Abstract
For many animal groups, both sexes have been reported to attempt to mate with members of their own sex. Such behaviour challenges theories of sexual selection, which predict optimization of reproductive success. We tested male mate choice between opposite- and same-sex members in the damselfly Ischnura elegans. Binary choice experiments were conducted following exposure periods in insectaries with only males or with both sexes present. We show that switches in choice between the opposite sex and the same sex can be induced and reversed again by changing the social context. We argue that the observed reversibility in male–male- and male–female-directed mating behaviour is maladaptive and a consequence of strong selection on a male's ability to alter choice between different female colour morphs.
Keywords: damselfly, colour polymorphism, mate choice, sex recognition, sexual selection
1. Introduction
Individuals are not expected to mate indiscriminately, but rather choose their mates carefully (Widemo & Saether 1999). For the competing sex, enhanced sensory adaptations to improve sex recognition and selection for mate quality are expected (Andersson 1994). Mate choice, then, should only occur when the characteristics of the detected animal matches the characteristics of the choosing animal's internal template (Widemo & Saether 1999). Although traditional models of mate choice assume preference for quality to be fixed (reviewed by Andersson 1994; Kokko et al. 2003), a sexually selected genotype that does best in one environment might have a lower fitness in a different environment (e.g. David et al. 2000). State-dependent reproductive decisions in response to social or environmental changes, therefore, may be beneficial for fitness optimization (Qvarnström 2001). One such example is frequency-dependent plasticity in mate choice for different female colour morphs (Miller & Fincke 1999; Van Gossum et al. 2001).
Here, we propose such plasticity in mate choice as a new proximate explanation for the often-observed male–male mating behaviours in animals (Bagemihl 1999). Several adaptive and non-adaptive explanations have been suggested to explain same-sex mating behaviour. Such behaviour is sometimes beneficial and may function as an alternative tactic to increase reproductive success (reviewed in Harari et al. 2000). For example, female beetles may mount other females to provide a greater opportunity for them to mate with larger males (Harari & Brockmann 1999). An alternative explanation is that such behaviour can be maladaptive and results from, for example, an absence or scarcity of opposite-sex partners (see §4).
Males of species with female polymorphism have been shown to exhibit plastic frequency-dependent mate choice (Miller & Fincke 1999; Van Gossum et al. 2001). Further, female morph frequencies may change rapidly (Fincke 1994), suggesting that flexible male behaviour is likely to be beneficial. We hypothesize that this same flexibility may also account for the regular occurrence of same-sex mating behaviour in species with polymorphic females. To evaluate this hypothesis, we conducted experiments in outdoor insectaries in which we alternately exposed males to situations with only males present and situations with males and females present, and examined resulting frequencies of male–male mating behaviour. We hypothesized that the recent social environment (absence or presence of females) would determine male mate choice and this choice would reverse after changing the social environment.
We tested this idea in a damselfly with a scramble competition mating system, where male–male sexual behaviour is common (Stoks & De Bruyn 1998). Competition among males for access to females is intense, with a high proportion of males never mating (Cordero et al. 1997). Male–male mating behaviour is probably costly, both in terms of time wasted and because it may result in body damage to one or both actors. Our study species, Ischnura elegans, shows discrete female colour morphs and males show flexibility in preference for female morphs whereby the most common female morph in a population is preferred (Van Gossum et al. 1999a, 2001, but see Cordero et al. 1998). This positive frequency-dependent mate preference is thought ultimately to result in negative frequency-dependent selection on female morphs (Van Gossum et al. 2001).
2. Study species and Methods
Three adult female colour morphs coexist in I. elegans populations: two types of G-females (gynochrome) and one type of A-female (androchrome; Cordero et al. 1998). While A-females resemble the males completely in body coloration and pattern, G-females do not. As shown recently, males do not discriminate between the two G-morphs, probably because these morphs differ only slightly (Cordero et al. 1998; Van Gossum et al. 1999a). We treated G-females as one group for this study.
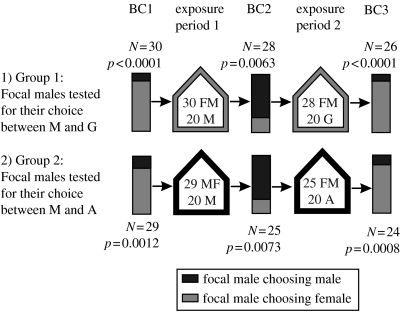
Male mate choice was examined directly after capture in a field population (32% of females were androchrome) in Niel, Belgium, using a first binary choice experiment (BC1; figure 1). All focal males (FM) received a small dot on the left forewing using a waterproof marker prior to being tested for their choice. Captured animals were stored in black film canisters for the time (15–45 min) between capture and the choice experiment to prevent visual or physical contact with conspecifics. Choice experiments were carried out in small cages (30 cm3; Van Gossum et al. 1999a). Individual males are not always eager to mate if presented with a female, which provided us with the opportunity to use such non-responsive males as non-focal males in our experiment. For every BC trial, one female and one such unresponsive male were brought into the cage prior to introducing the FM. All individuals could freely move around in this cage. FMs were randomly assigned to one of two groups. FMs of group 1 were tested for their choice between an unresponsive male and a G-female, while FMs of group 2 could choose between an unresponsive male and an A-female (figure 1). Before introducing an FM into the cage, we first held it by hand at the entrance of the cage while facing the individuals inside for approximately 10 s to enable visual experience with both individuals prior to choice. The majority of FMs approached an individual and clasped it at the pro-thorax with their anal appendages within the first 30 s (this is the tandem formation; females decide whether copulation occurs or not following tandem formation (Corbet 1999)). We considered the tandem formation as indication of male mate choice. The initiative to form a tandem was always taken by the FM. The trial was stopped when the tandem formation was realized or after the FM had been in the cage for 120 s (see also Van Gossum et al. 1999a, 2001). For each trial, a new set of animals was used.
