Abstract
In traumatic insemination, males pierce females with hypodermic genitalia and ejaculate into the body cavity rather than into the genital tract. This has resulted in the evolution of female counter-adaptations in the form of paragenitalia to reduce the direct physical costs of mating. While rare in the animal kingdom, traumatic insemination is oddly prevalent in the true bug infraorder Cimicomorpha (Heteroptera), where it occurs in six families and is thought to have arisen twice. Here, we report the discovery of traumatic insemination and elaborate paragenital development in the plant bug genus Coridromius (Miridae), representing a third, independent emergence of traumatic insemination in this infraorder.
Keywords: entomology, evolution, traumatic insemination, sexual conflict, Miridae
1. Introduction
Conflict between the sexes over mating can result in coevolutionary ‘arms races’, where the need to control mating favours the rapid evolution of sexually antagonistic structures and behaviours (Parker 1979; Rice & Holland 1997; Arnqvist & Rowe 2005). In ‘traumatic insemination’ (TI) males pierce females with hypodermic genitalia and ejaculate into their body cavity rather than into their genital tract (Carayon 1966, 1977). It is thought that TI initially evolved as a coercive mating strategy, providing males with a means to circumvent female pre- or post-mating resistance (Stutt & Siva-Jothy 2001; Arnqvist & Rowe 2005). In bedbugs (Cimicidae), this form of insemination is known to impose costs on the female in the form of physical damage and an increased risk of infection (Stutt & Siva-Jothy 2001; Morrow & Arnqvist 2003; Reinhardt et al. 2003). To mitigate these costs females of many species have evolved unique internal and external paragenital adaptations (Morrow & Arnqvist 2003; Reinhardt et al. 2003) collectively known as the ‘spermalege’ (Carayon 1959, 1966, 1977).
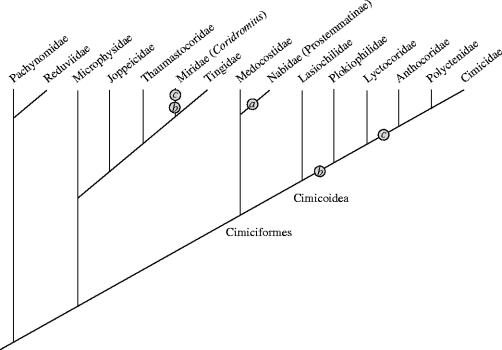
Although uncommon in the animal kingdom, TI occurs across a wide range of invertebrates (see Eberhard 1985; Arnqvist & Rowe 2005). It is most prevalent in the Heteropteran infraorder Cimicomorpha, of which the bedbugs are the most well studied (e.g. Carayon 1959, 1966, 1977; Hinton 1964; Stutt & Siva-Jothy 2001; Morrow & Arnqvist 2003; Reinhardt et al. 2003). In the Cimicomorpha TI is thought to have arisen twice; once in Prostemmatinae (Nabidae) and then again in the Cimicoidea (Anthocoridae, Plokiophilidae, Lyctocoridae, Polyctenidae and Cimicidae; Schuh & Štys 1991; figure 1).
Figure 1.
Phylogeny of the Cimicomorpha (Heteroptera). Taxa in bold are those which practice TI, while numbers indicate characters associated with TI. (a) Intragenital TI: in the Prostemmatinae the male inserts his genitalia into female genital opening and punctures the wall of the genital tract with a sharp process on the aedeagus (the acus). (b) Extragenital TI: in these taxa TI is extragenital (i.e. the male pierces the female body wall with his genitalia, never entering her genital tract). (c) Paramere and aedeagus coupled: the Plokiophilidae and Lyctocoridae puncture the female with an acus, while in the Anthocoridae, Polyctenidae, Cimicidae as well as the mirid genus Coridromius, the male's left paramere is coupled with the aedeagus, forming a scythe-like piercing intromittent organ. Adapted from Schuh & Štys 1991.
In the Prostemmatinae, mating begins normally with the insertion of the male genitalia into the female's reproductive tract. Instead of ejaculating into the female's genital cavity, the male pierces the wall of the bursa copulatrix with a sharp genitalic spicule (the acus) and deposits his semen in the haemocoel. The sperm then migrate to the ovaries, where fertilization takes place (Carayon 1966, 1977). In the Cimicoidea TI is extragenitalic, with the male puncturing the female's body wall and circumventing her genitalia entirely. In the more basal taxa (Plokiophilidae and Lyctocoridae) this is done using a sharpened spicule on the aedeagus, while in the Anthocoridae, Polyctenidae and Cimicidae the male's left clasping structure (paramere) is coupled with the aedeagus forming a scythe-like intromittent organ (Carayon 1966, 1977; Schuh & Štys 1991). These traits are mapped onto the Cimicoid phylogeny in figure 1.
