Abstract
Both male and female field crickets (Gryllus bimaculatus) autotomize front (tympanal) limbs more slowly than hind limbs. Arguably, this pattern could reflect possible differences in the mechanism of limb autotomy. However, we demonstrate that, for females, limb autotomy is also dependent on their mating status: virgin females autotomize front legs significantly more slowly than mated females. This response suggests a central control for leg autotomy in these animals, and less readiness to autotomize a front leg, possibly because the tympanum is crucial for mate location.
Keywords: phonotaxis, monaural, autotomy, Gryllidae, sex
1. Introduction
Crickets can, and will, autotomize their limbs when attacked by predators (Dixon 1989; Bateman & Fleming 2005, in press-a). This enables them to escape death (Bateman & Fleming in press-a), but imposes an immediate cost on future escape speed, and on mating ability (Bateman & Fleming 2005). Having lost a leg, crickets become less prepared to autotomize a second limb (Bateman & Fleming 2005, unpublished work), presumably due to increasing accrued costs.
Crickets suffer from the loss of even a single leg. By contrast, some arachnid species suffer minimal costs from autotomy of up to two legs, which has lead to the suggestion that that these animals may have a certain number of ‘spare legs’ (Macías-Ordoñez 1997; Guffey 1999). The difference between the groups is probably due to the more specialized function of each pair of legs in Orthoptera compared to arachnids. In Orthoptera, the hind legs are used for jumping: loss of one hind leg not only reduces their speed, but also the number of jumps performed during escape; loss of the second hind leg means that the cricket cannot jump at all (Gryllidae: Gryllus bimaculatus: Bateman & Fleming 2005; Acheta domestica: Bateman & Fleming in press-a). Loss of a middle leg reduces running escape speed to the same degree as losing a hind leg (Bateman & Fleming 2005). The front legs in these insects are possibly the most specialized of all, bearing the tympana.
Bateman & Fleming (2005) did not consider the cost to crickets of losing a front leg. Not only is the loss of a front leg likely to compromise locomotion, but possibly more importantly, loss of a tympanum-bearing front leg may compromise hearing and, therefore, phonotactic ability. Dixon (1989) found that the bushcricket Scudderia texensis (Tettigoniidae) took longer to autotomize tympanal legs than non-tympanal legs, and males took longer to autotomize a tympanal leg than did a female. This may be because male S. texensis perform phonotaxis when duetting with females. Also, Dixon (1989) suggested that, to females that have already mated (some tettigoniid females only mate once; Feaver 1982), loss of a tympanal leg would be trivial.
Female G. bimaculatus mate multiply (Tregenza & Wedell 1998) and are silent, but perform all the phonotaxis (Simmons 1988a; Weber & Thorson 1989). However, having mated, the phonotactic response immediately begins to decline in female G. bimaculatus (Loher et al. 1993), as for Gryllus integer (Lickman et al. 1998) and the tettigoniid Steropleurus stali (Bateman 2001). Older, unmated females of G. integer maintain a high phonotactic response, and also become less discriminatory over male song (Prosser et al. 1997). This suggests that, depending on their mating history, females may have a different perception of the costs involved with losing a tympanal leg. By contrast, although Gryllus males perform positive phonotaxis to other calling males in order to maintain rival-free zones (Simmons 1988b; Hissman 1990), directionality of hearing for males may not be as important as it is for females, and male crickets are, therefore, likely to accrue fewer costs (only locomotory) from the loss of a tympanal leg.
We have previously shown that G. bimaculatus alter their behaviour according to their physical condition: hind limb autotomized males and females take longer to emerge from shelter than intact individuals, and autotomized males take longer to resume singing after disturbance compared with intact males (Bateman & Fleming in press-b). We were also interested in discovering whether G. bimaculatus were able to make trade-offs between predation risk and compromised mating opportunities due to the loss of a tympanal leg. We predicted that both male and female G. bimaculatus may be less willing to lose a front (tympanal) leg compared with a hind limb, and that time to autotomize a limb would also be affected by mating history, with virgin females taking longest to autotomize an entrapped tympanal leg.
