Abstract
Several authors suggest that dolphins use information obtained by eavesdropping on echoes from sonar signals of conspecifics, but there is little evidence that this strategy is used by dolphins in the wild. Travelling rough-toothed dolphins (Steno bredanensis) either exhibit asynchronous movements or an extremely synchronized swimming behaviour in tight formations, which we expect to facilitate eavesdropping. Therefore, we determined, whether either one or more dolphins were echolocating in subgroups that were travelling with asynchronous and synchronized movements. Since, the number of recording sequences in which more than one animal produced sonar signals was significantly lower during synchronized travel, we conclude that the other members of a subgroup might get information on targets ahead by eavesdropping. Synchronized swimming in tight formations might be an energetic adaptation for travelling in a pelagic dolphin species that facilitates eavesdropping.
Keywords: eavesdropping, echolocation, sonar, synchronized behaviour, communication
1. Introduction
Several authors suggest that dolphins might use information obtained by passive listening to echoes from sonar signals of conspecifics (Jerison 1986; Dawson 1991). Barret-Lennard et al. (1996) found a negative correlation between group size and echolocation rate per member in killer whales (Orcinus orca). They concluded that that echolocation information might be shared but there was no hint (e.g. no change in behaviour or spatial organization of the group) that the non-echolocating group members gathered information by eavesdropping. Eavesdropping has only been demonstrated in an experiment where a bottlenose dolphin (Tursiops truncatus) was trained to recognize certain objects from a sample with its melon exposed to air, while another dolphin was ensonifying the target (Xitco & Roitblat 1996).
Rough-toothed dolphins exhibit an extremely synchronized swimming behaviour in tight subgroups (Ritter 2002). We propose that this behaviour would be advantageous to facilitate eavesdropping. Kuc (2002) pointed out that the geometrical problem of calculating the position of an object by passive listening to sonar signals produced by other individuals is ‘ill-posed’ if the distance between the echolocating and listening animal is not known. In spite of this an individual should be able to estimate a minimum distance to a possible obstacle Kuc (2002). However, if two animals swim close together in synchrony and only one animal locates a target, the other animal receives a pulse–echo pair (pulse from the neighbour and echo from the target) which encodes target distance with a high degree of certainty. The direction, which is also necessary for localization is indicated by the echo direction. This indirect localization is improved when all group members swim in synchrony. Thus, the behaviour has the additional advantage that the animal has no difficulties in attributing the returning echoes to the emitted pulses, which is necessary in calculating the distance to a target.
If this is the case the number of echolocating animals per subgroup should be lower during synchronized travelling behaviour. Therefore, we determined, whether either one or more dolphins were echolocating in subgroups that were travelling with synchronized and asynchronous movements.
2. Material and methods
During a field study near La Gomera (Canary Islands), we investigated the echolocation behaviour of free-ranging rough-toothed dolphins (Steno bredanensis). The swimming and echolocation behaviour of rough-toothed dolphins was recorded using 10 s focal group time-sampling (called recording sequence). Recordings were made from a 13 m steel ketch during six different sightings (over a three month period) and sampling intervals between consecutive recording sequences were at least 5 min to minimize pseudo-replication of data (see Hulbert 1984). We recorded several different subgroups during each encounter but we have no photo ID data to exclude the possibility that the same group has been re-sampled during another sighting. Sequences were only recorded when all visible members of a focal group could be observed to echolocate in the direction of the hydrophone during travelling behaviour (as observed from the spreader of the main mast). Travelling was defined as a behaviour when all subgroups were moving in the same direction for at least 5 min. Swimming speeds were estimated by adjusting the boat speed to the travelling speed of the group. Since these readings do not necessarily reflect the exact swimming speed of the recorded subgroup, we only give a range of swimming speeds for both behaviours and did not perform any statistical analysis. Although echolocation clicks are very directional, this is only the case for the high frequency peaks in the spectrum (see Au 1993) and it is, therefore, unlikely that we missed click trains from individuals that were slightly off-axis. However, the fact that most recorded click trains contained clicks with high-frequency components (120 kHz) makes it very likely that in fact all analysed sequences were from the focal group that was observed to approach the hydrophone. Echolocation clicks were picked up with a HS 150 hydrophone (Sonar Research & Development Ltd, Beverley, UK) that showed a ±6 dB frequency response up to 200 kHz. Clicks were sampled at a rate of 480 kHz with a custom-made data acquisition card (‘PCTape’, University of Tübingen, resolution 16 bits), and stored digitally for subsequent analysis as a wave file on a laptop computer (Medion MD 9783, Windows 2000 system, custom-written recording software). With a custom-made sound analysis program (Selena, Tübingen, Germany) we displayed each of the recorded sequences in colour sonagrams. Since echolocation pulses emitted by one animal are either similar or gradually changing in their structure within a click train (e.g. Ford & Fisher 1978), we were able to determine whether one or more animals were echolocating by comparing waveforms, frequency structure and amplitudes of consecutive clicks (see electronic supplementary material).
