Abstract
Parasites influence host biology and population structure, and thus shape the evolution of their hosts. Parasites often accelerate the evolution of host defences, including direct defences such as evasion and sanitation and indirect defences such as the management of beneficial microbes that aid in the suppression or removal of pathogens. Fungus-growing ants are doubly burdened by parasites, needing to protect their crops as well as themselves from infection. We show that parasite removal from fungus gardens is more complex than previously realized. In response to infection of their fungal gardens by a specialized virulent parasite, ants gather and compress parasitic spores and hyphae in their infrabuccal pockets, then deposit the resulting pellet in piles near their gardens. We reveal that the ants' infrabuccal pocket functions as a specialized sterilization device, killing spores of the garden parasite Escovopsis. This is apparently achieved through a symbiotic association with actinomycetous bacteria in the infrabuccal pocket that produce antibiotics which inhibit Escovopsis. The use of the infrabuccal pocket as a receptacle to sequester Escovopsis, and as a location for antibiotic administration by the ants' bacterial mutualist, illustrates how the combination of behaviour and microbial symbionts can be a successful defence strategy for hosts.
Keywords: actinomycete, behavioural ecology, Escovopsis, host–parasite interaction, mutualism, pathogen
1. Introduction
Parasites are direct agents of natural selection; they regulate host populations and influence community and ecosystem dynamics (Poulin & Morand 2004). Social insects have a high risk of parasitism due to the high density and genetic homogeneity of individuals in colonies, thus they have developed a range of defensive mechanisms. Termites (Coptotermes) wall off nestmates infected by nematodes (Fujii 1975), honey bees remove larvae infected by foulbrood (Spivak & Reuter 2001) and ants have glands (metapleural) with antibiotic properties active against general microbial infections (Bot et al. 2002; Poulsen et al. 2002).
Fungus-growing ants (New World tribe Attini) provide their fungal cultivar with substrate for growth and, in return, the fungus serves as the ants' main food source (Weber 1972). The obligate nature of this symbiosis requires the ants to protect both themselves and their fungal mutualist from parasites. Fungus garden health is constantly threatened by microbes inhabiting the soil and substrates collected to manure the cultivar. To separate new gardens from soil, queens use hygienic strategies during nest construction (Fernandez-Marin et al. 2004). The fungus garden is also threatened by parasitic fungi in the genus Escovopsis (Ascomycota, Hypocreales), infections of which can be lethal if not controlled (Currie et al. 1999a). Leaf-cutter ants (the two most derived genera of fungus-growing ants) use specialized hygienic behaviours called fungus grooming and weeding to remove Escovopsis spores and infected garden material (Currie & Stuart 2001), and waste-management tasks are partitioned to prevent the spread of potentially harmful microbes from the refuse into the garden (Hart & Ratnieks 2001). To further defend against Escovopsis infection, fungus-growing ants have a mutualistic association with filamentous bacteria (actinomycete) housed on the ants' cuticle that produce antibiotics that specifically inhibit Escovopsis (Currie et al. 1999b).
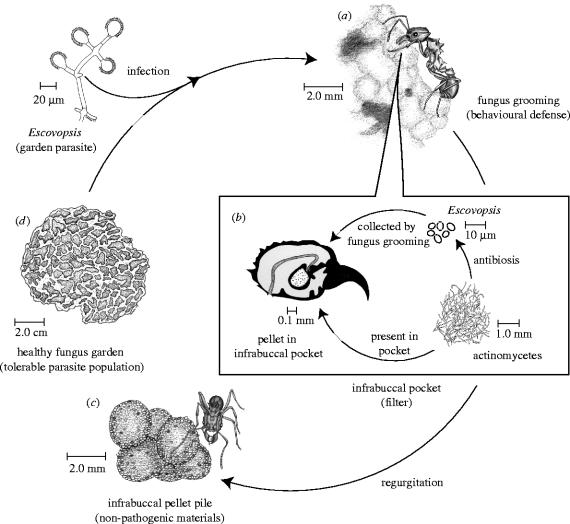
The infrabuccal pocket, a filtering structure within the oral cavity of ants, is a key component in leaf-cutter hygienic behaviours (Quinlan & Cherrett 1978). Detritus and potentially hazardous debris that ants gather while cleaning themselves, the nest area, or the fungus garden is accumulated in the pocket (Bailey 1920). Once full, the compressed material is expelled from the pocket as a pellet. To prevent microbes from re-establishing infection in the garden, leaf-cutter ants deposit their infrabuccal pellets in refuse piles segregated from their nest (Febvay & Kermarrec 1981). In contrast, ants in the more phylogenetically basal fungus-growing ant lineages stack and maintain their pellets in piles near their gardens (Little et al. 2003). It is currently unclear why most genera of fungus-growing ants build piles of infrabuccal pellets. In this study, we experimentally examine whether the piling of infrabuccal pellets is a response to garden infection by microbial parasites and we explore the microbial ecology of the infrabuccal pocket to further understand its role in fungus-growing ant nest hygiene.