Figure 1.
Mate choice by focal males (FM) for unresponsive males (M) or female morphs (A=androchrome, G=gynochrome) in binary choice (BC) experiments. The three successive BC experiments were separated by two exposure periods in insectaries. A binomial test was done for every BC. N values refer to FM preferring males versus FM preferring females.
After the choice experiment (BC1), the FMs that tried to clasp an individual were moved to an outdoor insectary with 20 unmarked males. Outdoor insectaries (3×3×2.5 m) contained small ponds and vegetation (for more details see Van Gossum et al. 1999b). The unmarked males were newly caught in the same source population in order to obtain males without BC experience. FMs and unmarked males were free to interact during a first exposure period of 2 days (exposure period 1; figure 1). A second BC experiment (BC2) was then conducted by again placing the FMs in small cages with freshly captured males and either G- (group 1) or A-females (group 2) from the source population. Afterwards, the FMs were returned to insectaries containing either 20 G- (group 1) or 20 A- (group 2) females (as in BC1) for a second exposure period (exposure period 2). Finally, the BC was repeated for a third time in an identical way (BC3). This experimental design resulted in our testing the mate choice decisions of responsive FMs after being exposed to males only or to males and females.
3. Results
In BC1, males of both group 1 (choice between unresponsive male and G-female) and group 2 (choice between unresponsive male and A-female) showed strong preference for females (BC1; figure 1). In group 1, 26 out of 30 FMs formed a tandem with a G-female while four FMs chose a male. In group 2, 23 FMs preferred an A-female while six FMs chose a male. After the first exposure period of 2 days in the insectary with only males, the FMs formed a tandem with males in the majority of the second BC trials (BC2; figure 1). In group 1, 21 FMs chose a male and seven FMs chose a G-female (two did not choose). In group 2, 19 FMs formed a tandem with a male while six FMs chose an A-female (four did not choose). Finally, after the second exposure period of 2 days in insectaries with both males and females, FMs preferred females in the majority of the third BC trials (BC3; figure 1). In group 1, out of 26 FMs, 23 formed a tandem with a G-female and three with a male. In group 2, out of 24 FMs, 20 preferred an A-female and four chose a male.
4. Discussion
Our results support the idea that male–male mating behaviour may be a consequence of adaptive plasticity in mate choice. Following each exposure period, FMs altered their choice drastically. Each time the social context was changed, the majority of males changed sex choice, initially choosing females, then choosing males in the second BC trial and again choosing females in the third trial. The lack of clear differences in results between group 1 and group 2 suggests that males can discriminate both between A-females (coloured as the male) and males and between G-females and males.
One often-used explanation for same-sex mating behaviour is that it only occurs because opposite-sex partners are absent or scarce (Bagemihl 1999). Hence, such behaviour should disappear once opposite-sex partners are available. The nature of our experiment, nonetheless, guaranteed availability of females in each choice experiment. However, following the first exposure period, the majority of FMs showed a preference for males if examined for their choice. Also after a longer confrontation with females (exposure period 2), some FMs still showed a preference for males. Furthermore, in the field where males and females were abundant, males mounted males regularly (note that 17% of males chose a male in BC1, indicating male–male mating behaviour to be common in I. elegans under natural conditions). Further, the extremely male-biased sex ratio may have caused males to become indiscriminate, resulting in more mating attempts with males because of their conspicuousness. However, A-females are also conspicuous but our results for group 1 and group 2 did not differ substantially.
Alternatively, some males may have an innate preference to mate other males. Rather than having an innate preference to mate males, males may adopt a preference for males in a critical period during early life (e.g. subadult phase after metamorphosis for damselflies) through imprinting (e.g. Kendrick et al. 1998) or at some point prior to adulthood (Hebets 2003). Although innate preferences or imprinting can explain why male–male mating behaviour was observed in all choice experiments, the switch in sex choice made by the majority of males cannot be explained in this way. A more flexible state-dependent explanation is needed to explain switches between the sexes in male mate choice in I. elegans.