3. Results
Similar to Ritter (2002) we found formations of 2–7 animals swimming in tight subgroups with exactly synchronized movements and diving patterns (figure 1, see also electronic supplementary material). The range of estimated travelling speeds was slightly lower in formations with synchronized movements (1.5–3 m s−1) in contrast to groups with asynchronous travelling behaviour (2–3.5 m s−1): The typical spatial organization of the two types of formations is shown in figure 1.
Figure 1.
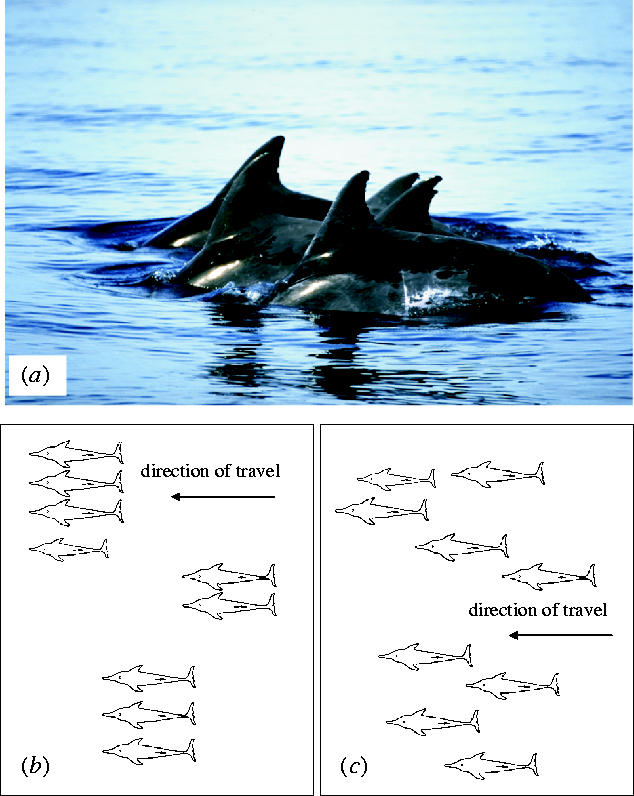
Spatial organization of a rough toothed dolphin group arranged in tight subgroups during slow travel with (a,b) synchronized movements and (c) during asynchronous travel. (Photograph courtesy of Fabian Ritter, MEEReV.)
The number of individuals per subgroup did not differ between synchronized (mean, 3.8±0.5) and asynchronous (mean, 4.1±0.6) swimming behaviour (Mann–Whitney U, p=0.28, n=23). In contrast, the number of recording sequences in which more than one individual produced echolocation clicks was significantly lower when the dolphins were travelling in tight subgroups with synchronized movements (figure 2; Fisher's exact test, χ2=7.61, p=0.006, n=23). During asynchronous travel there was always more than one animal echolocating.
Figure 2.
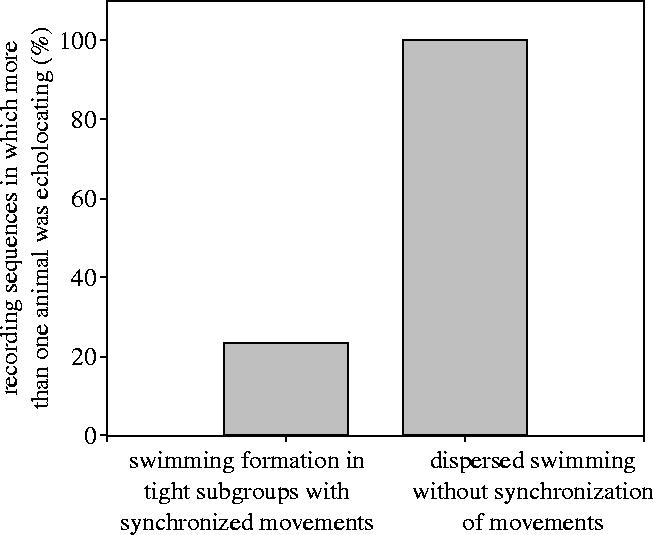
Percentage of recording sequences in which more than one individual was echolocating.