2. Material and methods
(a) Study organisms
To examine the behavioural and microbial ecology associated with infrabuccal pellets, Trachymyrmex cf. Zeteki, which frequently build infrabuccal pellet piles (IPPs), was used as a model species (Little et al. 2003). In January 2003, 40 queenright colonies with 75–250 workers and a single fungus chamber were collected in Gamboa, Panama. Colonies were maintained at the University of Kansas in plastic dual chambers (one housing the garden and one for feeding, foraging and dumping of refuse) connected by plastic tubes, and placed on islands surrounded by mineral oil to prevent potential transfer of microbes between colonies via vectors (e.g. mites). Colonies were fed a mixture of dried oats and oak catkins and watered once a week.
(b) Experimental infections
We examined the function of IPPs by experimentally infecting gardens of 40 T. cf. zeteki colonies. To determine whether pellet piling is a response to general microbial parasites or to the specialized parasite Escovopsis, colonies were randomly assigned to one of four treatments: (i) Escovopsis, (ii) Trichoderma viride, a generalist mycoparasitic fungus closely related to Escovopsis (Currie et al. 2003a,b), (iii) non-viable Escovopsis (UV irradiated for 1 h to kill spores) or (iv) sterilized, distilled water. Pre-existing IPPs were removed prior to treatment. Escovopsis and T. viride strains (sub-cultured from single colony-forming units) were isolated in Gamboa from a T. cf. zeteki colony and the soil, respectively. Isolates were grown on potato dextrose agar (PDA) (Difco, Sparks, MD) with 1000 iu ml−1 of penicillin–streptomycin (MP Biomedicals Inc., Aurora, OH). Spores were added to ddH2O with Tween 20 [5×10−5] (Fisher Scientific, Pittsburgh, PA) to evenly disperse spores in solution. Each colony received 0.5 ml of solution (ca 40 000 spores) via mist inoculation, then colonies were monitored at regular intervals for 48 h.
(c) Microbial ecology
To further examine the role of IPPs in T. cf. zeteki garden hygiene, we isolated microbes from pellets dissected from the infrabuccal pocket and those in IPPs built after experimental treatments. From each pile present 48 h after treatments (15 piles total), 12 randomly selected pellets were removed. Six pellets per pile were particle-plated on PDA (with 1000 iu ml−1 penicillin–streptomycin) and six were particle-plated on actinomycete specific medium (chitin agar with 1000 iu ml−1 Nystatin, Sigma-Aldrich, St. Louis, MO) and incubated at 21 °C for 10 days and four weeks, respectively. Growth was monitored, and the presence of fungal cultivar, Escovopsis or actinomycetes was scored (present/absent) and then sub-cultured into pure culture.
Forty-eight hours post treatment, the heads of six ants from each of three (randomly chosen) Escovopsis-treated colonies were aseptically dissected and the pellets removed to identify viable microbes in infrabuccal pellets prior to regurgitation from the infrabuccal pocket. Three pellets from each colony were particle-plated on PDA, while the other three were particle-plated on chitin agar. Plates were incubated, and microbes were scored and sub-cultured as above.
To determine whether actinomycetes isolated from the infrabuccal pocket produce Escovopsis-inhibiting antibiotics, we challenged one actinomycete isolate (representative of the most common morphotype found during experimental isolation of T. cf. zeteki pellets) in vitro with Escovopsis (Currie et al. 1999b). Challenges were scored 10 days after Escovopsis inoculation, and growth/inhibition of Escovopsis was scored as complete inhibition or a reduction of growth when compared to Escovopsis growth in the absence of the actinomycete (n=15).
3. Results
(a) Experimental infections
In response to experimental infections, IPPs are built very quickly. Piles were present within 3 h following treatment in 60% of Escovopsis-infected colonies (figure 1). IPP construction differed significantly between experimental treatments (G-test, G=13.05, p<0.005, d.f.=3; figure 1). After 48 h, 80% of colonies exposed to Escovopsis had built IPPs, significantly more than control colonies (G=16.12, p<0.001, d.f.=1). The proportion of colonies that constructed IPPs was also significantly higher when exposed to viable Escovopsis than when treated with non-viable Escovopsis (G=7.72, p<0.010, d.f.=1). Although some piles were built in colonies exposed to Trichoderma and non-viable Escovopsis, their proportions were not significantly different from the control nests (G=2.776, p>0.050, d.f.=1; G=0, p>0.100, d.f.=1, respectively).