2. Material and methods
Penultimate instar male and female G. bimaculatus nymphs were maintained individually in 2 l plastic jars with food (fish flakes) and water ad libitum; all experiments were carried out on crickets between 10 and 12 days post-eclosion.
A total of 160 male and female crickets were randomly allocated to one of four trials, involving autotomy of either a front or a hind limb, remaining unmated or after mating.
(a) Virgin treatments
Crickets were induced to autotomize a randomly selected (left or right) front (tympanal) leg (n=20 males, n=20 females) or a hind leg (n=20 males, n=20 females) by holding the cricket between finger and thumb, gripping the leg firmly with forceps and releasing the cricket on a surface of rough cardboard so that it could only escape by autotomizing the entrapped limb. Time to autotomize the limb (s) was recorded with a stopwatch.
(b) Mated treatments
Each female was mated once each with two males, and each male was mated once each with two females. Twenty-four hours later, each cricket was induced to autotomize a randomly selected front leg (n=20 males, n=20 females) or a hind leg (n=20 males, n=20 females), as described previously, and the time to autotomize the limb recorded.
Time to autotomize a limb was analysed for differences between the sexes, status (virgin and mated individuals) and limb identity (front and hind legs) by three-way factorial analysis of variance (ANOVA). Analyses were carried out on log(log)-transformed autotomy time data, which did not violate the assumptions of the statistic. There were significant interactions between sex×status and sex×leg; males and females were therefore re-analysed separately by two-way ANOVA. Post hoc analysis was carried out by Tukey's Honest Significant Difference (Tukey's HSD) test. The level of statistical significance was set at α<0.05.
3. Results
Significant effects of both mating status and leg identity were observed for females (figure 1a). Hind legs were lost considerably faster than front legs (leg: F1,76=37.78, p<0.001), while virgins autotomized their legs more slowly than females that had already mated (status: F1,76=17.99, p<0.001). A significant interaction between these two factors (F1,76=9.48, p=0.003) reflected virgin females autotomizing their front legs significantly more slowly than did mated females.
Figure 1.
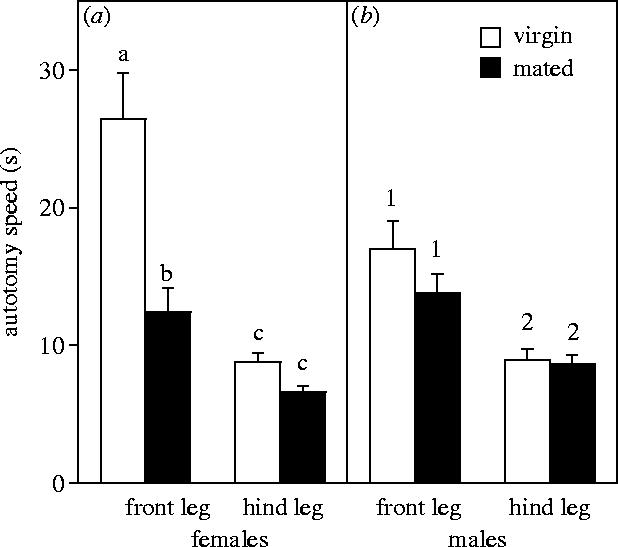
Mean+1 s.e. time to autotomize limb (s) for virgin and mated field crickets, Gryllus bimaculatus (n=20 for each treatment group). Identical letters and numbers link groups that were not significantly different from each other (post hoc Tukey's HSD analyses for (a) females and (b) males calculated separately).
Males (figure 1b) autotomized their hind limbs faster than their front legs (leg: F1,76=25.29, p<0.001). However, there was no significant effect of mating status on time to autotomize a limb for males (status: F1,76=1.78, p=0.186). The interaction between these factors was also not significant (F1,76=1.14, p=0.289).
4. Discussion
Our prediction that crickets would autotomize their tympanal legs less willingly than other limbs (due to the hearing specialization of these limbs) was supported by these data. Both males and females took longer to autotomize a front (tympanal) leg than a hind leg. We also recorded a significant effect of mating history for females; virgin females took longer to autotomize a front leg compared with mated females, but did not differ in the time taken to autotomize a hind limb. Finally, a males' mating history had no influence on autotomy speed.