4. Discussion
In 80% of the sequences recorded during synchronous swimming only one animal in the formation was echolocating. We deduce that this behaviour is more advantageous than if every animal uses its own echolocation system. Echolocation of several dolphins would produce ambiguous echo scenery if the animals could not discriminate the echoes of their own signals from the echoes of the others. Most likely it would be unclear which echo was produced by which dolphin and the processing of wrong pulse–echo pairs would create virtual targets. However, ‘eavesdropping’ in terms of the analysis of the pulse–echo pairs of the one echolocating dolphin could deliver sufficient information on real targets ahead and would be facilitated by the synchronous swimming behaviour (see Kuc 2002). We, therefore, assume that synchronous swimming in connection with echolocation by only one animal suggests eavesdropping behaviour in rough-toothed dolphins.
Alternatively there is a chance that other group members were just physically following the echolocating animal without listening to returning echoes or that some individuals in a slowly travelling group were even sleeping (see Sekiguchi & Kohshima 2003). We think that the latter explanation is less likely since swimming speeds were usually higher than those used as a definition for resting behaviour in the wild (Mann & Watson-Capps 2005) and those reported for ‘swim–rest’ (associated with eye closure) in captive bottlenose dolphins (Sekiguchi & Kohshima 2003). Apart from that sleeping in whales and dolphins is almost exclusively unihemispheric with the contra-lateral eye opened most of the time (Lyamin et al. 2002) which means that some unilateral sensory input (e.g. from one ear) is still maintained. We believe that the given explanations are not mutually exclusive, since eavesdropping might occur at different cognitive levels. Non-echolocating group members could physically follow the movements of the subgroup not paying attention all the time but a strong echo return (e.g. from prey or a landmark) might catch their attention and could initiate ‘eavesdropping’.
Eavesdropping in terms of interpreting echoes from conspecifics' sonar signal was first raised by Jerison (1986) and then suggested by Dawson (1991) as a mechanism by which information in a dolphin group could be shared. The major concern about the hypothesis was based on the fact that neurobiological data on echolocation in bats indicated that neural feedback loops initiated by the sound production mechanism might be important in processing of pulse–echo pairs (see Suga 1990). Nevertheless, Xitco & Roitblat (1996) showed that a bottlenose dolphin was able to perform an object classification task without producing its own echolocation signals. There may still be constraints in what kind of situations eavesdropping can be used. The spatial arrangement in Xitco & Roitblat's (1996) experiments with both animals almost being in touch with each other is strikingly similar to the synchronized swimming pattern we found in the wild. Interestingly, the authors even reported that the listening animal (when positioned at a bite-plate) tended to slide as close to the inspecting animal as possible. This indicates that proximity seems to be of crucial importance for successful eavesdropping performance. At least target classification is likely to be limited to situations when the animals are closely aligned (Xitco & Roitblat 1996), since the structure of an echo of an object strongly depends on the direction from which it was ensonified and a distant dolphin would hear an entirely different echo signature (Helweg et al. 1996). Therefore, it would be interesting to test whether the performance of the listening animal deteriorates if both dolphins are further apart or differently orientated to each other. Additionally future studies in the wild using more sophisticated methods could provide further evidence in support of the hypothesis. This could be done by localizing individuals with hydrophone arrays (e.g. Schotten et al. 2002) and testing the influence of the spatial arrangement of group members on echolocation use in different behavioural situations.
A possible reason for travelling in subgroups with synchronized movements in a pelagic dolphin species might be the saving of energy caused by hydrodynamic effects, as shown for mother–calf pairs of spinner dolphins (Stenella longirostris, Weihs 2004). A follow up behaviour would be the ceasing of echolocation by all but one dolphin to avoid ambiguous echo scenery, and the retrieving of information by ‘eavesdropping’ conducted by the silent conspecifics. This behaviour would possibly even lead to further energy savings: There is preliminary data indicating that echolocation may have a measurable impact on resting metabolic rates (Cole and Speakesman 1993) but future studies will have to quantify the actual relevance in travelling dolphins.