The female paragenitalia (the spermalege), is composed of two distinct structures, the inner mesospermalege and the outer ectospermalege (Carayon 1959, 1966, 1977). In its simplest form the mesospermalege, which is pierced during mating, is a semi-enclosed organ lying beneath the site of copulation. This structure contains haemocytes that absorb seminal fluid and possibly digest sperm (Hinton 1964; Carayon 1966). Sperm that are not absorbed diffuse out of the mesospermalege into the haemolymph, eventually migrating to the ovaries for fertilization (Carayon 1966, 1977). In some taxa the mesospermalege is fully enclosed and connected to the genital tract via a conducting tube; thus the sperm never enter the haemolymph (Carayon 1959, 1966, 1977). The ectospermalege, when present, varies from a simple groove for guiding the male intromittent organ, to a copulatory tube formed by an invagination of the cuticle (Carayon 1966). In those species with a well-developed ectospermalege, the mesospermalege also tends to be more well defined and is never absent (Carayon 1966).
TI has never before been documented in other members of the Cimicomorpha, though some traits associated with this form of mating have been observed in a few phytophagus plant bugs (Miridae, Carayon 1984). Here, we report the discovery of TI and elaborate paragenitalia in the plant bug genus Coridromius (Miridae), representing a third, independent emergence of TI in the Cimicomorpha.
2. Study organisms
Coridromius is found throughout the Old World tropics and subtropics, spanning the Pacific Islands, Australia, Asia and Africa. Easily recognized by their stout-bodies and muscular jumping hindlimbs, these minute insects (2–4 mm in length) are found on a wide variety of plants (e.g. Euphorbiaceae, Epacridaceae, Proteaceae, Chenopodiaceae and Mimosaceae), where they appear to feed on pollen. Coridromius is unique among the Miridae in that the male's left paramere and aedeagus together form a hypodermic copulatory organ, analogous to that found in the Cimicidae, Anthocoridae and Polyctenidae. There are currently 11 known species, while another 20–30 remain to be described.
Our observations of Coridromius variegatus in New Caledonia suggest a mating system of intense intersexual conflict, where males attempt to mate with females without any indication of prior courtship. Males will pounce on the dorsum of passing females, attempting to stab them on the right side of the abdomen with their needle-like genitalia. Females respond by struggling vigorously in an effort to dislodge these suitors. Males are indiscriminate and will occasionally mount other males, provoking a similar response.
3. Material and methods
This discovery was made while examining pinned specimens from the following institutions: the American Museum of Natural History, New York; the National Museum of Natural History, Smithsonian Institution, Washington; the Zoological Institute, St Petersburg; the Bishop Museum, Hawaii; Nankai University, Tianjin; Naturhistorisches Museum, Vienna; Université Libre de Bruxelles, Brussels; the Natural History Museum, London, the Queensland Museum, Brisbane; the Australian Museum, Sydney. In total 25 species were examined, 17 of which are currently being described by the senior author.
Mirids are typically glued by the right side of the body onto cardboard ‘points’, thereby obscuring that side of the body. Thus, to locate the female ectospermalege specimens were first removed from their mounts by soaking in warm water. Hemelytra and hind wings were then removed with forceps, and specimens were remounted in such a way that any external paragenital modifications could be observed. When possible specimens were examined through dissection and scanning electron microscopy.
4. Results
Coridromius males exhibit classic TI genitalia which vary considerably between species (figure 2b,d,f,h,j), whereas the female genitalia are unspectacular and not unlike those of other non-traumatically inseminating plant bugs. However, closer examination reveals that while the female genitalia are unmodified the abdomen is often asymmetrical, with any irregularities always found on the right side of the body. These asymmetries appear to represent various components of the female ectospermalege.
