Abstract
Mating success in plants depends largely on the efficiency of pollen dispersal. For hermaphrodite plants, self-pollination, either within or among flowers, can reduce mating opportunities because of pollen and ovule discounting and inbreeding depression. Self-pollination may be particularly detrimental in plants such as orchids and asclepiads that package each flower's pollen into one or more pollinia which, together with accessory structures, comprise a pollinarium. Darwin proposed that physical reconfiguration of pollinaria serves as a mechanism for reducing the likelihood of self-pollination. To be effective, the time taken for pollinarium reconfiguration would need to exceed that spent by a pollinator on a plant. We investigated pollinarium reconfiguration (including pollinarium bending, pollinium shrinking and anther cap retention) in 19 species and found a strong positive relationship between reconfiguration time and the duration of pollinator visits. Reconfiguration times were also consistently longer than pollinator visit times. These results provide strong support for Darwin's idea that this mechanism promotes cross-pollination.
Keywords: self-pollination, out-crossing, pollinia, pollination
1. Introduction
…the movement of depression in the pollinia does not commence (as I know by trial) until the pollinia are fairly withdrawn out of their cells [anthers]; nor will the movement be completed, and the pollinia be fitted to strike the stigmatic surfaces, until about half a minute has elapsed, which will give ample time for the moth to fly to another plant, and thus effect a union between two distinct individuals (Darwin 1862, p. 31, commenting on the common European orchid Orchis [Anacamptis] pyramidalis.)
Pollinator-mediated self-pollination can strongly depress fitness in plants. Female fitness is most obviously affected if it leads to inbreeding depression in progeny (Charlesworth & Charlesworth 1987; Darwin 1878; Keller & Waller 2002), but self-pollination can also reduce the pool of pollen available for export to other plants and can thus also reduce male fitness through pollen discounting (Barrett 2002a; Herlihy & Eckert 2002). Many plants, regardless of their degree of genetic self-incompatibility, possess physical mechanisms for promoting cross-pollination (Barrett 2002b). Well-documented mechanisms include dichogamy (differences in maturity of male and female organs; Bertin & Newman 1993), herkogamy (the spatial separation of male and female organs (Barrett 2002b), which includes stylar polymorphisms such as di- and tristyly (Cesaro & Thompson 2004; Barrett & Harder 2005)); ‘flexistyly’ (Li et al. 2001) and enantiomorphy (Jesson & Barrett 2002); rewardlessness (Dressler 1981; Johnson & Nilsson 1999; Johnson et al. 2004); and unisexuality (Barrett 2002b).
For orchids and asclepiads, which package their pollen into pollinia, self-pollination is potentially disastrous for three reasons. First, self-deposition of an entire pollinium may eliminate most or all of the opportunity for a flower's pollen to be exported (Johnson & Edwards 2000). Second, for self-compatible species, such as most orchids, ovules self-fertilized en masse are rendered unavailable for cross-fertilization (Barrett 2002a). Third, self-fertilization in orchids typically leads to rates of embryo abortion that are double those in seeds arising from cross-fertilization (Tremblay et al. 2005).
Pollinaria (comprising the pollen packets—the pollinia—as well associated accessory structures) often reorient gradually after withdrawal from the anther (figure 1a,b). This is typically due to bending or twisting of an accessory structure (such as a stipe or caudicle) that connects the pollinium to a sticky pad (the viscidium) in orchids (Johnson & Edwards 2000) or mechanical clamp (corpusculum) in asclepiads (Bookman 1981). These structures, in turn, attach the pollinium to the body of the pollinator. In orchids, the pollinium is rotated through an arc of 30–120° depending on the particular species. This movement is necessary for the pollinium to become orientated correctly for insertion into a stigma (figure 1c). In asclepiads the paired pollinia are initially flared at right angles, but reconfigure to be closely appressed to one another in the correct position to be inserted into the stigmatic chamber (Bookman 1981). Darwin was intrigued by this phenomenon and referred to it as a ‘beautiful contrivance’ that would function to reduce self-pollination if the time taken for its completion exceeds the duration of a pollinator's visit to a plant (Darwin 1862, p. 16). In the only previously published test of this idea, Johnson et al. (2004) confirmed that self-pollination in the European orchid Anacamptis morio does not take place unless pollinator visits exceed the time taken for pollinaria to undergo their bending movement. Other mechanisms of pollinarium reconfiguration may serve a similar function, including pollinia that shrink gradually to the correct size to be inserted into the stigmatic cavity (Borba & Semir 1999), and anther-caps that cover the pollinaria for a period following the pollinarium's removal (Catling & Catling 1991).
