Abstract
Twenty years ago, the highest active glucose transport rate and lowest passive glucose permeability in vertebrates were reported in Rufous and Anna's hummingbirds (Selasphorus rufus, Calypte anna). These first measurements of intestinal nutrient absorption in nectarivores provided an unprecedented physiological foundation for understanding their foraging ecology. They showed that physiological processes are determinants of feeding behaviour. The conclusion that active, mediated transport accounts for essentially all glucose absorption in hummingbirds influenced two decades of subsequent research on the digestive physiology and nutritional ecology of nectarivores. Here, we report new findings demonstrating that the passive permeability of hummingbird intestines to glucose is much higher than previously reported, suggesting that not all sugar uptake is mediated. Even while possessing the highest active glucose transport rates measured in vertebrates, hummingbirds must rely partially on passive non-mediated intestinal nutrient absorption to meet their high mass-specific metabolic demands.
Keywords: hummingbird, digestion, glucose absorption, paracellular intestinal permeability
1. Introduction
Karasov, Diamond and colleagues (Diamond et al. 1986; Karasov et al. 1986) noted that hummingbird diets contain sugar at concentrations far above blood levels. They raised the question of whether sugar absorption could occur without any energy expenditure (by passive, non-mediated paracellular movement down a concentration gradient from intestinal lumen to blood) in hummingbirds, contrary to the contemporary prevailing view that absorption of water-soluble nutrients in vertebrates must occur by active, mediated transport, the rate of which saturates at low substrate concentrations (Karasov & Diamond 1983a, 1988). Using an in vitro technique where a sleeve of intestinal tissue is everted, mounted on a glass rod and incubated in a stirred solution containing radio-labelled solutes (Karasov & Diamond 1983b), they measured d-glucose uptake and apparent passive permeability to l-glucose, a stereoisomer of glucose that is not metabolized or transported by mediated pathways in birds (Chang et al. 2004). Their findings led them to conclude that the hummingbird intestine is adapted to a high-sugar diet in two ways: (i) low passive permeability to prevent harmful diarrhoea caused by concentrated sugar solutions passing through the gut and (ii) maximum density of glucose active transport sites (e.g. the sodium–glucose cotransporter SGLT1; Hediger 1994).
A mismatch between the maximal mediated glucose uptake capacity extrapolated from in vitro measurements and the metabolic demands of hummingbirds in the field (Powers & Nagy 1988) and the finding of significant passive non-mediated absorption of water-soluble nutrients in avian species with diverse diets and taxonomic affiliations, including frugivores and nectarivores (McWhorter 2005), led us to reexamine passive non-mediated glucose uptake in hummingbirds. Glucose uptake rates in Anna's hummingbirds measured in vitro were approximately fourfold lower than glucose absorption rates observed in vivo. Consider the following argument. (i) Assuming that absorption occurs at the saturating rate along the entire length of the intestine, the maximal rate of active glucose absorption by the small intestine was estimated as 1.46 μmoles min−1 (Karasov et al. 1986). (ii) This results in a glucose energy assimilation rate of ca 4.33 J min−1. Hummingbird diets are generally sucrose-rich (Pyke & Waser 1981) and sugar assimilation efficiencies are high (≥95%; McWhorter & Martínez del Rio 2000; Schondube & Martínez del Rio 2003). Assuming that an equal concentration of fructose is assimilated with equal efficiency (McWhorter & Martínez del Rio 2000), this results in a maximal energy assimilation rate of ca 8.66 J min−1, or 8.3 kJ day−1, assuming that 16 h of foraging time are available to the bird. (iii) The field metabolic rate of the Anna's hummingbird is ca 32 kJ day−1 measured using doubly labelled water (Powers & Nagy 1988). Therefore, the levels of active transport reported by Karasov, Diamond and colleagues (Diamond et al. 1986; Karasov et al. 1986) could not have accounted for all glucose uptake.
We conducted two experiments on broad-tailed hummingbirds (Selasphorus platycercus). The first experiment measured the fractional absorption or bioavailability of l-glucose (an in vivo test of passive non-mediated glucose absorption) in hummingbirds feeding on a sucrose solution close to the median concentration found in the wild (approximately 20% by mass; Pyke & Waser 1981). The second experiment probed the effect of food energy density (and thus, indirectly, digesta retention time; López-Calleja et al. 1997) on passive non-mediated glucose absorption.