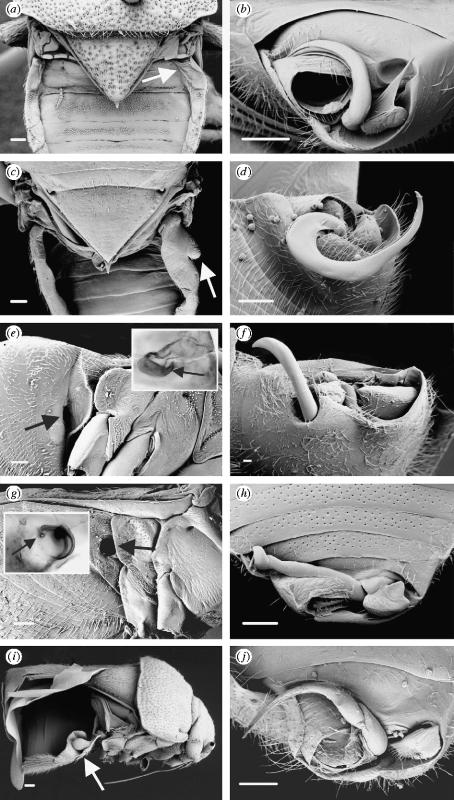
Figure 2.
Coridromius female paragenitalia (a,c,e,g,i) and corresponding male genitalia (b,d,f,h,j). Paragenital structures are indicated by arrows. C. chinensis (a,b): in the female, the first right lateral tergite is slightly swollen and thinly sclerotized (a). The site of copulation (not visible) is high on the anterior margin of the first visible ventral sternite, into which the male's scythe-like paramere (b) is inserted. Undescribed species from the Philippines (c,d): the paragenitalia of this species include swelling of both the dorsolateral tergites and the corresponding abdominal sternites. Undescribed species from Tahiti (e,f): in this species the female ectospermalege takes the form of a prominent copulatory tube (e), which opens into a thinly sclerotized cavity (e inset). C. zetteli (g,h): in C. zetteli, males have a corkscrewed intromittent organ (h) which is inserted into the female copulatory tube (g) located on the right side of the abdomen. This corkscrewed copulatory tube is punctured at its apex (g inset), indicating the point of insemination. Undescribed species from South Africa (i,j): in this species both females and males possess unusual ‘cup and bulb’ paragenitalia (i), the function of which is unknown. All scale bars, 100 μm.
Many species (e.g. C. variegatus) show little or no female paragenital development. Instead, mated females are marked by small melanized scars on the first right dorsolateral abdominal sclerite, indicating the site of copulation. Several other species, however, do exhibit a variety of paragenitalic modifications.
In some, such as Coridromius chinensis, the first right dorsolateral sclerite is slightly swollen and desclerotized, resembling a deflated sac (figure 2a). Dissections reveal the mesospermalege as a clear, membrane-bound organ nested within this structure. Rounded and balloon-like anteriorly, the mesospermalege narrows into a tube, its borders becoming ill defined as it approaches the common oviduct. Externally, a minute longitudinal depression leading to the anterior margin of the first visible abdominal sternite indicates the probable site of copulation. Other species, such as an unidentified species from the Philippines (figure 2b,c), exhibit much more extensive swelling and desclerotization of the lateral tergites, which is occasionally accompanied by lateral swelling of the corresponding abdominal sternites (figure 2c).
In other Coridromius the ectospermalege is expressed as a well-defined copulatory tube. In an undescribed species from Tahiti the posterior margin of the second abdominal sternite is flared outwards, exposing the opening of the ectospermalege (figure 2e). This cuticular invagination extends into the body cavity and opens up into a thinly sclerotized chamber (figure 2e inset). In this species no mesospermalege could be confirmed due to poor specimen quality.
Even more striking is the paragenital system of Coridromius zetteli, previously known only from male specimens. In C. zetteli males possess a corkscrewed intromittent organ (figure 2h), in contrast to the scythe-like structure most typical of the genus (e.g. figure 2b,d,f). Our discovery of the female of C. zetteli reveals a complementary corkscrewed copulatory tube on the first visible abdominal sternite (figure 2g), which is punctured apically at the point of insemination (figure 2g inset). The apex of the copulatory tube appears to be embedded in a long, narrow mesospermalege, but it is unknown how this organ interacts with the genital tract.
Some species possess paragenital structures that are much harder to decipher, as in an undescribed species from South Africa, where we find a cuticular ‘cup and bulb’ extending out of the second and third lateral tergites and concealed beneath the wings (figure 2i). Intriguingly, males also possess similar but reduced paragenitalia. This is comparable to males of the bedbug Afrocimex which also possess a female-like ectospermalege (Carayon 1959, 1966) and may reflect an evolutionary response to the costs of homosexual copulation attempts. In this species, the site of insemination is unknown.