Figure 1.
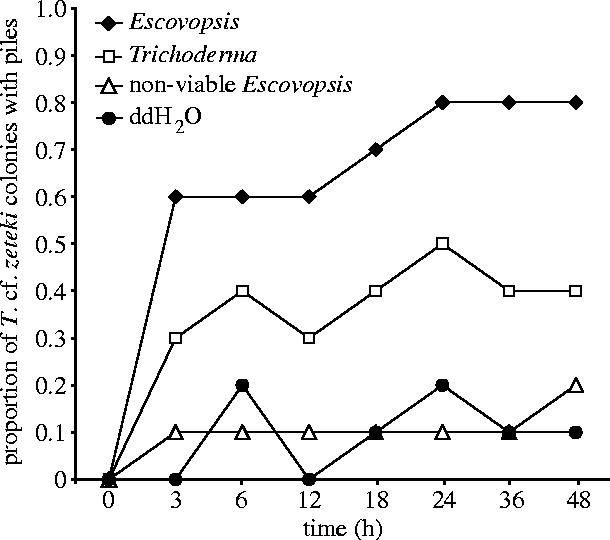
Proportion of Trachymyrmex cf. zeteki colonies that built infrabuccal pellet piles after exposure to one of four infection treatments over a 48 h period.
(b) Microbial ecology
Microbial isolations reveal that pellets in IPPs do not contain viable fungal cultivar tissue and very few contain viable Escovopsis (6.25%); however, many contain viable actinomycetes (64.44%) (table 1). Similarly, isolations from pellets dissected from the infrabuccal pocket do not contain viable cultivar or Escovopsis, but 88.9% contain actinomycetes (table 1). There were significantly more actinomycetes isolated from pellets in piles and pockets of Escovopsis-treated colonies than statistically expected when compared to sham-treated colonies (G=8.71, p<0.005, d.f.=1 for both non-viable Escovopsis and ddH2O). The proportion of actinomycetes isolated from pellets formed during Escovopsis treatment were significantly greater than those formed during Trichoderma treatments (G=3.88, p<0.050, d.f.=1).
Table 1.
Mean numbers of isolates per pellet, given as proportions, in which fungus-growing ant–microbe symbionts, cultivar, Escovopsis and actinomycetes were isolated following exposure to one of four infection treatments.
| proportion of infrabuccal pellets from which isolates were obtained±s.d. | |||||
|---|---|---|---|---|---|
| treatment | pellet location | sample size | cultivar | Escovopsis | actinomycetes |
| Escovopsis | pellet pile | 48 | 0.0 | 0.06±0.16 | 0.79±0.17 |
| infrabuccal pocket | 9 | 0.0 | 0.0 | 0.89±0.19 | |
| Trichoderma | pellet pile | 24 | 0.0 | 0.0 | 0.58±0.22 |
| non-viable Escovopsis | pellet pile | 12 | 0.0 | 0.0 | 0.33±0.24 |
| distilled water | pellet pile | 6 | 0.0 | 0.0 | 0.33±0.0 |
| total | 99 | ||||
Strong inhibition of Escovopsis by the infrabuccal actinomycete isolate was found in bioassay challenges as seen in Currie et al. (1999b), Germination and growth of Escovopsis were completely suppressed in 40% of challenges, while fungal growth in the remaining challenges was inhibited by 85.32±3.29% (mean±s.e.) when compared to Escovopsis growth in the absence of actinomycetes.
4. Discussion
The success of agriculture by ants has, in part, been attributed to efficient removal of microbial parasites from fungus gardens by workers (Currie & Stuart 2001). Our study shows that the removal of microbial parasites from fungus gardens is even more complex than previously realized and that the infrabuccal pocket plays a key role (figure 2). Experimental infection of T. cf zeteki gardens resulted in a fivefold increase in IPP abundance compared to baseline pile abundance in sham-treated nests (distilled water and non-viable Escovopsis). Examination of pellets dissected from the infrabuccal pocket, and of those collected after expulsion, confirms that pellets contain Escovopsis spores used to infect the garden (A. Little, personal observation). However, in addition to storing and compacting spores, we found that the infrabuccal pocket also appears to function as a specialized sterilization device, killing spores of the garden parasite Escovopsis. Microbial isolations of pellets taken from IPPs and those dissected from the infrabuccal pocket reveal that pellets rarely contain viable spores of Escovopsis.