2. Material and methods
Male broad-tailed hummingbirds (body mass=3.69±0.19 g, N=17) were captured in Albany County, Wyoming, USA. Routine animal husbandry and experimental housing, diets and sample collection were as previously published (Hartman Bakken et al. 2004). All animal experiments adhered to appropriate institutional regulations. In experiment 1, we measured the fractional absorption of 14C radio-labelled l-glucose (N=8) following Karasov & Cork (1994). Tests with 14C labelled l-glucose find that it is absorbed by non-mediated pathways, not catabolized, excreted quantitatively, and nearly all (>90%) label remains on the mother compound (Chang et al. 2004). Fractional absorption (F) was calculated as
| (2.1) |
where S is the probe distribution space (μl plasma), P is the steady-state feeding concentration of 14C l-glucose in plasma (radioactive disintegrations per minute (dpm) μl−1), kel is the elimination rate constant for l-glucose (per unit time), and I is the label intake rate (dpm per unit time). This indirect method, adapted from pharmacokinetic models of absorption, was used because l-glucose is excreted unaltered in urine, which is mixed with faeces in birds. Thus, traditional balance methods for measuring assimilation cannot be employed. For details of how we measured experimental parameters, see electronic supplementary material A.
To vary food intake rate in experiment 2, we fed birds 292 mM (N=5) and 876 mM (N=4) sucrose solutions and measured P and I as in experiment 1. Because this experiment simply tested for a diet treatment effect, we assumed that S and kel did not change with treatment. This was confirmed by finding that l-glucose distribution pool size and kel under the same treatments obtained in previous separate experiments (Hartman Bakken et al. 2004) did not differ significantly between diet treatments (F1,7=1.16, p=0.32 and F1,7=1.99, p=0.2, respectively).
3. Results
(a) Experiment 1
Hummingbirds ingesting the radio-labelled sugar solution reached steady state by 60 min. At this time there was a relatively high level of labelled l-glucose in their blood (372±16 dpm μl−1), indicating absorption. Taking into consideration their label ingestion rates (12 697±687 dpm min−1) and measured values for distribution space (747±64 μl plasma or 24.5±1.7% of body mass) and elimination rate constant (0.027±0.002 min−1), their calculated l-glucose fractional absorption was 0.59±0.05. Fractional absorption was significantly correlated with the ratio of blood plasma label concentration (P) to label ingestion rate (I) (F1,6=17.81, p=0.006, y=17.48x+0.044, r2=0.75; figure 1), an observation expected from the pharmacokinetic model we used and one we put to use in experiment 2 to infer the effect of diet treatment on l-glucose fractional absorption.
Figure 1.
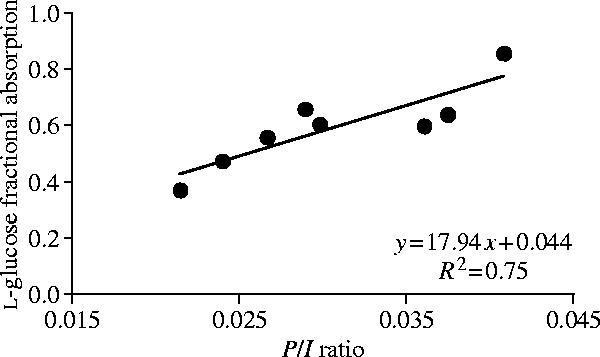
Fractional absorption, or bioavailability, of radio-labelled l-glucose in hummingbirds was significantly correlated with the ratio of the blood plasma label concentration (P) to label ingestion rate (I), an observation expected from pharmacokinetic models of absorption that allows one to infer the effect of diet treatment on l-glucose fractional absorption. Absorption of this non-transported glucose stereoisomer is an indicator of the passive (non-mediated) permeability of the gut to glucose.
(b) Experiment 2
Label ingestion rate was significantly greater when birds were feeding on the more dilute diet (11 290±670 dpm min−1 versus 7490±1472 dpm min−1 for 292 mM and 876 mM sucrose solutions, respectively; F1,5=9.47, p=0.028, figure 2a). There was no effect of body mass on marker ingestion rate; however, there was a significant interaction between diet treatment and body mass (F1,5=0.7, p=0.442 and F1,5=8.67, p=0.032, respectively). Steady-state plasma l-glucose concentration did not differ significantly between diet treatments (281±23 and 284±50 dpm μl−1, F1,7=0.004, p=0.95, figure 2b). The ratio of P to I was significantly greater in hummingbirds ingesting 876 mM sucrose compared with 292 mM sucrose (0.039±0.003 versus 0.025±0.002, respectively, F1,7=13.75, p=0.008, figure 2c), indicating that fractional absorption, which is directly proportional to this ratio (see figure 1), was increased on the higher sugar diet. When P/I ratio data from both experiments were combined there was also a significant treatment effect (F2,15=5.5, p=0.016); the 292 and 876 mM sucrose diet treatments were significantly different from each other but neither was significantly different from the 584 mM sucrose diet treatment (Tukey/Kramer HSD; figure 2c).