Acknowledgments
We would like to thank Claudio (Claus Heinrichs) and the Club de Mar de La Gomera for the opportunity to use their boats and substantial support during field work. We would like to thank Saskia Knillmann, Martina Jacobson, Sabine Hartig and Bernd Metzler for their dedicated assistance during field work.
Supplementary Material
References
- Au W.W.L. Springer; New York: 1993. The sonar of dolphins. [Google Scholar]
- Barret-Lennard L.G, Ford J.K.B, Heise K.A. The mixed blessing of echolocation: differences in sonar use by fish-eating and mammal-eating killer whales. Anim. Behav. 1996;51:553–565. doi:10.1006/anbe.1996.0059 [Google Scholar]
- Cole, K. R. & Speakman, J. R. 1993 Do cetaceans have elevated resting metabolic rates? Abstracts 10th Biennial Conf. on the biology of marine mammals. Galveston, Texas, 12–15 November 1993 The Society for Marine Mammology.
- Dawson S.M. Clicks and communication: the behavioural and social contexts of Hector's dolphin vocalizations. Ethology. 1991;88:265–276. [Google Scholar]
- Ford J.K.B, Fisher H.D. Underwater acoustic signals of narwhals (Monodon monoceros) Can. J. Zool. 1978;56:552–560. [Google Scholar]
- Helweg D.A, Roitblat H.L, Nachtigall P.E, Hautus M.J. Recognition of aspect-dependent three-dimensional objects by an echolocating Atlantic bottlenose dolphin (Tursiops truncatus) J. Exp. Psychol.: Anim. Behav. Process. 1996;22:19–31. doi:10.1037/0097-7403.22.1.19 [PubMed] [Google Scholar]
- Hulbert S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984;54:187–211. [Google Scholar]
- Jerison H.J. The perceptual worlds of dolphins. In: Schusterman R.J, Thomas J.A, Woods F.G, editors. Dolphin cognition and behaviour: a comparative approach. Erlbaum; Hillsdale, NJ: 1986. pp. 141–166. [Google Scholar]
- Kuc R. Object localization from acoustic emissions produced by other sonars. J. Acoust. Soc. Am. 2002;112:1753–1755. doi: 10.1121/1.1508792. doi:10.1121/1.1508792 [DOI] [PubMed] [Google Scholar]
- Lyamin O.I, Mukhametov L.M, Siegel J.M, Nazarenko E.A, Polyakova I.G, Shpak O.V. Unihemispheric slow wave sleep and the state of the eyes in a white whale. Behav. Brain Res. 2002;129:125–129. doi: 10.1016/s0166-4328(01)00346-1. doi:10.1016/S0166-4328(01)00346-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J, Watson-Capps J.J. Surviving at sea: ecological and behavioural predictors of calf mortality in Indian Ocean bottlenose dolphins, Tursiops sp. Anim. Behav. 2005;69:899–909. doi:10.1016/j.anbehav.2004.04.024 [Google Scholar]
- Ritter F. Behavioural observations in rough-toothed dolphins (Steno bredanensis) off La Gomera (Canary Islands) with special reference to their interactions with humans. Aquat. Mamm. 2002;28:65–71. [Google Scholar]
- Schotten M, Au W.W.L, Lammers M.O, Aubauer R. Echolocation recordings and localizations of wild spinner dolphins (Stenella longirostris) and pantropical spotted dolphins (Stenella attenuata) using a four hydrophone array. In: Thomas J, Moss C, Vater M, editors. Echolocation in bats and dolphins. University of Chicago press; Chicago: 2002. pp. 340–393. [Google Scholar]
- Suga N. Cortical computational maps for auditory imaging. Neural Netw. 1990;3:3–21. doi:10.1016/0893-6080(90)90043-K [Google Scholar]
- Sekiguchi Y, Kohshima S. Resting behaviors of captive bottlenose dolphins (Tursiops truncatus) Physiol. Behav. 2003;79:643–653. doi: 10.1016/s0031-9384(03)00119-7. doi:10.1016/S0031-9384(03)00119-7 [DOI] [PubMed] [Google Scholar]
- Weihs D. The hydrodynamics of dolphin drafting. J. Biol. 2004;3:8. doi: 10.1186/jbiol2. doi:10.1186/jbiol2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xitco M.J, Roitblat H.L. Object recognition through eavesdropping: passive echolocation in bottlenose dolphins. Anim. Learn. Behav. 1996;24:355–365. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