Figure 2.
Removal of the fungus garden parasite Escovopsis by Trachymyrmex cf. zeteki. Following infection, ants collect parasitic spores in their infrabuccal pocket via fungus grooming (a). In the pocket, Escovopsis spores are exposed to inhibitory antibiotics produced by actinomycetous bacteria (b). Once the pocket is full, the non-pathogenic contents are regurgitated in the form of pellets and stacked together in piles tended by the ants (c), leaving the fungus garden with tolerable levels of the parasite (d).
Our finding of actinomycetes in infrabuccal pellets probably explains the absence of viable Escovopsis; antibiotics produced by actinomycetes in the infrabuccal pocket probably kill Escovopsis spores. The infrabuccal pocket is a confined space that permits the ants to place parasitic material in quarantine, subjecting it to antibiotics produced by their mutualistic bacteria, then expelling the resultant non-pathogenic material. Whether actinomycetous bacteria are housed within the infrabuccal pocket regularly or they are acquired from the ants' cuticle in response to infection is unknown. Other factors, such as enzymes in the salivary secretions of fungus-growing ants, may also aid in the inhibition of Escovopsis in the oral cavity (Febvay et al. 1984). Fungal inhibition in the infrabuccal pocket may be specific to Escovopsis; the fungal cultivar is successfully transmitted to new nests via the infrabuccal pocket (Weber 1972). Our data provide the first evidence that fungus-growing ants actively distinguish between viable and non-viable spores of Escovopsis, suggesting that the ants may recognize a secondary metabolite produced by Escovopsis, perhaps related to germination.
This study clearly indicates that the piling of infrabuccal pellets by T. cf. zeteki is a behavioural response to microbial infection of the fungus garden, particularly to Escovopsis. Interestingly, actinomycetes are found significantly more often in infrabuccal pellets formed after exposure to viable Escovopsis than in those formed after exposure to Trichoderma, non-viable Escovopsis or water. This could be the result of increased uptake of actinomycetes into the infrabuccal pocket from an external source, such as the exoskeleton, during times of infection. Alternatively, if actinomycete populations are maintained in the infrabuccal pocket it is possible that Escovopsis collected during infection stimulates the growth of actinomycetes in the infrabuccal pocket, as seen on the ants' cuticle during infection (Currie et al. 2003a).
Behaviour is an important part of an organism's parasite defence strategy and, in this case, hygienic behaviours are critical. The development of hygienic practices was very important in decreasing the prevalence of human disease (Ewald 1994), and sanitary measures, such as deep ploughing, pruning and burning, have been used for centuries to eradicate or decrease parasitic inoculum in agriculture (Agrios 1997). Apparently, natural selection pressures have led fungus-growing ants to evolve a similar suite of parasite defence strategies (Currie & Stuart 2001; Mueller et al. in press; this study). The association between fungus-growing ants, their cultivar and Escovopsis is ancient (Currie et al. 2003b). It is likely that prior to domestication by fungus-growing ants, the fungi they cultivate were parasitized by Escovopsis, thus the ants have had to adapt to the presence of Escovopsis for millions of years. Our data suggest that the development of the infrabuccal pocket as a receptacle for parasites and locale for antibiotic administration by the ants' bacterial mutualist may be one of the key evolutionary innovations in fungus-growing ants. This combination of behaviours and microbial symbionts, which constitutes the fungus-growing ants' parasite defence strategy, has undoubtedly allowed for their enduring success.
Acknowledgments
This study was supported by the NSF [IRCEB DEB-0110073]. We thank STRI, the Autoridad Nacional del Ambiente of the Republic of Panama for granting research permits and M. Leone, O. Arosemena, and STRI for logistical support. We are grateful to E. Davenport, S. Ingram, H. Reynolds for ant care and to S. Hoover, D.P. Hughes, and an anonymous reviewer for helpful comments on this manuscript.