Figure 1.
Pollinarium bending—the most common form of pollinarium reconfiguration. (a) A pollinarium of the orchid Eulophia parviflora freshly affixed to a cetoniid beetle will bend in the direction of the yellow arrow as indicated in (b) with the pollinarium half bent. (c) After ca 100 s the pollinarium has reconfigured and the paired pollinia can be inserted into the stigma (white arrow) as the beetle backs out of a flower in the direction of the white arrow (shown in cross-section). Scale bar, 5 mm.
The timing of pollinarium reconfiguration varies extensively in orchids and asclepiads. If this duration is adaptive, then two logical predictions from Darwin's hypothesis are: (i) that variation in the timing of pollinarium reconfiguration reflects the duration of visits by a plant's pollinators, and (ii) that reconfiguration times of any particular species exceed the average duration of pollinator visits to plants of that species. Using data from 17 orchid and two asclepiad species, we explore the strength of support for Darwin's hypothesis that gradual reconfiguration of pollen after its removal from flowers serves as an anti-selfing mechanism that is finely tuned to the duration of pollinator visits to plants.
2. Methods
Data on the timing of pollinarium reconfiguration and pollinator visits to plants of 19 species were obtained from the literature (9 species) and our own observations in Sweden and South Africa (10 species; details and references given in table 1). Bending times of orchid pollinaria were recorded by withdrawing them from the anthers on the head of a pin and measuring their movement at suitable intervals with a protractor. Angles were plotted against time to determine when the pollinaria had stopped moving, which is unclear when pollinaria move slowly. The changing orientation of asclepiad pollinia was recorded by removing them from anthers with a hooked tip of an insect pin and photographing them at timed intervals using a dissecting microscope. Angles were measured from the subsequent photographs. In orchids in which pollinium insertion into stigmas is prevented by anther cap retention, the time taken for the anther cap to dry and fall off a pollinarium after it had been withdrawn was recorded. The duration of pollinator visits to inflorescences was recorded in the field with a portable voice recorder.
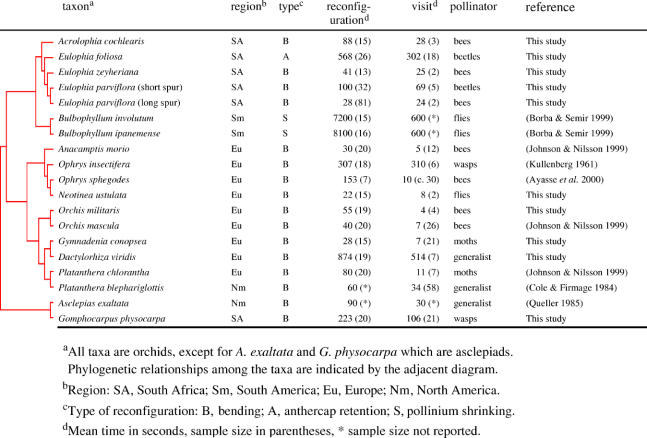
Table 1.
Characteristics and phylogenetic relationships of the study species.
The relationship between observed values of pollinator visit time and pollinarium reconfiguration time was analysed using standard major axis regression (Legendre 2001), while the relationship between standardized linear phylogenetically independent contrasts (PICs) of these values was analyzed in the program PDTREE (Garland et al. 1992). The latter were based on a phylogeny of the 19 study species (table 1) obtained from existing phylogenetic trees (Cameron et al. 1999; Bateman et al. 2003) trimmed to include just the 19 species of interest. Branch lengths were assigned according to Pagel's arbitrary method (Pagel 1992). Relationships within Eulophia have not been fully resolved and are based on an existing classification (Hall 1965). Changing the position of the three Eulophia species in the phylogeny had no influence on the results using PICs. The variance homogeneity of contrasts was verified by examining the linear relationship between the absolute value of the standardized contrasts and the sum of the squares of branch lengths (Garland et al. 1992).