In addition to loss of mobility and speed, loss of a tympanal front leg carries another cost in terms of loss of directionality of hearing—a cost that is likely to be most extreme for unmated females. Female crickets performing phonotaxis orient towards a male song using both tympana to detect the direction of the calls. A single tympanum means that, rather than approaching the song in a relatively straight line, female crickets lose orientation, turning away from the calling male or circling towards the intact side (e.g. Schmitz 1989; Kohne et al. 1992). Arguably, therefore, a front leg is more important to virgin females than other groups, reflected in their longer latency until autotomizing one of these limbs.
It might be argued that different autotomy mechanisms may account for delay in autotomizing front legs compared with other limbs. For example, autotomy has been associated with the location of specialized breakage planes in a number of taxa (see review by McVean 1975). Anatomical differences could easily account for differences between limbs, without invoking any central control over autotomy. Dixon (1989), for example, pointed out distinct differences in autotomy mechanisms between legs in the bushcricket S. texensis: the animal severs trapped front and middle legs with its mandibles, while hind legs are autotomized by dislocation. In the case of S. texensis, it would, therefore, be impossible to determine whether differences in time to autotomize a limb were a realistic reflection of ‘readiness’ to lose a limb. In G. bimaculatus, all pairs of legs are autotomized by dislocation. Furthermore, our data argue for a central control over limb autotomy, since the observed differences between front and hind legs were significantly modified by the reproductive status of females. This result is particularly exciting, since it supports the premise that tympanal legs are more important to virgin females than to mated females, linking reproduction and autotomy (a predation response) behaviour.
Central control over autotomy ‘decisions’ has previously only been demonstrated for crabs, to our knowledge. Robinson et al. (1970) demonstrated differences between crab species in readiness to autotomize chelipeds. For two crab species (Gecarcinus quadratus and Potamocarcinus richmondi), chelipeds latched onto an approaching putative predator (a toy bear), with limbs which were then were autotomized before the toy was pulled back (Robinson et al. 1970). The authors termed this ‘attack autotomy’, since the chelipeds remained attached to the predator, thereby serving to increase the harm of the encounter. This is a telling observation, since it demonstrates critical control of the autotomy reflex by higher nervous centres (McVean 1975). Inhibition to autotomize subsequent legs after already having autotomized a limb (demonstrated for crickets G. bimaculatus and A. domestica: Bateman & Fleming 2005; unpublished data; and in Hemigrapsus oregonensis crabs: Easton 1972) likewise points to a degree of central influence on autotomy (McVean 1975).
We have previously demonstrated that autotomy status affects reproductive behaviour in G. bimaculatus, with the loss of a single limb making crickets significantly more wary about emergence from cover, and with autotomized males remaining silent for significantly longer after disturbance than intact males (Bateman & Fleming in press-b). The present study adds data to the link between sensitivity to predation and reproductive behaviour, suggesting that virgin females will undergo a greater degree of risk (significantly longer autotomy time) before autotomizing tympanal legs. Reproductive history can, therefore, influence responses to putative predators in terms of the extreme form of escape, through sacrifice of a limb by autotomy.