Figure 2.
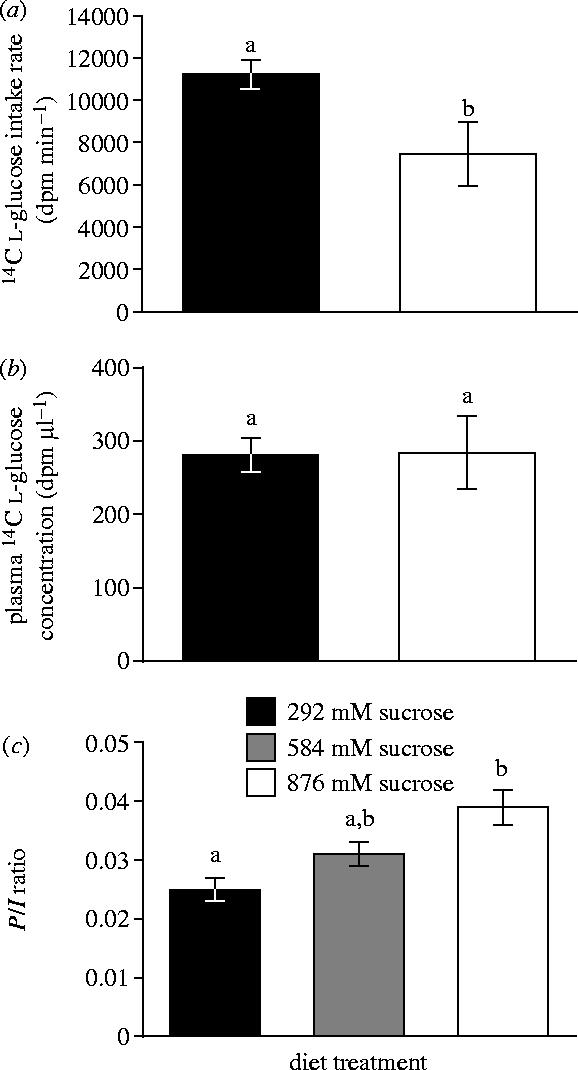
(a) Radio-labelled l-glucose ingestion rate was significantly greater when broad-tailed hummingbirds were feeding on the more energy-dilute 292 mM sucrose diet. This is expected, because food intake rate is inversely correlated with energy density in many vertebrates. (b) There was a relatively high concentration of radio-labelled l-glucose in the blood plasma of hummingbirds feeding at steady state, indicating absorption. Plasma label concentration did not differ significantly between birds feeding on the 292 and 876 mM sucrose diets. (c) The ratio of blood plasma label concentration (P) to label ingestion rate (I), and hence l-glucose fractional absorption, increased in stepwise fashion with diet sucrose concentration when data from both experiments were combined. This suggests that fractional absorption of l-glucose is correlated with the retention time of digesta in the gut. Error bars indicate ±1 s.e.m. Letters above error bars indicate statistically significant differences.
4. Discussion
Why should l-glucose fractional absorption increase on the higher sugar diet? The simplest explanation is that as diet energy density increases, so does digesta retention time in hummingbirds (López-Calleja et al. 1997) and thus the ‘contact time window’ for l-glucose to be absorbed increases. This suggests that l-glucose fractional absorption varies continuously with food intake rate, which is inversely related to digesta retention time. The passive permeability of the hummingbird intestine probably does not change with digesta transit or retention time, but rather the absolute amount absorbed via non-mediated pathways depends on contact time with absorptive surfaces (McWhorter & Martínez del Rio 2000). Sugar assimilation efficiencies measured in hummingbirds using traditional balance methods are high (≥95%) and independent of diet energy density (McWhorter & Martínez del Rio 2000; Schondube & Martínez del Rio 2003) and are thus independent of digesta retention time. This suggests that the relative contribution of non-mediated to total glucose uptake in hummingbirds may increase with diet energy density. The proportional contribution of non-mediated to total glucose uptake, impossible to estimate in hummingbirds in vivo because of their small size, is at least 50% of total glucose uptake in other species of birds with significant intestinal passive glucose permeability (Chang & Karasov 2004; McWhorter et al. 2005).