3. Results
The average time taken for a species' pollinaria to reconfigure varied positively with the average time that pollinators spent visiting individual plants, both when actual values and phylogenetically independent contrasts are considered (figure 2a,b). Importantly, the duration of pollinarium reconfiguration almost always (in 18/19 cases, two-tailed sign test, p<0.001) exceeded pollinator residency times (figure 2b). Pollinarium reconfiguration times varied from 22 to 8100 s (mean=952 s, median=88 s). On average, reconfiguration times were 1.58 times longer than pollinator residency times.
Figure 2.
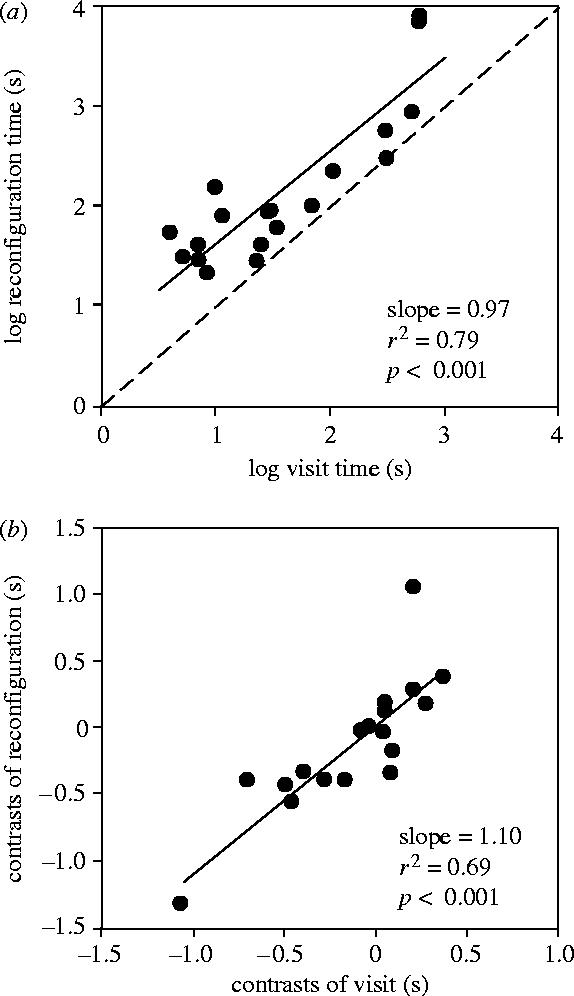
(a) There is a positive relationship between pollinarium reconfiguration and pollinator visit times. The data points are above the dashed line of unity, indicating that pollinarium reconfiguration tends to take place after the end of a pollinator visit. (b) The positive relationship between phylogentically independent contrasts of pollinarium reconfiguration and pollinator visit times.
4. Discussion
The findings of this study provide strong empirical support for the idea that promotion of cross-pollination underlies the evolution of pollinarium reconfiguration. As predicted by Darwin's hypothesis, pollinarium reconfiguration times are both positively related to, and invariably exceed, pollinator visit times (figure 2a,b).
Self-pollination could also be prevented effectively by very long pollinarium reconfiguration times; however, reconfiguration times that greatly exceed pollinator residency times could be detrimental. In particular, cross-pollination opportunities could diminish if pollinia are lost quickly from pollinators and/or if pollinators leave a patch of conspecific plants before reconfiguration is complete. Thus mating opportunities should be maximized if pollinaria reconfigure shortly after pollinators have departed from the source plant, so that pollinia are ready for insertion into stigmas of the next plant visited. The positive relationship between reconfiguration and pollinator visit times (figure 2a,b) is consistent with this theoretical prediction.