References
- Agrios G.N. Plant pathology. 4th edn. Academic Press; San Deigo, CA: 1997. Control of plant diseases; pp. 174–218. [Google Scholar]
- Bailey I.W. Some relations between ants and fungi. Ecology. 1920;1:174–189. [Google Scholar]
- Bot A.N.M, Ortius-Lechner D, Finster K, Maile R, Boomsma J.J. Variable sensitivity of fungi and bacteria to compounds produced by the metapleural glands of leaf-cutting ants. Insect Soc. 2002;49:363–370. [Google Scholar]
- Currie C.R, Stuart A.E. Weeding and grooming of pathogens in agriculture by ants. Proc. R. Soc. B. 2001;268:1033–1039. doi: 10.1098/rspb.2001.1605. doi:10.1098/rspb.2001.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie C.R, Mueller U.G, Malloch D. The agricultural pathology of ant fungus gardens. Proc. Natl Acad. Sci. USA. 1999a;96:7998–8002. doi: 10.1073/pnas.96.14.7998. doi:10.1073/pnas.96.14.7998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie C.R, Scott J.A, Summerbell R.C, Malloch D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature. 1999b;398:701–704. doi:10.1038/19519 [Google Scholar]
- Currie C.R, Bot A.N.M, Boomsma J.J. Experimental evidence of a tripartite mutualism: bacteria protect ant fungus gardens from specialized parasites. Oikos. 2003a;101:91–102. doi:10.1034/j.1600-0706.2003.12036.x [Google Scholar]
- Currie C.R, Wong B, Stuart A.E, Schultz T.R, Rehner S.A, Mueller U.G, Sung G.-H, Spatafora J.W, Straus N.A. Ancient tripartite coevolution in the attine ant–microbe symbiosis. Science. 2003b;299:386–388. doi: 10.1126/science.1078155. doi:10.1126/science.1078155 [DOI] [PubMed] [Google Scholar]
- Ewald P.W. Oxford University Press; Oxford, UK: 1994. Evolution of infectious disease. [Google Scholar]
- Febvay G, Kermarrec A. Morphologie et fonctionnement du filter infrabuccal chez une attine Acromyrmex octospinosus (Reich) (Hymenoptera: Formicidae): role de la poche infrabuccale. Int. J. Insect Morphol. Embryol. 1981;10:441–449. doi:10.1016/0020-7322(81)90024-6 [Google Scholar]
- Febvay G, Decharme M, Kermarrec A. Digestion of chitin by the labial glands of Acromyrmex octospinosus Reich (Hymenoptera: Formicidae) Can. J. Zool. 1984;62:229–234. [Google Scholar]
- Fernandez-Marin H, Zimmerman J.K, Wcislo W.T. Ecological traits and evolutionary sequence of nest establishment in fungus-growing ants (Hymenoptera Formicidae, Attini) Biol. J. Linnean Soc. 2004;81:39–48. doi:10.1111/j.1095-8312.2004.00268.x [Google Scholar]
- Fujii, J. K. 1975 Effects of an entomogenous nematode, Neoaplectana carpocapsae Weiser, on the Formosan subterranean termite Coptotermes formosanus Shiraki, with ecological and biological studies on C. formosanus Ph.D. dissertation, University of Hawaii, Honolulu, Hawaii.
- Hart A.G, Ratnieks F.L.W. Task partitioning, division of labor and nest compartmentalization collectively isolate hazardous waste in the leaf-cutting ant Atta cephalotes. Behav. Ecol. Sociobiol. 2001;49:387–392. doi:10.1007/s002650000312 [Google Scholar]
- Little A.E.F, Murakami T, Mueller U.G, Currie C.R. The infrabuccal pellet piles of fungus-growing ants. Naturwissenschaften. 2003;90:558–562. doi: 10.1007/s00114-003-0480-x. doi:10.1007/s00114-003-0480-x [DOI] [PubMed] [Google Scholar]
- Mueller, U. G., Gerardo, N. M., Aanen, D. K., Six, D. L. & Schultz, T. R. In press. The evolution of agriculture in insects. Annu. Rev. Ecol. Syst
- Poulin R, Morand S. Smithsonian Books; Washington: 2004. Parasite biodiversity. [Google Scholar]
- Poulsen M, Bot A.N.M, Nielsen M.G, Boomsma J.J. Experimental evidence for the costs and hygienic significance of the antibiotic metapleural gland secretion in leaf-cutting ants. Behav. Ecol. Sociobiol. 2002;52:151–157. doi:10.1007/s00265-002-0489-8 [Google Scholar]
- Quinlan R.J, Cherrett J.M. Studies on the role of the infrabuccal pocket of the leaf-cutting ant Acromyrmex octospinosus (Reich) (Hym Formicidae) Insect Soc. 1978;25:237–245. [Google Scholar]
- Spivak M, Reuter G.S. Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie. 2001;32:555–565. doi:10.1051/apido:2001103 [Google Scholar]
- Weber N.A. Gardening ants: the attines. vol. 92. The American Philosophical Society; Philadelphia: 1972. [Google Scholar]