Starck et al. (2000) recently reported that the everted sleeve method may cause serious damage to intestinal tissues and lead to underestimates of in vivo uptake rates, which may partly account for the discrepancy between in vitro glucose uptake estimates and in vivo metabolic demands. However, the present study provides robust evidence for significant passive non-mediated absorption of water-soluble nutrients by hummingbirds and is similar to findings in other birds of less than 1 kg in body mass (McWhorter 2005). The everted sleeve method does not incorporate processes that can influence passive uptake, such as solvent drag across the paracellular junction between enterocytes (Pappenheimer 1993), which helps explain the immeasurably low passive glucose permeability previously reported (Diamond et al. 1986; Karasov et al. 1986). McWhorter & Martínez del Rio (1999) found that hummingbirds essentially completely absorb their exceptional preformed water loads in their intestines and dispose of excess water with their kidneys and via evaporative loss. This finding makes the intestinal osmotic diarrhoea scenario proposed by Diamond, Karasov and colleagues (Diamond et al. 1986; Karasov et al. 1986) unlikely because there would not be large volumes of fluid passing through the lower intestine.
Hummingbirds' mediated glucose absorption capacity is undoubtedly enormous, perhaps even higher than measured (Starck et al. 2000). Reliance on passive, non-mediated absorption provides the birds with an additional, non-saturating, energy-efficient absorptive capacity that is automatically matched to nutrient load (Karasov & Cork 1994). Mediated absorption may be important for setting up conditions for extensive passive absorption by paracellular diffusion and solvent drag (Pappenheimer 1993), and active transport becomes especially important for achieving quantitative extraction at lower substrate concentrations near the end of absorption for a meal (Chang et al. 2004). Nonetheless, the finding of significant non-mediated absorption of glucose in avian species with diverse diets and taxonomic affiliations suggests that nutritionally significant non-mediated absorption of water-soluble nutrients may be a general pattern in small birds.
How does the finding of significant non-mediated glucose uptake in hummingbirds influence our perspective on their digestive physiology and ecology and that of vertebrates in general? The conclusion reached by Diamond, Karasov and colleagues (Diamond et al. 1986; Karasov et al. 1986) that digestive processing time, rather than food collection, limits energy assimilation by hummingbirds is robust independently of the mechanism of nutrient uptake. This study and other recent research underscore how digestive physiology is an important determinant of feeding behaviour (e.g. nutrient absorption rate may limit energy assimilation and feeding rates in nectarivorous birds; Martínez del Rio et al. 2001; Fleming et al. 2004; Schondube & Martínez del Rio 2004), and are providing more correct mechanistic explanations of digestion in these exceptional animals. Hummingbirds exhibit the highest capacity for active, mediated glucose uptake among vertebrates, yet they still must rely partially on non-mediated passive uptake to meet their metabolic demands.
Acknowledgments
We thank Ella Tsahar for assistance with experiments. This work was supported by NSF grants IBN-0216709 to W.H.K., and IBN-0110416 to C.M.R.
Supplementary Material
References
- Chang M.H, Karasov W.H. How the house sparrow Passer domesticus absorbs glucose. J. Exp. Biol. 2004;207:3109–3121. doi: 10.1242/jeb.01154. doi:10.1242/jeb.01154 [DOI] [PubMed] [Google Scholar]
- Chang M.H, Chediack J.G, Caviedes-Vidal E, Karasov W.H. l-glucose absorption in house sparrows (Passer domesticus) is nonmediated. J. Comp. Physiol. B. 2004;174:181–188. doi: 10.1007/s00360-003-0403-3. doi:10.1007/s00360-003-0403-3 [DOI] [PubMed] [Google Scholar]
- Diamond J.M, Karasov W.H, Phan D, Carpenter F.L. Digestive physiology is a determinant of foraging bout frequency in hummingbirds. Nature. 1986;320:62–63. doi: 10.1038/320062a0. doi:10.1038/320062a0 [DOI] [PubMed] [Google Scholar]