The evolutionary lability in the timing of pollinarium reconfiguration is evidenced from the variation observed in this trait among two subspecies of Eulophia parviflora. One subspecies pollinated by slow-moving beetles has pollinaria that take an average of 100 seconds to reconfigure (figure 1), while the other subspecies pollinated by rapidly moving bees has pollinaria that reconfigure in just 28 s (t111=17.53, p<0.0001). The mechanism(s) behind the bending movements of both orchid and asclepiad pollinaria remain largely undocumented, but preliminary work by the authors supports the suggestion by Darwin (1862) that reconfiguration in some orchids involves differential drying of layers of tissue of the accessory structures attaching the pollinia to the pollinators. Darwin noted that tissue of the viscidium and base of the stipe may be important in causing reconfiguration. In other orchids, tissue in the middle of the stipe appears to be responsible for reconfiguration. In some asclepiads, differential drying of tissue at the base of the translator arms may change the orientation of the pollinia (C. I. Peter, unpublished data).
Mechanisms that reduce the likelihood of self-pollination appear to be particularly prevalent in plant families in which pollen is aggregated as pollinia (cf. Johnson & Nilsson 1999; Harder & Johnson in press). Reconfiguration mechanisms have evolved at least four times (pollinarium bending in orchids and asclepiads, anther cap retention and pollinium shrinking in orchids) and perhaps many more times in these families. In this study, for example, the mechanism of bending in Eulophia and Acrolophia does not appear to be homologous with the mechanism found in the orchidoid species examined. While pollinarium reconfiguration is a mechanism confined to orchids and asclepiads, the remarkable evolutionary fine-tuning of this trait in response to pollinator visit times, as demonstrated in this study, conveys a broader message about the central role that pollinator behaviour plays in the evolution of plant traits that promote cross-pollination.
Acknowledgments
We thank Barry Lovegrove for assistance with the software to calculate contrasts and Lawrence Harder and Bruce Anderson for commenting on the manuscript. The work was support by grants from the National Research Foundation (South Africa) and Rhodes University.
References
- Ayasse M, Schiestl F.P, Paulus H.F, Lofstedt C, Hansson B, Ibarra F, Francke W. Evolution of reproductive strategies in the sexually deceptive orchid Ophrys sphegodes: how does flower-specific variation of odor signals influence reproductive success? Evolution. 2000;54:1995–2006. doi: 10.1111/j.0014-3820.2000.tb01243.x. [DOI] [PubMed] [Google Scholar]
- Barrett S.C.H. Sexual interference of the floral kind. Heredity. 2002a;88:154–159. doi: 10.1038/sj.hdy.6800020. doi:10.1038/sj.hdy.6800020 [DOI] [PubMed] [Google Scholar]
- Barrett S.C.H. The evolution of plant sexual diversity. Nat. Rev. Genet. 2002b;3:274–284. doi: 10.1038/nrg776. doi:10.1038/nrg776 [DOI] [PubMed] [Google Scholar]
- Barrett S.C.H, Harder L.D. The evolution of polymorphic sexual systems in daffodils (Narcissus) New Phytol. 2005;165:45–53. doi: 10.1111/j.1469-8137.2004.01183.x. doi:10.1111/j.1469-8137.2004.01183.x [DOI] [PubMed] [Google Scholar]
- Bateman R.M, Hollingsworth P.M, Preston J, Yi-Bo L, Pridgeon A.M, Chase M.W. Molecular phylogenetics and evolution of Orchidinae and selected Habenariinae (Orchidaceae) Bot. J. Linnean Soc. 2003;142:1–40. doi:10.1046/j.1095-8339.2003.00157.x [Google Scholar]
- Bertin R.I, Newman C.M. Dichogamy in angiosperms. Bot. Rev. 1993;59:112–152. [Google Scholar]
- Bookman S.S. The floral morphology of Asclepias speciosa (Asclepiadaceae) in relation to pollination and a clarification in terminology for the genus. Am. J. Bot. 1981;68:675–679. [Google Scholar]