References
- Bateman P.W. Changes in phonotactic behaviour of a bushcricket with mating history. J. Insect Behav. 2001;14:333–343. doi:10.1023/A:1011167128430 [Google Scholar]
- Bateman P.W, Fleming P.A. Direct and indirect costs of limb autotomy in field crickets Gryllus bimaculatus. Anim. Behav. 2005;69:151–159. doi:10.1016/j.anbehav.2004.04.006 [Google Scholar]
- Bateman, P. W. & Fleming, P. A. In press-a A cost of limb autotomy: increased susceptibility to predation for autotomized house crickets (Acheta domestica). Ethology
- Bateman, P. W. & Fleming, P. A. In press-b Sex, intimidation and severed limbs: the effect of simulated predator attack and limb autotomy on calling behavior and level of caution in the field cricket Gryllus bimaculatus Behav. Ecol. Sociobiol
- Dixon K. Effect of leg type and sex on autotomy in the Texas bush katydid, Scudderia texensis. Can. J. Zool. 1989;67:1607–1609. [Google Scholar]
- Easton D.M. Autotomy of walking legs in the Pacific shore crab, Hemigrapsus oregonensis. Mar. Behav. Physiol. 1972;1:209–217. [Google Scholar]
- Feaver M. Pair formation in the katydid Orchelimum nigripes (Orthoptera: Tettigoniidae) In: Gwynne D.T, Morris G.K, editors. Orthopteran mating systems: sexual selection in a diverse group of insects. Westview Press; Boulder, CO: 1982. pp. 205–239. [Google Scholar]
- Guffey C. Costs associated with leg autotomy in the harvestmen Leiobunum nigripes and Leiobunum vittatum (Arachnida: Opiliones) Can. J. Zool. 1999;77:824–830. doi:10.1139/cjz-77-5-824 [Google Scholar]
- Hissman K. Strategies of mate finding in the European field cricket (Gryllus campestris) at different population densities: a field study. Ecol. Entomol. 1990;15:281–291. [Google Scholar]
- Kohne R, Atkins S, Stout J, Atkins G. Enhanced calling song syllable period discrimination during one-eared phonotaxis by the female cricket (Acheta domesticus) J. Comp. Physiol. 1992;170:357–362. doi:10.1007/BF00191424 [Google Scholar]
- Lickman K, Murray A.-M, Cade W.H. Effect of mating on female phonotactic response in Gryllus integer (Orthoptera: Gryllidae) Can. J. Zool. 1998;76:1263–1268. doi:10.1139/cjz-76-7-1263 [Google Scholar]
- Loher W, Weber T, Huber F. The effect of mating on phonotactic behaviour in Gryllus bimaculatus (De Geer) Physiol. Entomol. 1993;18:57–66. [Google Scholar]
- Macías-Ordoñez R. 1997. The mating system of Leiobunum vittatum Say 1821 (Arachnida: Opiliones: Palpatores): resource defence polygyny in the striped harvestman. Ph.D. thesis, Lehigh University, Bethlehem, PA. [Google Scholar]
- McVean A. Autotomy. Comp. Biochem. Physiol. A. 1975;51:497–505. doi: 10.1016/0300-9629(75)90332-1. doi:10.1016/0300-9629(75)90332-1 [DOI] [PubMed] [Google Scholar]
- Prosser M.R, Murray A.-E, Cade W.H. The influence of female age on phonotaxis during single and multiple song presentations in the field cricket, Gryllus integer (Orthoptera: Gryllidae) J. Insect Behav. 1997;10:437–449. [Google Scholar]
- Robinson M.H, Abele L.G, Robinson B. Attack autotomy: a defence against predators. Science. 1970;169:301–302. doi: 10.1126/science.169.3942.300. [DOI] [PubMed] [Google Scholar]
- Schmitz B. Neuroplasticity and phonotaxis in monaural adult female crickets (Gryllus bimaculatus de Geer) J. Comp. Physiol. 1989;164:343–358. doi:10.1007/BF00612994 [Google Scholar]
- Simmons L.W. The calling song of the field cricket, Gryllus bimaculatus (De Geer): constraints on transmission and its role in intermale competition and female choice. Anim. Behav. 1988a;36:380–394. [Google Scholar]
- Simmons L.W. Male size, mating potential and lifetime reproductive success in the field cricket, Gryllus bimaculatus (De Geer) Anim. Behav. 1988b;36:372–379. [Google Scholar]
- Tregenza T, Wedell N. Benefits of multiple mates in the cricket Gryllus bimaculatus. Evolution. 1998;52:1726–1730. doi: 10.1111/j.1558-5646.1998.tb02252.x. [DOI] [PubMed] [Google Scholar]
- Weber T, Thorson J. Phonotactic behavior of walking crickets. In: Huber F, Moore T.E, Loher W, editors. Cricket behavior and neurobiology. Cornell University Press; Ithaca, Comstock: 1989. pp. 310–339. [Google Scholar]