- Fleming P.A, Hartman Bakken B, Lotz C.N, Nicolson S.W. Concentration and temperature effects on sugar intake and preferences in a sunbird and a hummingbird. Funct. Ecol. 2004;18:223–232. doi:10.1111/j.0269-8463.2004.00818.x [Google Scholar]
- Hartman Bakken B, McWhorter T.J, Tsahar E, Martínez del Rio C. Hummingbirds arrest their kidneys at night: diel variation in glomerular filtration rate in Selasphorus platycercus. J. Exp. Biol. 2004;207:4383–4391. doi: 10.1242/jeb.01238. doi:10.1242/jeb.01238 [DOI] [PubMed] [Google Scholar]
- Hediger M.A. Structure, function and evolution of solute transporters in prokaryotes and eukaryotes. J. Exp. Biol. 1994;196:15–49. doi: 10.1242/jeb.196.1.15. [DOI] [PubMed] [Google Scholar]
- Karasov W.H, Cork S.J. Glucose absorption by a nectarivorous bird: the passive pathway is paramount. Am. J. Physiol. 1994;267:G16–G26. doi: 10.1152/ajpgi.1994.267.1.G18. [DOI] [PubMed] [Google Scholar]
- Karasov W.H, Diamond J.M. Adaptive regulation of sugar and amino acid transport by vertebrate intestine. Am. J. Physiol. 1983a;245:G443–G462. doi: 10.1152/ajpgi.1983.245.4.G443. [DOI] [PubMed] [Google Scholar]
- Karasov W.H, Diamond J.M. A simple method for measuring intestinal solute uptake in vitro. J. Comp. Physiol. 1983b;152:105–116. [Google Scholar]
- Karasov W.H, Diamond J.M. Interplay between physiology and ecology in digestion. Bioscience. 1988;38:602–611. [Google Scholar]
- Karasov W.H, Phan D, Diamond J.M, Carpenter F.L. Food passage and intestinal nutrient absorption in hummingbirds. Auk. 1986;103:453–464. [Google Scholar]
- López-Calleja M.V, Bozinovic F, Martínez del Rio C. Effects of sugar concentration on hummingbird feeding and energy use. Comp. Biochem. Physiol. 1997;118A:1291–1299. doi:10.1016/S0300-9629(97)00243-0 [Google Scholar]
- Martínez del Rio C, Schondube J.E, McWhorter T.J, Herrera L.G. Intake responses in nectar feeding birds: digestive and metabolic causes, osmoregulatory consequences, and coevolutionary effects. Am. Zool. 2001;41:902–915. [Google Scholar]
- McWhorter T.J. Paracellular intestinal absorption of carbohydrates in mammals and birds. In: Starck J.M, Wang T, editors. Physiological and ecological adaptations to feeding in vertebrates. Science Publishers; Enfield, New Hampshire: 2005. pp. 113–140. [Google Scholar]
- McWhorter T.J, Martínez del Rio C. Food ingestion and water turnover in hummingbirds: how much dietary water is absorbed? J. Exp. Biol. 1999;202:2851–2858. doi: 10.1242/jeb.202.20.2851. [DOI] [PubMed] [Google Scholar]
- McWhorter T.J, Martínez del Rio C. Does gut function limit hummingbird food intake? Physiol. Biochem. Zool. 2000;73:313–324. doi: 10.1086/316753. doi:10.1086/316753 [DOI] [PubMed] [Google Scholar]
- McWhorter T.J, Karasov W.H, Green A.K. How the American robin absorbs glucose. FASEB J. 2005;19:A752. [Google Scholar]
- Pappenheimer J.R. On the coupling of membrane digestion with intestinal absorption of sugars and amino acids. Am. J. Physiol. 1993;265:G409–G417. doi: 10.1152/ajpgi.1993.265.3.G409. [DOI] [PubMed] [Google Scholar]
- Powers D.R, Nagy K.A. Field metabolic rate and food consumption by free-living Anna's hummingbirds (Calypte anna) Physiol. Zool. 1988;61:500–506. [Google Scholar]
- Pyke G.H, Waser N.M. The production of dilute nectars by hummingbird and honeyeater flowers. Biotropica. 1981;13:260–270. [Google Scholar]
- Schondube J.E, Martínez del Rio C. Concentration-dependent sugar preferences in nectar-feeding birds: mechanisms and consequences. Funct. Ecol. 2003;17:445–453. doi:10.1046/j.1365-2435.2003.00749.x [Google Scholar]
- Schondube J.E, Martínez del Rio C. Sugar and protein digestion in flowerpiercers and hummingbirds: a comparative test of adaptive convergence. J. Comp. Physiol. B. 2004;174:263–273. doi: 10.1007/s00360-003-0411-3. doi:10.1007/s00360-003-0411-3 [DOI] [PubMed] [Google Scholar]
- Starck J.M, Karasov W.H, Afik D. Intestinal nutrient uptake measurements and tissue damage: validating the everted sleeves method. Physiol. Biochem. Zool. 2000;73:454–460. doi: 10.1086/317738. doi:10.1086/317738 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