- Borba E.L, Semir J. Temporal variation in pollinarium size after its removal in species of Bulbophyllum: a different mechanism preventing self-pollination in Orchidaceae. Plant Syst. Evol. 1999;217:197–204. doi:10.1007/BF00984365 [Google Scholar]
- Cameron K.M, Chase M.W, Whitten W.M, Kores P.J, Jarrell D.C, Albert V.A, Yukawa T, Hills H.G, Goldman D.H. A phylogenetic analysis of the Orchidaceae: evidence form RBCL nucleotide sequences. Am. J. Bot. 1999;86:208–224. [PubMed] [Google Scholar]
- Catling P.M, Catling V.R. Anther-cap retention in Tipularia discolor. Lindleyana. 1991;6:113–116. [Google Scholar]
- Cesaro A.C, Thompson J.D. Darwin's cross-promotion hypothesis and the evolution of stylar polymorphism. Ecol. Lett. 2004;7:1209–1215. doi:10.1111/j.1461-0248.2004.00683.x [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Ann. Rev. Ecol. Syst. 1987;18:237–268. doi:10.1146/annurev.es.18.110187.001321 [Google Scholar]
- Cole F.R, Firmage D.H. The floral ecology of Platanthera blephariglottis. Am. J. Bot. 1984;71:700–710. [Google Scholar]
- Darwin C. 1st edn. John Murray; London: 1862. On the various contrivances by which British and foreign orchids are fertilised by insects and on the good effects of intercrossing. [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The effects of cross- and self-fertilization in the vegetable Kingdom. 2nd edn. John Murray; London: 1878. [Google Scholar]
- Dressler R.L. The Orchids: natural history and classification. Harvard University Press; Cambridge, MA: 1981. [Google Scholar]
- Garland T, Harvey P.H, Ives A.R. Procedures for anlaysis of comparative data using phylogenetically independent constrasts. Syst. Biol. 1992;41:18–32. [Google Scholar]
- Hall A.V. Studies of the South African species of Eulophia. J. S. Afr. Bot. 1965 Supplementary volume No. V. [Google Scholar]
- Harder, L. D. & Johnson, S. D. In press. Adaptive plasticity of floral display size in animal-pollinated plants. Proc. R. Soc. Bdoi:10.1098/rspb.2005.3268 [DOI] [PMC free article] [PubMed]
- Herlihy C.R, Eckert C.G. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature. 2002;416:320–323. doi: 10.1038/416320a. doi:10.1038/416320a [DOI] [PubMed] [Google Scholar]
- Jesson L.K, Barrett S.C.H. Solving the puzzle of mirror-image flowers. Nature. 2002;417:707. doi: 10.1038/417707a. doi:10.1038/417707a [DOI] [PubMed] [Google Scholar]
- Johnson S.D, Edwards T.J. The structure and function of orchid pollinia. Plant Syst. Evol. 2000;222:243–269. doi:10.1007/BF00984105 [Google Scholar]
- Johnson S.D, Nilsson L.A. Pollen carryover, geitonogamy and the evolution of deceptive pollination systems in orchids. Ecology. 1999;80:2607–2619. [Google Scholar]
- Johnson S.D, Peter C.I, Agren J. The effects of nectar addition on pollen removal and geitonogamy in the non-rewarding orchid Anacamptis morio. Proc. R. Soc. B. 2004;271:803–809. doi: 10.1098/rspb.2003.2659. doi:10.1098/rspb.2003.2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L.F, Waller D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002;17:230–241. doi:10.1016/S0169-5347(02)02489-8 [Google Scholar]
- Kullenberg B. Studies in Ophrys pollination. Zool. Bidr. Uppsala. 1961;34:57. [Google Scholar]
- Legendre, P. 2001 Model II regression—user's guide. Département de sciences biologiques, Université de Montréal. Available at http://www.fas.umontreal.ca/biol/legendre/
- Li Q.J, Xu Z.F, Kress W.J, Xia Y.M, Zhang L, Deng X.B, Gao J.Y, Bai Z.L. Pollination: flexible style that encourages outcrossing. Nature. 2001;410:432. doi: 10.1038/35068635. doi:10.1038/35068635 [DOI] [PubMed] [Google Scholar]
- Pagel M.D. A method for the analysis of comparative data. J. Theor. Biol. 1992;156:431–442. [Google Scholar]
- Queller D.C. Proximate and ultimate causes of low fruit production in Asclepias exalta. Oikos. 1985;441:373–381. [Google Scholar]
- Tremblay R.L, Ackerman J.D, Zimmerman J.K, Calvo R.N. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biol. J. Linnean Soc. 2005;84:1–54. doi:10.1111/j.1095-8312.2004.00400.x [Google Scholar]