5. Discussion
While TI has never before been recorded in the Miridae, Carayon (1984) described certain characteristics associated with this form of mating in several plant bugs, including: scarring and puncturing of the female genital tract by the male genitalia, modifications of the female genital wall to accommodate such damage, the escape of sperm from the genital tract through these copulatory wounds, and the survival of this sperm in the haemocoel. These he considered precursors to the emergence of TI, envisioning a transition from wounding of the female genital tract leading to intragenital TI (as in the Prostemmatinae) and culminating in extragenital TI with the simultaneous coevolution of the female spermalege. With Coridromius we show that TI does in fact occur in at least one genus of Miridae, representing a third, independently evolved case of TI in the Cimicomorpha. Rather than being more akin to what we see in the Prostemmatinae, TI in Coridromius is most similar to the more derived system of the Cimicoidea (i.e. extragenital TI), with piercing male parameres matched against elaborate female paragenital structures.
While undoubtedly a rare and novel outcome of intersexual conflict, within this infraorder at least TI is more common than previously believed. In the context of the current Cimicomorphan phylogeny, this discovery suggests that those traits which might predispose taxa to this form of mating are present fairly basally in the infraorder. Alternatively, we must also consider the possibility that the present phylogeny does not reflect the true evolutionary history of this group. The discovery of TI in the Miridae will facilitate comparative studies aimed at unravelling the selective forces that have led to this remarkable form of sexual conflict.
Acknowledgments
We wish to thank Sue Lindsay at the Australian Museum for SEM imaging and all those who loaned us material for this study.
References
- Arnqvist G, Rowe L. Sexual conflict. In: Krebs J.R, Clutton-Brock T.H, editors. Monographs in Behavior and Ecology. Princeton University Press; Princeton, NJ: 2005. [Google Scholar]
- Carayon J. Insémination par ‘spermalège’ et cordon conducteur de spermatozoids chez Stricticimex brevispinosus Usinger (Heteroptera, Cimicidae) Rev. Zool. Bot. Afr. 1959;60:81–104. [Google Scholar]
- Carayon J. Traumatic insemination and the paragenital system. In: Usinger R.L, editor. Monograph of the Cimicidae (Hemiptera–Heteroptera) Entomological Society of America; College Park, MD: 1966. pp. 81–166. [Google Scholar]
- Carayon J. Insémination extragénitale traumatique. In: Grassé P.P, editor. Traité de Zoologie 8(V-A) Masson; Paris: 1977. pp. 351–390. [Google Scholar]
- Carayon J. Faits remarquables accompagnant l'insémination chez certains Hétéroptères Miridae. Bulletin de la Société entomologique de France. 1984;89:982–998. [Google Scholar]
- Eberhard W.G. Harvard University Press; Cambridge, MA: 1985. Sexual selection and animal genitalia. [Google Scholar]
- Hinton H.E. Sperm transfer in insects and the evolution of haemocoelic insemination. R. Entomol. Soc. Lond. 1964;2:95–107. [Google Scholar]
- Morrow E.H, Arnqvist G. Costly traumatic insemination and a female counter-adaptation in bed bugs. Proc. R. Soc. B. 2003;270:2377–2381. doi: 10.1098/rspb.2003.2514. doi:10.1098/rspb.2003.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G.A. Sexual selection and sexual conflict. In: Blum M.S, Blum N.A, editors. Sexual selection and reproductive competition in insects. Academic Press; London: 1979. pp. 123–166. [Google Scholar]
- Reinhardt K, Naylor R, Siva-Jothy M.T. Reducing a cost of traumatic insemination: female bedbugs evolve a unique organ. Proc. R. Soc. B. 2003;270:2371–2375. doi: 10.1098/rspb.2003.2515. doi:10.1098/rspb.2003.2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R, Holland B. The enemies within: intergenomic conflict, interlocus contest evolution (ICE), and the intraspecific Red Queen. Behav. Ecol. Sociobiol. 1997;41:1–10. doi:10.1007/s002650050357 [Google Scholar]
- Schuh R.T, Štys P. Phylogenetic analysis of Cimicomorphan family relationships (Heteroptera) J. NY Entomol. Soc. 1991;99:298–350. [Google Scholar]
- Stutt A.D, Siva-Jothy M.T. Traumatic insemination and sexual conflict in the bed bug Cimex lectularius. Proc. Natl Acad. Sci. USA. 2001;98:5683–5687. doi: 10.1073/pnas.101440698. doi:10.1073/pnas.101440698 [DOI] [PMC free article] [PubMed] [Google Scholar]