001.1813. doi:10.1098/rspb.2001.1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier G, de Lope F, Møller A.P, Ninni P. Structural coloration and sexual selection in the barn swallow Hirundo rustica. Behav. Ecol. 2002;13:728–736. [Google Scholar]
- Roulin A, Dijkstra C, Riols C, Ducrest A.L. Female- and male-specific signals of quality in the barn owl. J. Evol. Biol. 2001;14:255–266. [Google Scholar]
- Shawkey M.D, Estes A.M, Siefferman L.M, Hill G.E. Nanostructure predicts intraspecific variation in ultraviolet-blue plumage colour. Proc. R. Soc. B. 2003;270:1455–1460. doi: 10.1098/rspb.2003.2390. doi:10.1098/rspb.2003.2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon B.C, Andersson S, Griffith S.C, Örnborg J, Sendecka J. Ultraviolet colour variation influences blue tit sex ratios. Nature. 1999;402:874–877. [Google Scholar]
- Siefferman L, Hill G.E. Structural and melanin coloration indicate parental effort and reproductive success in male eastern bluebirds. Behav. Ecol. 2003;14:855–861. [Google Scholar]
- Siitari H, Honkavaara J, Huhta E, Viitala J. Ultraviolet reflection and female mate choice in the pied flycatcher, Ficedula hypoleuca. Anim. Behav. 2002;63:97–102. [Google Scholar]
- Veiga J.P, Moreno J, Arenas M, Sánchez S. Reproductive consequences for males of paternal vs territorial strategies in the polygynous spotless starling under variable ecological and social conditions. Behaviour. 2002;139:677–693. [Google Scholar]