Previously, male mate choice in I. elegans was shown to be plastic and dependent on female morph frequency (Van Gossum et al. 1999a; Van Gossum et al. 2001). Therefore, we suggest that the male–male mating behaviour described here might be maladaptive and a consequence of intense selection on plasticity in male mate choice of female morphs. We speculate that any selection for male mate choice patterns that avoid same-sex mating behaviour may be opposed by strong selection to preserve a flexible frequency-dependent choice pattern for female morphs. If true, this new hypothesis may apply to the many species showing female colour polymorphism, such as other damselflies and dragonflies as well as beetles, butterflies, hummingbirds and lizards.
Acknowledgments
The experiment described in this paper was performed according to the laws and ethical guidelines of Flanders (AMINAL-Flanders). H.V.G. and R.S. are postdoctoral fellows with the Fund for Scientific Research-Flanders (FWO). We are grateful to C. Beatty, T. Birkhead, S. Davis, O. Fincke, K. Lauwers, H. Leirs, T. Nilsson, J. Scheirs, M. Siva-Jothy and two anonymous referees for their comments on previous drafts of the manuscript.
References
- Andersson A. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Bagemihl B. St Martin's Press; New York: 1999. Biological exuberance: animal homosexuality and natural diversity. [Google Scholar]
- Corbet P.S. Harley Books; Colchester, UK: 1999. Dragonflies: behaviour and ecology of Odonata. [Google Scholar]
- Cordero A, Santolamazza S.S, Utzeri C. Male mating success in a natural population of Ischnura elegans (Vander Linden) (Odonata: Coenagrionidae) Odonatologica. 1997;26:459–465. [Google Scholar]
- Cordero A, Santolamazza S.S, Utzeri C. Mating opportunities and mating costs are reduced in androchrome female damselflies, Ischnura elegans (Odonata) Anim. Behav. 1998;55:185–197. doi: 10.1006/anbe.1997.0603. [DOI] [PubMed] [Google Scholar]
- David P, Bjorksten T, Fowler K, Pomiankowski A. Condition-dependent signalling of genetic variation in stalk-eyed flies. Nature. 2000;406:186–188. doi: 10.1038/35018079. [DOI] [PubMed] [Google Scholar]
- Fincke O.M. Female colour polymorphism in damselflies: failure to reject the null hypothesis. Anim. Behav. 1994;47:1249–1266. [Google Scholar]
- Harari A.R, Brockmann H.J. Male beetles attracted by females mounting. Nature. 1999;401:762–763. [Google Scholar]
- Harari A.R, Brockmann H.J, Landolt P.J. Intrasexual mounting in the beetle Diaprepes abbreviatus (L.) Proc. R. Soc. B. 2000;267:2071–2079. doi: 10.1098/rspb.2000.1251. doi:10.1098/rspb.2000.1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebets E.A. Subadult experience influences adult mate choice in an arthropod: exposed female wolf spiders prefer males of a familiar phenotype. Proc. Natl Acad. Sci. 2003;100:13 390–13 395. doi: 10.1073/pnas.2333262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick K.M, Hinton M.R, Atkins K, Haupt M.A, Skinner J.D. Mothers determine sexual preferences. Nature. 1998;395:229–230. doi: 10.1038/26129. [DOI] [PubMed] [Google Scholar]
- Kokko H, Brooks R, Jennions M.D, Morley J. The evolution of mate choice and mating biases. Proc. R. Soc. B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. doi:10.1098/rspb.2002.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.N, Fincke O.M. Cues for mate recognition and the effect of prior experience on mate recognition in Enallagma damselflies. J. Insect Behav. 1999;12:801–814. [Google Scholar]
- Qvarnström A. Context-dependent genetic benefits from mate choice. Trends Ecol. Evol. 2001;16:5–7. doi: 10.1016/s0169-5347(00)02030-9. [DOI] [PubMed] [Google Scholar]
- Stoks R, De Bruyn L. Unusual reproductive associations of Ischnura elegans. Notuale Odonatol. 1998;5:3–7. [Google Scholar]
- Van Gossum H, Stoks R, Matthysen E, Valck F, De Bruyn L. Male choice for female colour morphs in Ischnura elegans (Odonata: Coenagrionidae): testing the hypotheses. Anim. Behav. 1999a;57:1229–1232. doi: 10.1006/anbe.1999.1100. [DOI] [PubMed] [Google Scholar]
- Van Gossum H, Stoks R, De Bruyn L. Small outdoor insectaries as a tool for lifetime studies on damselflies. Belg. J. Zool. 1999b;129:317–324. [Google Scholar]
- Van Gossum H, Stoks R, De Bruyn L. Reversible frequency-dependent switches in male mate choice. Proc. R. Soc. B. 2001;268:83–85. doi: 10.1098/rspb.2000.1333. doi:10.1098/rspb.2000.1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widemo F, Saether S.A. Beauty in is the eye of the beholder: causes and consequences of variation in mating preferences. Trends Ecol. Evol. 1999;14:26–31. doi: 10.1016/s0169-5347(98)01531-6. [DOI] [PubMed] [Google Scholar]